This observational study describes the characteristics of spontaneous spinal cord infarction and proposes diagnostic criteria.
Key Points
Questions
What are the clinical and neuroimaging findings of patients with a spontaneous spinal cord infarction (SCI), and can diagnostic criteria be established to facilitate an accurate diagnosis?
Findings
Of 133 patients with a spontaneous SCI, 102 (77%) reached nadir within 12 hours, while others had a stuttering decline. Magnetic resonance imaging shows confirmatory (eg, vertebral body infarct) and supportive findings while excluding other etiologies; cerebrospinal fluid was usually noninflammatory (92%).
Meaning
The validation of spinal cord infarction diagnostic criteria supports their utility.
Abstract
Importance
Spinal cord infarction (SCI) is often disabling, and the diagnosis can be challenging without an inciting event (eg, aortic surgery). Patients with a spontaneous SCI are often misdiagnosed as having transverse myelitis. Diagnostic criteria for SCI are lacking, hindering clinical care and research.
Objective
To describe the characteristics of spontaneous SCI and propose diagnostic criteria.
Design, Setting, and Participants
An institution-based search tool was used to identify patients evaluated at Mayo Clinic, Rochester, Minnesota, from January 1997 to December 2017 with a spontaneous SCI. Patients provided written consent to use their records for research. Participants were 18 years and older with a diagnosis of spontaneous SCI (n = 133), and controls were selected from a database of alternative myelopathy etiologies for validation of the proposed diagnostic criteria (n = 280).
Main Outcomes and Measures
A descriptive analysis of SCI was performed and used to propose diagnostic criteria, and the criteria were validated.
Results
Of 133 included patients with a spontaneous SCI, the median (interquartile range) age at presentation was 60 (52-69) years, and 101 (76%) had vascular risk factors. Rapid onset of severe deficits reaching nadir within 12 hours was typical (102 [77%]); some had a stuttering decline (31 [23%]). Sensory loss occurred in 126 patients (95%), selectively affecting pain/temperature in 49 (39%). Initial magnetic resonance imaging (MRI) spine results were normal in 30 patients (24%). Characteristic MRI T2-hyperintense patterns included owl eyes (82 [65%]) and pencil-like hyperintensity (50 [40%]); gadolinium enhancement (37 of 96 [39%]) was often linear and located in the anterior gray matter. Confirmatory MRI findings included diffusion-weighted imaging/apparent diffusion coefficient restriction (19 of 29 [67%]), adjacent dissection/occlusion (16 of 82 [20%]), and vertebral body infarction (11 [9%]). Cerebrospinal fluid showed mild inflammation in 7 of 89 patients (8%). Diagnostic criteria was proposed for definite, probable, and possible SCI of periprocedural and spontaneous onset. In the validation cohort (n = 280), 9 patients (3%) met criteria for possible SCI, and none met criteria for probable SCI.
Conclusions and Relevance
This large series of spontaneous SCIs provides clinical, laboratory, and MRI clues to SCI diagnosis. The diagnostic criteria proposed here will aid clinicians in making the correct diagnosis and ideally improve future care for patients with SCI. The validation of these criteria supports their utility in the evaluation of acute myelopathy.
Introduction
Spinal cord infarctions (SCI) cause acute myelopathy with high morbidity.1 A confident diagnosis is challenging without an inciting event such as a surgical procedure.2 Onset is more protracted and radiologic distinction from competing diagnoses is more difficult than with cerebral infarction. Thus, patients with a spontaneous (ie, nonprocedural, nontraumatic) SCI often receive misdiagnoses. Although generally considered rare,3 recent literature suggests underdiagnosis of spontaneous SCI, with 2 large studies showing 14% to 16% of patients referred for the evaluation of transverse myelitis ultimately are diagnosed as having SCI.4,5 Misdiagnosis may expose patients to unnecessary and possibly deleterious treatments, as well as missed treatment opportunities and secondary stroke prevention. Moreover, the lack of diagnostic criteria hinders progress in the field of SCI. Based on an analysis of 75 cases of periprocedural SCIs,2 we applied insights from these definite cases to spontaneous SCI to better characterize its clinical, laboratory, and radiologic features and ultimately propose criteria for diagnosis of SCI.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the institutional review board of Mayo Clinic, Rochester, Minnesota. All patients provided written consent to the use of their medical records for research.
Patients and Inclusion Criteria
We used an institution research tool to identify patients with a spontaneous SCI evaluated at Mayo Clinic, Rochester, Minnesota, from January 1, 1997, to December 1, 2017. We searched clinical notes in the Impression and Diagnosis sections for the terms spinal cord infarction, spinal cord stroke, anterior spinal artery, posterior spinal artery, and vascular myelopathy. We subsequently reviewed the data of all patients to verify the diagnosis. None of the included cases had spinal cord trauma, compression, or a recent procedure within 1 month. Inclusion criteria were final diagnosis of spontaneous SCI and adequate clinical (clear timeline and specificity of deficits, appropriate alternative etiologies excluded) and radiologic data (neuroimaging consistent with SCI and/or rule out alternative etiologies). Patients with periprocedural SCI were excluded but are reported elsewhere.2 Furthermore, we excluded 46 patients in whom SCI was suspected but had incomplete data per our inclusion criteria.
Myelopathy Diagnostic Evaluation
The Mayo Clinic electronic medical record was reviewed for clinical details, laboratory values, neuroimaging findings, electrophysiological tests, and cerebrospinal fluid (CSF) results. All testing was performed as deemed appropriate by the managing physicians (including N.L.Z., A.A.R., R.D.B., E.F.M.W., B.G.W., A.J.A., J.D.B., and E.P.F.). Representative neuroimaging was reviewed on Quick Query Radiographs and Photographs Electronic Analysis and Display Station Neuroimaging Program.
Laboratory Evaluation
Laboratory evaluation varied as deemed appropriate by each physician and included vitamin B12, copper, zinc, syphilis serology, Lyme disease serology, varicella-zoster virus serology, HIV serology, human T-lymphotropic virus 1 serology, antinuclear antibody, antibodies to extractable nuclear antigens, anti–cyclic citrullinated peptide, anti–neutrophil cytoplasmic antibody, angiotensin-converting enzyme, hypercoagulable profile including antiphospholipid antibody evaluation, aquaporin-4–IgG, myelin oligodendrocyte glycoprotein–IgG, and paraneoplastic autoantibody evaluation.
Cerebrospinal Fluid Evaluation
Cerebrospinal fluid evaluations included white blood cell count and differential, red blood cell count, protein, and glucose. Additional CSF testing included IgG index, oligoclonal bands, cytology, flow cytometry, Gram stain, bacterial culture, venereal disease research laboratory, Lyme disease serology and polymerase chain reaction (PCR), cryptococcal antigen, angiotensin-converting enzyme, paraneoplastic autoantibody evaluation, varicella-zoster virus PCR, Epstein-Barr virus PCR, cytomegalovirus PCR, enterovirus PCR, mycobacterium tuberculosis PCR, and culture.
Neuroimaging
Mayo Clinic neuroimaging was performed with 1.5- and 3-T magnetic resonance imaging (MRI) Siemens and General Electric machines. Neuroimaging prior to Mayo Clinic evaluation varied. Images from other diagnostic centers were reviewed and compared with Mayo Clinic MRIs. Typical Mayo Clinic MRI sequences included sagittal T1, T2, short-τ inversion recovery, and axial T2 sequences. Gadolinium was administered at the discretion of ordering and performing services. Diffusion-weighted imaging (DWI) of the spine was not routinely performed. Imaging findings were documented according to initial appearance, with day 0 considered the initial day of deficit onset. The conus medullaris was defined as the very distal end of the spinal cord below the level of the lumbosacral enlargement.
Other Diagnostic Evaluation
Other variable diagnostic evaluation included brain MRI, cervical arterial imaging (magnetic resonance angiography, computed tomography angiography), thoracoabdominal computed tomography angiography, full spinal magnetic resonance angiography, digital subtraction angiography, and electromyography/nerve conduction studies.
Clinical Evaluation
Records were reviewed to verify clinical details, neuroimaging findings, and confirm the diagnosis. All patients were seen by Mayo Clinic consulting physicians. Forty-five patients from this series were included in previous studies,1,5 and a subset of 15 patients with posterior spinal artery infarctions were included in a brief report elsewhere.34 Time of onset was delineated by the initial neurological deficit (eg, weakness), not the onset of pain. Disability was measured by the American Spinal Injury Association Impairment Scale6 and ambulatory outcomes. Descriptive analyses were performed using JMP Pro 13 (SAS Institute Inc).
Validation Cohort
For validation of the proposed SCI diagnostic criteria, we retrospectively analyzed a cohort of 280 adult patients from our institution with alternative intrinsic myelopathy etiologies selected from a myelopathy database. Our cohort included patients with the following diagnoses: spinal dural arteriovenous fistula, 83 (29.6%); multiple sclerosis, 81 (28.9%); spinal cord sarcoidosis, 34 (12.1%)7; aquaporin-4 –IgG seropositive myelitis, 33 (11.8%)8; spinal cord metastasis, 29 (10.4%); myelin oligodendrocyte glycoprotein–IgG seropositive myelitis, 10 (3.6%); infectious myelitis, 8 (2.9%) (varicella-zoster virus, 4; herpes simplex virus-2, 1; cytomegalovirus, 1; West Nile virus, 1; syphilis, 1); collapsin response-mediator protein-5 (CRMP5/anti-CV2), 1 (0.4%); and nitrous oxide toxicity, 1 (0.4%). Clinical and diagnostic information was reviewed to see if patients met the proposed SCI diagnostic criteria at the time of clinical presentation.
Results
Patient Demographics
We identified 133 patients 18 years and older with a spontaneous SCI; 71 (53%) were women, and 124 (93%) were white. Median (interquartile range [IQR]) age at presentation was 60 (52-69) years and 52 (39-61) years for patients with suspected fibrocartilaginous embolism. Median (IQR) time from symptom onset to evaluation at our facility was 18 (3-217) days; 77 patients (58%) were evaluated at our facility within the first 31 days. Median (IQR) follow-up was 1 (1-19) month.
Clinical Features
Spontaneous SCI presented with rapid severe deficits (eg, paralysis) (102 patients [77%] nadir within 12 hours) (Table 1); 31 patients (23%) had a more prolonged time to nadir; however, all but 1 patient had a component of rapid decline with severe deficits within 12 hours. Selective pain and temperature sensory loss was present in 49 of 126 patients (39%). Severe back/limb pain was reported at onset in 96 patients (72%). A specific physical maneuver (eg, lifting, Valsalva) was described at or proceeding onset in 33 patients (25%). Clinical features are shown in Table 1.
Table 1. Clinical Features in Spontaneous Spinal Cord Infarction.
| Clinical Features | Patients, No./Total No. (%) (N = 133) |
|---|---|
| Time to nadir deficit | |
| ≤4 h | 74 (56) |
| Stuttering/stepwise decline | 2/74 (5) |
| 4-8 h | 21 (16) |
| Stuttering/stepwise decline | 1/21 (5) |
| 8-12 h | 7 (5) |
| Stuttering/stepwise decline | 3/7 (43) |
| 12-24 h | 18 (14) |
| Stuttering/stepwise decline | 12/18 (67) |
| >24 h | 13 (10) |
| Stuttering/stepwise decline | 13/13 (100) |
| Associated pain | 96 (72) |
| ≥ 1 h Before deficit | 42 (32) |
| At deficit onset | 81 (61) |
| Activity/movement before onset | 33 (25) |
| Paraplegia at nadir | 54 (41) |
| Quadriplegia at nadir | 11 (8) |
| Bowel/bladder dysfunction | 110 (83) |
| Examination findingsa | |
| Weakness | 118 (89) |
| Bilateral | 97/118 (82) |
| Sensory loss | 126 (95) |
| Bilateral | 104/126 (78) |
| Selective pain/temperature loss | 49/126 (39) |
| Sensory level | 93/126 (74) |
| Reflexes | |
| Absent reflexes/flaccid tone | 33 (25) |
| Extensor plantar response | 60 (45) |
Median (interquartile range), 18 (0-3720) days after onset.
Vascular Risk Factors
A history of 1 or more vascular risk factors was present in 101 patients (76%): hypertension, 61 (46%); smoking, 61 (46%); hyperlipidemia, 57 (43%); and diabetes mellitus, 21 (16%). Co-occurring vascular disorders included coronary artery disease, 14 (11%); peripheral vascular disease, 10 (8%); atrial fibrillation, 8 (6%); and previous cerebral ischemia, 3 (2%).
Cerebrospinal Fluid Analysis
A lumbar puncture from the acute evaluation was available in 89 patients (66.9%) with the following abnormalities: elevated nucleated cell count (>5 cells/mcL), 7 (8%) (median, 14; range, 6-21; neutrophilic predominance, 3; monocytic, 1; lymphocytic, 1; unknown, 2); elevated protein (>35 mg/dL), 66 (74%) (median, 57; range, 36-655); supernumerary oligoclonal bands (≥3 supernumerary bands), 2 (2%); and elevated IgG index, 1 (1%). None had findings consistent with a specific infection, autoantibody-mediated myelopathy, or other etiology.
Neuroimaging
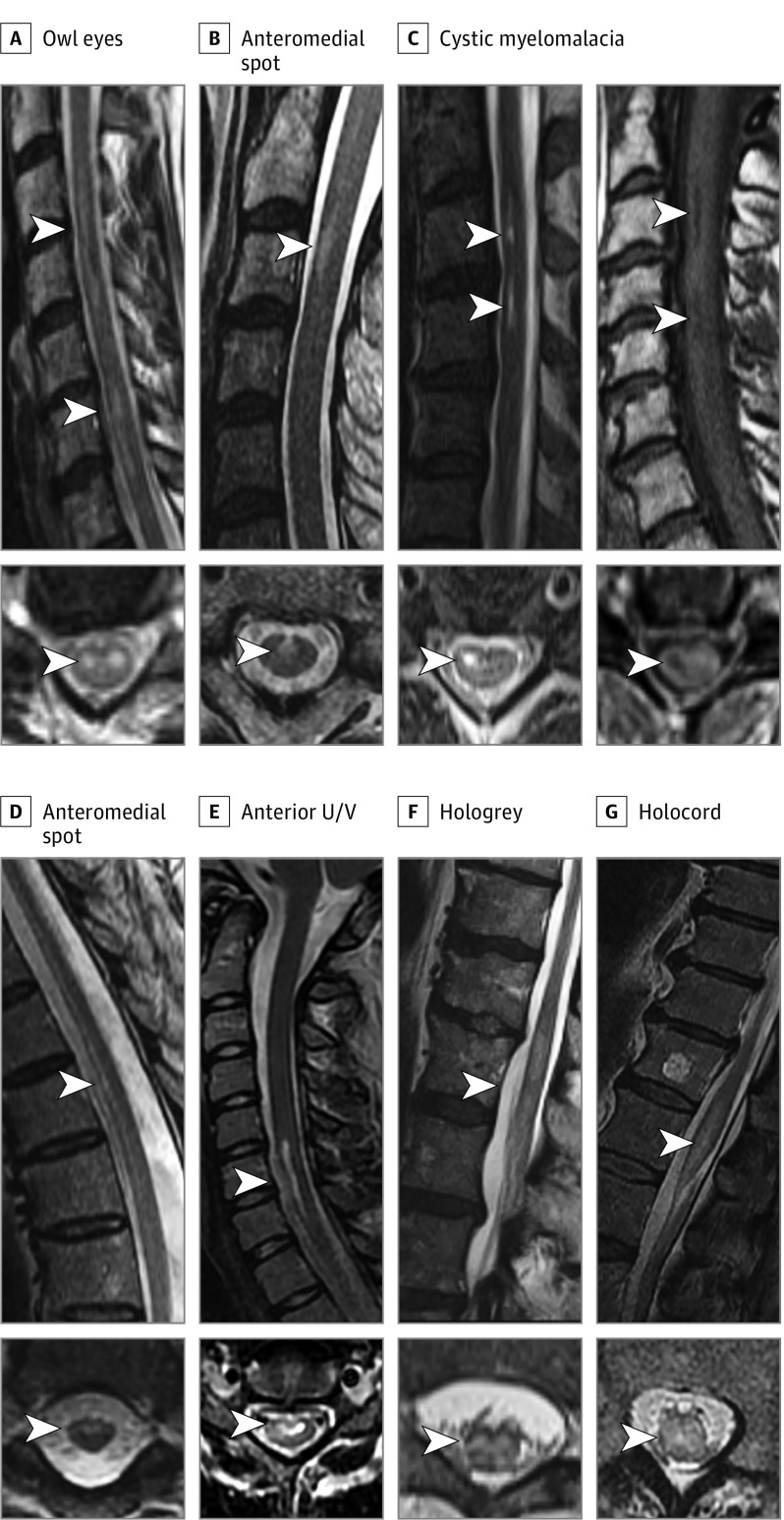
Magnetic resonance imaging spine findings were available in 126 patients (94.7%) (1.5-T, 112 [89%]; 3-T, 14 [11%]), (median [IQR] time to initial MRI was 1 [0-2] day); 7 patients (5.2%) did not have an MRI owing to a contraindication (eg, pacemaker, unstable condition). An intrinsic spinal cord lesion was demonstrated on MRI in all but 1 patient who had a normal MRI finding day 1 (flaccid paraplegia associated with aortic dissection). Thirty patients (24%) initially had a normal MRI result despite severe deficits (median day, 0; range day, 0-1). Subsequent imaging revealed T2-hyperintense cord lesions (median day, 4); 3 of these patients had acute DWI/apparent diffusion coefficient imaging, and 1 demonstrated restriction. The lesion axial localization and imaging pattern varied over the length of the lesion; a single infarct could have multiple patterns. The neuroimaging findings are shown in Table 2. The posterior one-third of the spinal cord was involved on 1 or more imaging slices in 57 patients (45%). Five patients with gadolinium enhancement and follow-up imaging available 1 to 3 months after SCI had resolution of enhancement (median, 2.5; range, 1.5-2.5 months). Focal cystic myelomalacia with bright T2-hyperintense signal accompanied by T1 hypointensity was seen in 31 of 82 cases (38%) on follow-up imaging (Figure 1). Vascular imaging (computed tomography angiography/magnetic resonance angiography/digital subtraction angiography) was performed in 82 patients and revealed a specific abnormality in 16 (20%): Stanford type A aortic dissection, 4; type B aortic dissection, 3; vertebral artery dissection, 4; anterior spinal artery occlusion, 3; aortoiliac occlusion, 1; and artery of Adamkiewicz occlusion, 1. Of patients with a suspected fibrocartilaginous embolism, 10 of 19 (53%) showed nearby/adjacent intervertebral disc extrusions.
Table 2. Neuroimaging Findings in Spontaneous Spinal Cord Infarction.
| MRI Findings | Patients, No./Total No. (%) (n = 126) | Median (IQR) Days After Deficit Onset |
|---|---|---|
| Confirmatory findings | ||
| DWI/ADC restriction | 19/29 (67) | 1 (0-5) |
| Vertebral body infarction | 11 (9) | 9 (1-16) |
| Adjacent arterial dissection/occlusion | 16/82 (20) | 0 (0-23) |
| T2-hyperintensity patterns | ||
| Owl eyes | 82 (65) | 3 (1-7) |
| Anterior pencil-like hyperintensity | 50 (40) | 8 (1-40) |
| Anterior U/V (letter shape) | 31 (25) | 20 (4-136) |
| Anteromedial spot | 30 (24) | 9 (4-148) |
| Hologrey | 24 (19) | 1 (1-10) |
| Holocord | 20 (16) | 5 (1-6) |
| Gadolinium enhancement | 37/96 (39) | 10 (4-18) |
| Linear strip (gray matter/arterial territory) | 34/37 (92) | NA |
| Nerve root enhancement | 8 (6) | 17 (6-31) |
| Other findings | ||
| Initial normal MRI results while symptomatic | 30 (24) | 0 (0-1) |
| Longitudinally extensive (≥3 vertebral segments) | 75 (60) | 5 (1-15) |
| Noncontiguous/patchy | 75 (60) | 9 (3-105) |
| Chronic spinal cord atrophy | 39/82 (48) | 158 (102-403) |
| Cystic myelomalacia | 31/82 (38) | 157 (92-221) |
| Edema/swelling | 27 (21) | 5 (2-6) |
| Concurrent acute cerebral infarct | 7/85 (8) | 2 (2-31) |
| Chronic cerebral infarct | 4/85 (5) | NA |
| Spinal level | ||
| Cervical | 37/125 (30) | NA |
| Thoracic | 34/125 (27) | NA |
| Thoracic through conus | 33/125 (26) | NA |
| Cervical through thoracic | 19/125 (15) | NA |
| Cervical through conus | 1/125 (1) | NA |
| Isolated conus | 1/125 (1) | NA |
Abbreviations: ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; IQR, interquartile range; MRI, magnetic resonance imaging; NA, not applicable.
Figure 1. Magnetic Resonance Imaging T2-Hyperintensity Patterns in Spinal Cord Infarctions.
Typical patterns of T2-hyperintense signal seen in spinal cord infarction (SCI) include owl eyes associated with noncontiguous anterior pencil-like hyperintensity (A); anteromedial spot confirmed with short anterior pencil-like hyperintensity on sagittal view (B); residual cystic myelomalacia with very bright T2-hyperintensity and associated T1-hypointensity seen longer than 1 month after SCI (C); anterior pencil-like hyperintensity with associated anteromedial T2-hyperintensity (D); anterior U/V with associated anterior pencil-like hyperintensity on sagittal view (E); hologrey pattern with associated edematous T2-hyperintensity on sagittal view (F); and holocord pattern with associated edematous T2-hypertensity extending through the conus on sagittal view (G).
Additional Diagnostic Evaluations
Ambulatory 24-hour Holter monitor was performed in 18 patients, and no specific contributing arrhythmia was identified. An echocardiogram in 52 patients (transesophageal, 35; transthoracic, 17) revealed the following findings: patent foramen ovale, 10; atrial enlargement, 3; Lambl excrescence, 2; spontaneous echo contrast, 1; atrial septal aneurysm, 1.
The following laboratory values were evaluated and abnormal: hemoglobin A1C (>5.6%; to convert to proportions of total hemoglobin, multiply by 0.01), 10 of 21 patients (48%); elevated low-density lipoprotein (>100 mg/dL; to convert to millimoles per liter, multiply by 0.0259), 29 of 55 patients (53%); hypercoagulable profile, 7 of 63 patients (11%) (antiphospholipid antibody positivity, 4; heterozygous prothrombin gene mutation, 2; low antithrombin, 1); connective tissue disease evaluation, 11 of 78 patients (14%) (mildly elevated antinuclear antibody, 10; cyclic citrullinated peptide elevation, 1); anti–neutrophil cytoplasmic antibody panel for vasculitis, 2 of 45 patients (4%) (both perinuclear anti–neutrophil cytoplasmic antibody positive); paraneoplastic autoantibody evaluation, 8 of 44 patients (nonspecific low titer elevations of no clinical significance in all). Aquaporin-4-IgG was negative in 54 patients evaluated. Electromyography/nerve conduction studies were performed in 19 patients, with neurogenic changes (large motor units, fibrillations) correlating to the area of ischemia in 13 of 19 patients (68%).
Suspected Mechanism of Infarction
The suspected mechanism of SCI was idiopathic with atherosclerotic risk factors, 91 (68%); fibrocartilaginous embolism, 19 (14%); aortic dissection, 7 (5%); hypercoaguability, 5 (4%); vertebral artery dissection, 4 (3%); systemic hypotension, 3 (2%); cardioembolic, 2 (2%); and vasculitis, 2 (2%).
Treatment
Immunotherapy was used for a suspected immune-mediated condition in 74 patients (56%): corticosteroids, 71; intravenous immunoglobulin, 16; plasma exchange, 12; azathioprine, 1; mycophenolate mofetil, 1; and rituximab, 1. Blood pressure augmentation was used in 9, lumbar drain in 8, and intravenous tissue plasminogen activator was administered to 2 patients. Anticoagulation was initiated in 11 patients, and at least 1 antiplatelet agent was used in 92 patients. Other treatments for suspected alternative diagnoses included spinal decompression, 3 (all patients worsened after surgery); cardiac stent placement, 1; and epidural corticosteroid injection for radiculopathy, 1.
Outcomes and Follow-up
At final follow-up at our facility, American Spinal Injury Association Impairment Scale outcomes were graded as follows: A, 12; B, 5; C, 23; and D, 93. Ambulatory outcome at last follow-up was: no gait aid, 63 (47%); cane, 13 (10%); walker, 20 (15%); wheelchair, 34 (26%); and death, 3 (2%). No patients developed recurrent episodes of SCI during follow-up.
Proposed Diagnostic Criteria
We propose diagnostic criteria for SCI in the Box, using the terms definite, probable, and possible SCI. Patients in our study met the following criteria: definite, 39 (29.3%); probable, 83 (62.4%); and possible SCI, 10 (7.5%). One patient did not meet diagnostic criteria, having severe pain without significant deficits.
Box. Proposed Spinal Cord Infarction (SCI) Diagnostic Criteria.
Criteria
-
Acute nontraumatic myelopathy (no preceding progressive myelopathy)
Onset to nadir severe deficitsa 12 h or less
If stuttering course is more than 12 h, severe deficitsa rapidly develop 12 h or less
-
Magnetic resonance imaging
No spinal cord compression
Supportive: Intramedullary T2-hyperintense spinal cord lesion (eBox 2 in the Supplement)
Specific (1 of): diffusion-weighted imaging/apparent diffusion coefficient restriction, associated vertebral body infarction, arterial dissection/occlusion adjacent to lesion
-
Cerebrospinal fluid
Noninflammatory (normal cell count, IgG index and no oligoclonal bands)
-
Alternative diagnoses
Alternative diagnosis is not more likely (eBox 1 in the Supplement)
Type of SCI
Definite spontaneous SCI (1, 2A, 2B, 2C, 4)
Probable spontaneous SCI (1, 2A, 2B, 3, 4)
Possible spontaneous SCI (1, 4)
Definite periprocedural SCI (1, 2A, 2B, 4)
Probable periprocedural SCI (1, 4)
Validation Cohort
Of 280 patients from our validation cohort, 9 patients (3.2%) met criteria for possible SCI at presentation, but diagnostic evaluation revealed an alternative diagnosis was more likely in all: spinal dural arteriovenous fistula, 5 (MRI flow voids); multiple sclerosis, 2 (multiple demyelinating lesions on MRI); sarcoidosis, 1 (inflammatory CSF, enhancement pattern); and cytomegalovirus, 1 (inflammatory CSF). No patients met criteria for definite or probable SCI.
Discussion
We describe the clinical and neuroimaging details of a large series of spontaneous SCIs, propose diagnostic criteria, and use a validation cohort to assess their utility. Features often considered to be atypical (eg, >4 hours to nadir, MRI and clinical evidence outside the anterior spinal artery territory, gadolinium enhancement) are actually common and should not preclude physicians from making the diagnosis of SCI. Spinal cord infarction is underrecognized and frequently misdiagnosed as transverse myelitis,4,5 highlighting the need for diagnostic criteria that can be broadly applied to patients with spontaneous and periprocedural SCI.
The proposed SCI diagnostic criteria emphasize 3 major components: (1) clinical: rapid development of severe deficits within 12 hours; (2) MRI spine: exclusion of compression (2A) with supportive features (2B), and specific imaging findings (2C); and (3) CSF: highlighting noninflammatory findings in most. The most critical component is the rapid accumulation of severe deficits within 12 hours because more gradual worsening favors alternative etiologies. In our validation cohort, this was the most powerful diagnostic discriminator, similar to what others have found4; it is rare for alternative myelopathy etiologies to present this rapidly with a severe deficit. A noninflammatory CSF profile is helpful to differentiate from infectious and inflammatory etiologies, although mild abnormalities might be encountered in SCI as seen in 8% of our patients. As with most other diagnostic criteria, a patient should not have a more likely alternative diagnosis (eBox 1 in the Supplement) based on the careful interpretation of all diagnostic information. Our validation cohort with only 9 patients (3.2%) initially meeting criteria for possible SCI suggests these criteria will be very helpful in discriminating SCI from other myelopathy etiologies. In addition, the criteria may have utility for enrollment in clinical trials and consensus of definitions for research in SCI.
Many prior cases of SCI have demonstrated a prolonged time to nadir over many hours, including autopsy-proven cases and periprocedural SCI.2,4,9 The slower evolution of deficits than in cerebral infarction might be explained by diverse collateralization of spinal arterial supply10 with transient preservation of tissue viability before infarction, while the relatively small area of the spinal cord may also make it more susceptible to the effects of edema contributing to clinical worsening.11,12 Pain was common at presentation (72%); it may be explained by SCI activation of the spinothalamic tract or by the contributing mechanism (fibrocartilaginous embolism, artery dissection).9,13,14
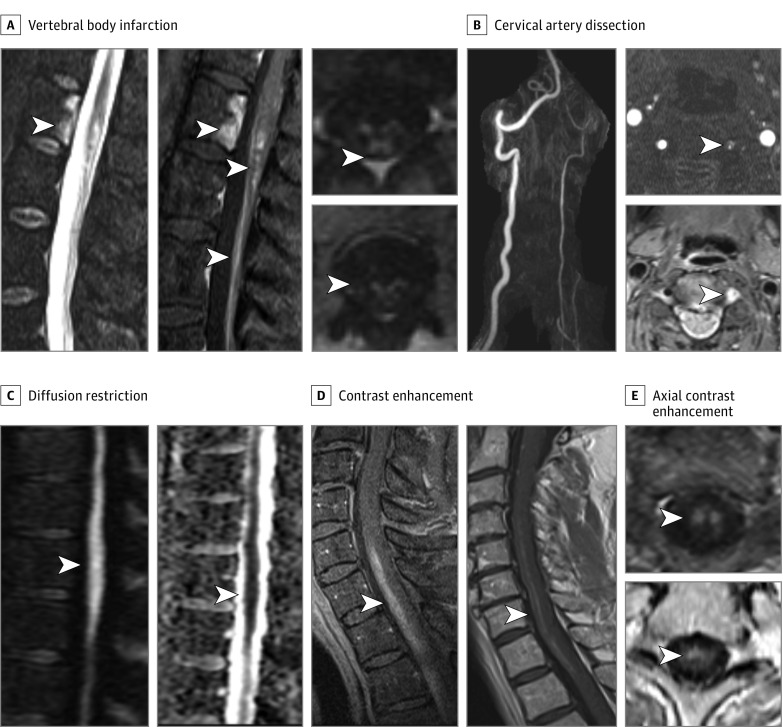
Given the high proportion of older patients with vascular risk factors in our study, traditional stroke mechanisms (atherothrombosis) likely have an important role in SCI,15 which may likely affect more proximal arteries than the spinal arteries themselves.16 Cervical artery dissections may be underdiagnosed given limitations of assessment with standard angiographic imaging techniques; T1 fat suppression imaging may visualize a vessel wall hematoma (Figure 2) and confirm a dissection.17 Detection of local disc protrusions may suggest a fibrocartilaginous embolism as the mechanism of SCI,9 which is still captured within and consistent with our diagnostic criteria; however, degenerative disc disease is very common in the general population.18
Figure 2. Confirmatory Magnetic Resonance Imaging Findings and Typical Gadolinium Enhancement Pattern in Spinal Cord Infarctions.
Confirmatory spinal cord infarction (SCI) findings are shown, including vertebral body infarction on short-τ inversion recovery imaging with associated gadolinium enhancement of the vertebral body infarct, SCI, and anterior cauda equina (A); cervical artery dissection with significantly decreased left vertebral flow with confirmed intramural hematoma shown on T1-fat suppression imaging (B) adjacent to SCI; and diffusion restriction on diffusion-weighted imaging with correlation on apparent diffusion coefficient (C). Spinal cord infarction gadolinium enhancement demonstrated with a typical craniocaudal linear strip on sagittal views (D) and corresponding anterior predominant gray matter and anteromedial spot (E) patterns on axial views, highlighting the predominant areas of ischemia.
Although some MRI features confirm SCI (criterion 2C), these features are often absent or sequences to detect these findings are not undertaken. Physicians should request DWI in patients with acute myelopathy in an attempt to confirm the diagnosis of SCI; however, DWI has incomplete sensitivity in SCI given limitations of spatial resolution and susceptibility artifacts, as has been shown in definite cases of periprocedural SCI.2 Classic findings of owl eyes19,20 or pencil-like hyperintensity21 are helpful in suspecting SCI but are neither specific20 nor required for the diagnosis. A variety of other T2-hyperintensity patterns can also be seen (Figure 1, Table 2, and eBox 2 in the Supplement), as previously characterized in the periprocedural setting.2 Magnetic resonance imaging is often normal acutely in SCI (24%), and thus repeating imaging days later is recommended. Although gadolinium enhancement is usually considered suggestive of transverse myelitis or other etiologies, many periprocedural SCIs (43%)2 and spontaneous SCIs (39%) demonstrate definite enhancement. A linear craniocaudal strip of enhancement is typical of SCI (Figure 2), highlighting the most predominant ischemic area (gray matter, arterial territory), and such a pattern is unusual with inflammatory etiologies. Other patterns of enhancement should lead clinicians to alternative diagnoses. When SCI remains in question after initial evaluation, follow-up imaging demonstrating chronic focal cystic myelomalacia (Figure 1) supports vascular sequelae as opposed to myelitis.22
Unfortunately, no therapies are proven as effective to treat spontaneous SCI.23,24 Lumbar drainage and blood pressure augmentation are used for SCI in the setting of aortic surgery,23,25,26 but there are no data to support these strategies in spontaneous SCI. Although previous cases15 and 2 of these patients received intravenous tissue plasminogen activator without harm, there is no evidence for efficacy in SCI. Empirical intravenous methylprednisolone treatment is reasonable when concern remains for a possible inflammatory myelopathy. However, some treatments, such as plasma exchange (which can reduce cord perfusion)27,28,29 and intravenous immunoglobulin (which is prothrombotic),30,31,32 could be harmful and should be avoided. Aggressive rehabilitation should be pursued,1 and many patients achieve a good outcome (47% without gait aid).
Limitations
Our study is limited by its retrospective design. The absence of a criterion standard for diagnosis of spontaneous SCI against which our diagnostic criteria can be assessed is the main limitation. The possibility exists that some cases with alternative myelopathy etiologies were misdiagnosed as SCI. The exhaustive workup undertaken to exclude alternative etiologies helped reduce this risk of misdiagnosis. Patients did not receive a standard battery of tests in all cases. Forty-six patients were excluded because they had incomplete data and thus did not meet inclusion criteria. The predominance of white individuals likely reflects our upper Midwest patient catchment area, which is also predominantly white (80%-90%) and impacts the generalizability to other racial/ethnic groups.33 The imaging sequences performed varied, as did their timing. A larger number of patients with DWI may change the apparent sensitivity, but the absence of diffusion restriction does not exclude SCI as shown in the periprocedural setting.2 Validation of these criteria will be needed in other cohorts and ideally would include greater racial/ethnic diversity.
Conclusions
In conclusion, our comprehensive review of a large series of patients with SCI identified several clinical and radiological clues to SCI and refines our understanding of the spectrum of SCI presentation. We proposed and validated a set of diagnostic criteria that may improve recognition of SCI cases, guide acute management, and facilitate future research.
eBox 1. Alternative acute myelopathy diagnoses
eBox 2. Typical SCI MRI findings
Footnotes
A severe acute deficit (motor and/or sensory) typically consists of loss of antigravity strength or worse, severe objective sensory loss impairing function (eg, severe sensory ataxia).
References
- 1.Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012;78(2):114-121. doi: 10.1212/WNL.0b013e31823efc93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zalewski NLRA, Rabinstein AA, Krecke KN, et al. Spinal cord infarction: clinical and imaging insights from the periprocedural setting. J Neurol Sci. 2018;388:162-167. doi: 10.1016/j.jns.2018.03.029 [DOI] [PubMed] [Google Scholar]
- 3.Sandson TA, Friedman JH. Spinal cord infarction: report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68(5):282-292. doi: 10.1097/00005792-198909000-00003 [DOI] [PubMed] [Google Scholar]
- 4.Barreras P, Fitzgerald KC, Mealy MA, et al. Clinical biomarkers differentiate myelitis from vascular and other causes of myelopathy. Neurology. 2018;90(1):e12-e21. doi: 10.1212/WNL.0000000000004765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalewski NL, Flanagan EP, Keegan BM. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology. 2018;90(2):e96-e102. doi: 10.1212/WNL.0000000000004796 [DOI] [PubMed] [Google Scholar]
- 6.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535-546. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan EP, Kaufmann TJ, Krecke KN, et al. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol. 2016;79(3):437-447. doi: 10.1002/ana.24582 [DOI] [PubMed] [Google Scholar]
- 8.Zalewski NL, Morris PP, Weinshenker BG, et al. Ring-enhancing spinal cord lesions in neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2017;88(3):218-225. doi: 10.1136/jnnp-2016-314738 [DOI] [PubMed] [Google Scholar]
- 9.AbdelRazek MA, Mowla A, Farooq S, Silvestri N, Sawyer R, Wolfe G. Fibrocartilaginous embolism: a comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. J Spinal Cord Med. 2016;39(2):146-154. doi: 10.1080/10790268.2015.1116726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griepp EB, Di Luozzo G, Schray D, Stefanovic A, Geisbüsch S, Griepp RB. The anatomy of the spinal cord collateral circulation. Ann Cardiothorac Surg. 2012;1(3):350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abou Al-Shaar H, AbouAl-Shaar I, Al-Kawi MZ. Acute cervical cord infarction in anterior spinal artery territory with acute swelling mimicking myelitis. Neurosciences (Riyadh). 2015;20(4):372-375. doi: 10.17712/nsj.2015.4.20150109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battey TW, Karki M, Singhal AB, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45(12):3643-3648. doi: 10.1161/STROKEAHA.114.006884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducrocq X, Lacour JC, Debouverie M, Bracard S, Girard F, Weber M. Cerebral ischemic accidents in young subjects: a prospective study of 296 patients aged 16 to 45 years [in French]. Rev Neurol (Paris). 1999;155(8):575-582. [PubMed] [Google Scholar]
- 14.Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J; CADISS trial investigators . Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367. doi: 10.1016/S1474-4422(15)70018-9 [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T, Wakida K, Nishida H. Cervical posterior spinal artery syndrome: a case report and literature review. J Stroke Cerebrovasc Dis. 2016;25(6):1552-1556. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 16.Tubbs RS, Blouir MC, Singh R, et al. Relationship between regional atherosclerosis and adjacent spinal cord histology. Cureus. 2015;7(9):e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuvinciuc V, Viallon M, Momjian-Mayor I, et al. 3D fat-saturated T1 SPACE sequence for the diagnosis of cervical artery dissection. Neuroradiology. 2013;55(5):595-602. doi: 10.1007/s00234-013-1141-1 [DOI] [PubMed] [Google Scholar]
- 18.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811-816. doi: 10.3174/ajnr.A4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udiya AK, Shetty GS, Singh V, Phadke RV. “Owl eye sign”: anterior spinal artery syndrome. Neurol India. 2015;63(3):459. doi: 10.4103/0028-3886.158286 [DOI] [PubMed] [Google Scholar]
- 20.Kister I, Johnson E, Raz E, Babb J, Loh J, Shepherd TM. Specific MRI findings help distinguish acute transverse myelitis of neuromyelitis optica from spinal cord infarction. Mult Scler Relat Disord. 2016;9:62-67. doi: 10.1016/j.msard.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 21.Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology. 2002;44(10):851-857. doi: 10.1007/s00234-002-0828-5 [DOI] [PubMed] [Google Scholar]
- 22.Reynolds JM, Belvadi YS, Kane AG, Poulopoulos M. Thoracic disc herniation leads to anterior spinal artery syndrome demonstrated by diffusion-weighted magnetic resonance imaging (DWI): a case report and literature review. Spine J. 2014;14(6):e17-e22. doi: 10.1016/j.spinee.2013.10.050 [DOI] [PubMed] [Google Scholar]
- 23.Nasr DM, Rabinstein A. Spinal cord infarcts: risk factors, management, and prognosis. Curr Treat Options Neurol. 2017;19(8):28. doi: 10.1007/s11940-017-0464-3 [DOI] [PubMed] [Google Scholar]
- 24.Rabinstein AA. Vascular myelopathies. Continuum (Minneap Minn). 2015;21(1 spinal cord disorders):67-83. doi: 10.1212/01.CON.0000461085.79241.e0 [DOI] [PubMed] [Google Scholar]
- 25.Coselli JS, LeMaire SA, Schmittling ZC, Köksoy C. Cerebrospinal fluid drainage in thoracoabdominal aortic surgery. Semin Vasc Surg. 2000;13(4):308-314. [PubMed] [Google Scholar]
- 26.Sugiura J, Oshima H, Abe T, et al. The efficacy and risk of cerebrospinal fluid drainage for thoracoabdominal aortic aneurysm repair: a retrospective observational comparison between drainage and non-drainage. Interact Cardiovasc Thorac Surg. 2017;24(4):609-614. [DOI] [PubMed] [Google Scholar]
- 27.Córdoba JP, Larrarte C, Medina MC. Experience in therapeutic plasma exchange by membrane filtration at an academic center in Colombia: registry of the first 500 sessions. J Clin Apher. 2015;30(6):347-352. doi: 10.1002/jca.21391 [DOI] [PubMed] [Google Scholar]
- 28.Lemaire A, Parquet N, Galicier L, et al. Plasma exchange in the intensive care unit: technical aspects and complications. J Clin Apher. 2017;32(6):405-412. doi: 10.1002/jca.21529 [DOI] [PubMed] [Google Scholar]
- 29.Clark SL, Rabinstein AA. Safety of intravenous immunoglobulin and plasma exchange in critically ill patients. Neurol Res. 2015;37(7):593-598. doi: 10.1179/1743132815Y.0000000017 [DOI] [PubMed] [Google Scholar]
- 30.Benadiba J, Robitaille N, Lambert G, Itaj NK, Pastore Y. Intravenous immunoglobulin-associated thrombosis: is it such a rare event? report of a pediatric case and of the Quebec Hemovigilance System. Transfusion. 2015;55(3):571-575. doi: 10.1111/trf.12897 [DOI] [PubMed] [Google Scholar]
- 31.Pollreisz A, Assinger A, Hacker S, et al. Intravenous immunoglobulins induce CD32-mediated platelet aggregation in vitro. Br J Dermatol. 2008;159(3):578-584. doi: 10.1111/j.1365-2133.2008.08700.x [DOI] [PubMed] [Google Scholar]
- 32.Barsheshet A, Marai I, Appel S, Zimlichman E. Acute ST elevation myocardial infarction during intravenous immunoglobulin infusion. Ann N Y Acad Sci. 2007;1110:315-318. doi: 10.1196/annals.1423.033 [DOI] [PubMed] [Google Scholar]
- 33.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-160. doi: 10.1016/j.mayocp.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zalewski NL, Rabinstein AA, Wijdicks EFM, et al. Spontaneous posterior spinal artery infarction: an under-recognized cause of acute myelopathy [published online July 25, 2018]. Neurology. doi: 10.1212/WNL.0000000000006084 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eBox 1. Alternative acute myelopathy diagnoses
eBox 2. Typical SCI MRI findings