Abstract
Background
Aggressive medical care at the end of life can be harmful to patients and families, but its prevalence in use among younger cancer patients is unknown. The goal of the study was to report on the use of aggressive care and hospice services for patients younger than age 65 years.
Methods
Using the HealthCore Integrated Research Database, we analyzed patients who died between 2007 and 2014 with metastatic lung (n = 12 764), colorectal (n = 5207), breast (n = 5855), pancreatic (n = 3397), or prostate (n = 1508) cancer. Based on published quality measures, we assessed uses of chemotherapy, intensive care, emergency room visits, and hospice care at the end of life. We examined additional items including radiotherapy, invasive procedures, hospitalization, and in-hospital deaths. Multivariable modified Poisson regression models were used to adjust for age, sex, geographic region, rural/urban location, year of death, and regional education and income measures.
Results
Across the five cancers, 10.1% to 14.1% of patients received chemotherapy within the last 14 days of life, 15.9% to 20.6% received intensive care in last 30 days, and 1.5% to 2.5% went to the emergency room two or more times in last 30 days. Hospice enrollment at least three days before death was 54.4% to 59.6%. However, 55.3% to 59.3% of patients had a hospital admission in the last 30 days, and one-third died (30.3%–35.4%) in the hospital.
Conclusions
There was low use of cancer-directed treatment at the end of life for younger cancer patients, and hospice use was higher than 50%. However, there was a relatively high utilization of hospital-based care. These results demonstrate an opportunity for continued improvements in the provision of high-value, patient-centered care at the end of life.
Aggressive treatment of incurable cancer at the end of life can be harmful to patients and their families. Prior studies have shown that provision of anticancer therapy at the end of life can decrease a patient’s quality of life (1), increase financial hardship (2), and is often contrary to patient and family wishes (3). Recognition of the importance of this issue has led to creation of National Quality Forum (NQF) and American Society of Clinical Oncology (ASCO) Quality Oncology Practice Initiative (QOPI) measures specifically related to different aspects of care that can be considered to be aggressive, including receipt of chemotherapy within the last two weeks of life (4), intensive care within the last 30 days of life (5), and more than one emergency room visit in the last 30 days of life (6). An additional measure assesses the enrollment in hospice care for more than three days before death (7,8), which is considered high-quality care.
The prevalence of aggressive end-of-life care for younger, non-Medicare-eligible cancer patients has not been studied on a national level. The present study addresses this knowledge gap by examining the prevalence of aggressive end-of-life care—chemotherapy, intensive care, emergency room visits—as well as hospice care in patients younger than age 65 years with five common metastatic cancers. In addition to these items, which are based on published quality measures, we further examined use of additional items that could be considered aggressive—including radiotherapy (9), hospital admission and in-hospital death (10), and invasive procedures—in order to more comprehensively depict patterns of care at the end of life for these younger patients.
Methods
Data Source and Patient Cohort
This study analyzed data from the HealthCore Integrated Research Database (HIRD), which includes single-payer administrative claims data and enrollment information for approximately 60 million lives with commercial insurance plans across 14 states in the Northeastern, Mid-Atlantic, Southeastern, Midwest, Central, and Western regions of the United States (11). The HIRD is a longitudinally integrated database of inpatient and outpatient medical claims. It also contains patient- and regional-level characteristics, including sex, date of birth, date of death (ascertained using the death master file from the US Social Security Administration), geographic region of patient residence, census tract-level measures of household income and educational attainment, and population density. This study was exempted by the University of North Carolina at Chapel Hill Institutional Review Board.
The analytic cohort included individuals who met the following criteria: 1) age 18 to 64 years at time of death; 2) died between January 1, 2007, and December 31, 2014; 3) were continuously insured for the 12 months prior to death; 4) had an International Classification of Disease, Ninth Edition (ICD-9) diagnosis code reflecting any of the five cancer types of interest during the 12 months prior to date of death: lung and bronchus, colon and rectum, breast (female only), pancreas, prostate; and 5) had a diagnosis code reflecting metastatic disease during the 12 months preceding death. Diagnosis codes are shown in Supplementary Table 1 (available online).
The five cancer types were chosen to represent the most common causes of cancer deaths in the United States (12). Further, inclusion criteria 4 and 5 were utilized to maximize specificity of the analyzed cohort in having an active, incurable cancer and therefore likely to have died from the cancer rather than a nonrelated cause. Among patients who met criteria 1 through 4 above, 86.3% of lung cancer (n = 12 764), 89.3% of colorectal (n = 5207), 89.1% of breast (n = 5855), 87.3% of pancreas (n = 3397), and 72.7% of prostate (n = 1508) cancer patients had a diagnosis code reflecting metastatic disease (criterion 5) and were included in the analytic cohort.
Outcome Measures and Statistical Analysis
Receipt of aggressive care and hospice were defined from inpatient and outpatient medical claims files using ICD-9 procedure codes, Current Procedures Terminology (CPT) codes, revenue codes, and Healthcare Common Procedure Coding System (HCPCS) codes (Supplementary Table 2, available online). Hospital admission and emergency department visits were ascertained using place of service revenue codes on facility claims. Based on published NQF and ASCO QOPI measures, the following items were assessed as our primary objective: chemotherapy in the last 14 days of life; intensive care in the last 30 days of life; two or more emergency room visits in last 30 days of life; and receiving hospice care at least three days before death. Additional, secondary items assessed included 1) radiotherapy in the last 30 days of life (9); 2) invasive procedures in the last 30 days of life, which included procedures requiring anesthesia, procedures for which pathology consultation occurred, interventions such as feeding tube placement or intubation, or other advanced life support measures such as cardiopulmonary resuscitation; 3) hospital admission (10); and 4) in-hospital death (10). For patients with inpatient admissions, in-hospital death was defined as patients with a death date between admission date and discharge date.
Modified Poisson regression models were used to estimate the risk for each outcome, adjusting for age, sex, geographic region, rural vs urban location, year of death, and regional education and income measures (13). Separate models were constructed by cancer site and by outcome, with binary indicators for each outcome. Risk adjustment was completed using direct adjustment. Specifically, we estimated the predicted probability of the event for each patient based on their observed characteristics and averaged the predicted probabilities over the cohort (14). The modified Poisson method provides estimates of the risk and risk ratios with 95% confidence intervals (CIs), which can be directly estimated by exponentiation of the intercept and beta coefficients from the output (15). Model-adjusted risks and 95% confidence intervals are reported.
Results
Patient characteristics are shown in Table 1. Across the five cancers, 83.1% of patients were age 50 to 64 years (ranging from 76.4% to 93.3%, depending on the cancer site). Consistent with overall HIRD coverage, there was representation within all regions of the country and both male and female patients were well represented in lung, colorectal, and pancreatic cancers.
Table 1.
Patient characteristics
| Lung cancer (n = 12 764) | Colorectal cancer (n = 5207) | Breast cancer (n = 5855) | Pancreas cancer (n = 3397) | Prostate cancer (n = 1508) | |
|---|---|---|---|---|---|
| Characteristic | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) |
| Age, y | |||||
| <40 | 338 (2.6) | 217 (4.2) | 268 (4.6) | 67 (2.0) | 13 (0.9) |
| 40–49 | 1515 (11.9) | 818 (15.7) | 1114 (19.0) | 427 (12.6) | 88 (5.8) |
| 50–64 | 10 911 (85.5) | 4172 (80.1) | 4473 (76.4) | 2903 (85.5) | 1407 (93.3) |
| Sex | |||||
| Male | 6682 (52.4) | 2883 (55.4) | NA | 1995 (58.7) | 1508 (100.0) |
| Female | 6082 (47.6) | 2324 (45.6) | 5855 (100) | 1402 (41.3) | NA |
| Year of death | |||||
| 2007 | 2101 (16.4) | 766 (14.7) | 978 (16.7) | 465 (13.7) | 213 (14.1) |
| 2008 | 2130 (16.7) | 867 (16.7) | 998 (17.0) | 494 (14.5) | 234 (15.5) |
| 2009 | 2027 (15.9) | 877 (16.8) | 966 (16.5) | 590 (17.4) | 252 (16.7) |
| 2010 | 1972 (15.4) | 770 (14.8) | 900 (15.4) | 516 (15.2) | 229 (15.2) |
| 2011 | 1962 (15.4) | 816 (15.7) | 848 (14.5) | 546 (16.1) | 250 (16.6) |
| 2012 | 984 (7.7) | 441 (8.5) | 486 (8.3) | 294 (8.7) | 117 (7.8) |
| 2013 | 861 (6.7) | 363 (7.0) | 353 (6.0) | 241 (7.1) | 123 (8.2) |
| 2014 | 727 (5.7) | 307 (5.9) | 326 (5.6) | 251 (7.4) | 90 (6.0) |
| Population density (RUCA 2.0)* | |||||
| Urban core | 8050 (63.1) | 3385 (65.0) | 3961 (67.7) | 2203 (64.9) | 998 (66.2) |
| Urban other | 2228 (17.4) | 836 (16.1) | 880 (15.0) | 548 (16.1) | 239 (15.8) |
| Large rural | 817 (6.4) | 302 (5.8) | 285 (4.8) | 193 (5.7) | 92 (6.1) |
| Small rural | 448 (3.5) | 168 (3.2) | 175 (3.0) | 125 (3.7) | 44 (2.9) |
| Isolated | 370 (2.9) | 154 (3.0) | 150 (2.6) | 101 (3.0) | 42 (2.8) |
| US Regions*† | |||||
| Northeast | 3019 (23.7) | 1179 (22.6) | 1304 (22.3) | 907 (26.7) | 385 (25.5) |
| Midwest | 3096 (24.3) | 1117 (21.5) | 1302 (22.2) | 731 (21.5) | 312 (20.7) |
| South | 3906 (30.6) | 1549 (29.7) | 1605 (27.4) | 934 (27.5) | 412 (27.3) |
| West | 1900 (14.9) | 1007 (19.3) | 1245 (21.3) | 603 (17.8) | 310 (20.6) |
Some percentages do not add to 100% due to missing data. RUCA = rural-urban commuting area.
Regions correspond to four census regions defined by the US Census Bureau (Northeast: CT, ME, NH, NY; Midwest: IN, MO, OH, WI; South: GA, KY, VA; West: CA, CO, NV).
Multivariable-adjusted receipt of each component of aggressive care at the end of life and hospice enrollment at least three days before death are summarized in Table 2. Chemotherapy was received by 10.1% to 14.1% of patients in the last two weeks of life. In the last 30 days of life, 15.9% to 20.6% of patients received intensive care. Few patients (1.5%–2.5%) had two or more emergency room visits. In addition, more than half (55.3%–59.3%) of patients were admitted to the hospital and about one-third (30.3%–35.4%) died in the hospital. Hospice use at least three days before death was received by 54.4% to 59.6% of patients.
Table 2.
Risk of receipt of aggressive care in last 30 days of life and hospice enrollment at least 3 days prior to death*
| Intervention | Lung cancer | Colorectal cancer | Breast cancer | Pancreas cancer | Prostate cancer |
|---|---|---|---|---|---|
| (n = 12 764) | (n = 5207) | (n = 5855) | (n = 3397) | (n = 1508) | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Chemotherapy† | 12.6 (9.8 to 16.0) | 10.9 (7.7 to 14.7) | 14.1 (11.0 to 18.0) | 12.5 (7.6 to 17.5) | 10.1 (6.6 to 15.2) |
| Intensive care | 20.6 (13.7 to 28.5) | 15.9 (10.0 to 21.3) | 17.5 (11.0 to 23.6) | 16.0 (10.1 to 22.1) | 18.0 (10.0 to 27.8) |
| ≥2 emergency room visits | 1.8 (0.9 to 3.3) | 1.5 (0.5 to 3.1) | 1.7 (0.5 to 3.9) | 1.6 (0.5 to 3.9) | 2.2 (0.4 to 6.7) |
| Radiotherapy | 20.6 (16.8 to 25.3) | 8.8 (5.8 to 12.9) | 15.7 (11.8 to 21.3) | 5.8 (2.5 to 11.1) | 13.1 (6.6 to 21.3) |
| Invasive procedure | 29.3 (22.6 to 36.0) | 26.5 (20.8 to 33.0) | 27.7 (21.8 to 33.4) | 31.1 (23.3 to 40.1) | 25.3 (17.0 to 36.8) |
| Hospitalization | 59.3 (51.7 to 66.9) | 56.3 (49.4 to 65.0) | 59.0 (51.7 to 67.2) | 59.2 (51.5 to 67.6) | 55.3 (44.9 to 68.3) |
| In-hospital death | 35.4 (26.7 to 46.8) | 30.8 (22.0 to 41.8) | 33.3 (25.3 to 43.3) | 30.3 (21.4 to 43.7) | 31.4 (20.3 to 44.4) |
| Hospice care‡ | 54.4 (45.5 to 63.5) | 58.2 (50.3 to 67.1) | 54.6 (45.6 to 63.4) | 59.6 (49.2 to 69.7) | 55.8 (43.6 to 70.4) |
Data presented are multivariable adjusted using a modified Poisson model. The model was adjusted for case-mix based on age, sex, geographic region, rural vs urban location, year of death, and regional education and income measures. CI = confidence interval.
Chemotherapy in last 14 days of life.
Hospice enrollment at least three days prior to death.
To examine the intensity of care at the end of life in more detail, histograms were created to show the proximity of aggressive care to patients’ date of death (Figure 1, all cancers combined). These plots demonstrate that there was use of all items of aggressive care for a proportion of patients within the last 14 days before death, seven days, and even up to the day of death. For example, 5.5% of patients had a hospital admission either on the day of death or the day before. The proportion of patients receiving their final dose of chemotherapy increased until approximately the week prior to death, at which point the proportion of patients receiving a final dose of chemotherapy began to decline; a similar pattern was seen for final treatment with radiotherapy. We found that more than 90% of invasive procedures on day of death were related to life-sustaining measures, including cardiopulmonary resuscitation, insertion of emergency airway, insertion of central venous catheter, and physician direction of advanced life support (CPT codes 92950, 31500, 36556, and 99288, respectively). The numbers of days before death at which half of patients received each aggressive care measure is reported in Supplementary Table 3 (available online).
Figure 1.
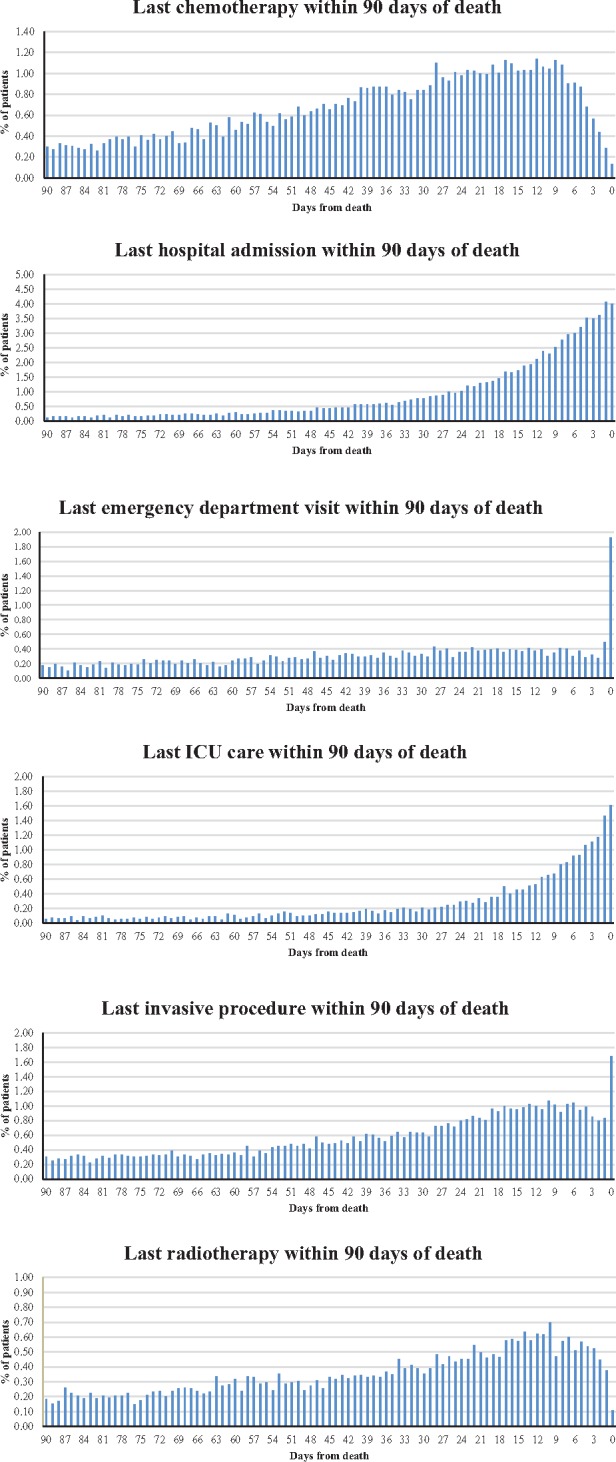
Proximity of aggressive care to the end of life. The histograms show proximity of aggressive care to patients’ date of death. In each graph, patients are “counted” only once, on the day when they last received each aggressive care measure before death. The y-axes represent the overall proportion of patients out of the entire analytic cohort. ICU = intensive care unit.
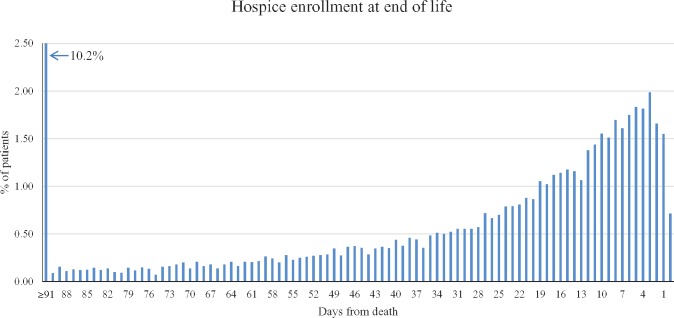
Date of hospice enrollment relative to date of death is shown in Figure 2. Overall, 5.9% of patients enrolled in the final three days of life, 45.1% enrolled between three and 90 days before death, and 10.2% enrolled more than three months before death.
Figure 2.
Proximity of hospice enrollment to the end of life. The histogram shows proximity of hospice enrollment to patients’ date of death. The first date on which hospice services were received was considered to be the date of hospice enrollment. The first bar on the histogram indicates the percentage of patients who were enrolled in hospice between 91 and 365 days before death. The y-axis represents the overall proportion of patients out of the entire analytic cohort.
Discussion
This is the first study to examine use of aggressive care and hospice services at end of life for contemporary patients younger than age 65 years across the United States. End-of-life care is recognized as an important issue across oncology, and there are multiple published guidelines that recommend limiting the use of aggressive treatments that may be more harmful than helpful to patients at the end of life, as well as increasing the use of palliative and hospice care (16–18).
Using published quality measures, we found overall low uses of chemotherapy, intensive care, and emergency room visits at the end of life across the five common cancers examined. These findings represent overall adherence to these metrics for aggressive end-of-life care. Indeed, 10% to 13% of patients receiving chemotherapy within the last 14 days of life may be clinically appropriate. Similarly, radiotherapy use at end of life can provide palliative benefit to patients. We showed that the proportion of patients receiving the last dose of chemotherapy decreased within the last days of life, likely demonstrating recognition of patients’ deteriorating conditions, leading to cessation of treatment. By comparison, intensive care utilization in the last month of life was mostly lower among this younger patient cohort (16%–21%) than what has been observed for the Medicare population (20%–27%) (19).
Prior studies have shown that early enrollment in hospice helps reduce aggressive care, improves patient quality of life, and improves the quality of end-of-life care (20–23)—thus, hospice is commonly considered to be high-value, patient-centered care. Using the published quality measure of hospice enrollment at least three days before death, we found that over half of patients received this high-quality care. However, it was notable that only 10.2% of patients enrolled in hospice more than 90 days before death. The rates of hospice use found in this study are similar to those shown in a recent publication from a large academic, comprehensive cancer center reporting greater than 45% use of hospice for more than three days (24). In prior studies of the Medicare population, hospice use has been reported to be 60% among cancer patients (19,20). While our numbers are similar to those from the Medicare population, more than 40% of younger patients with incurable cancers at the end of life did not receive hospice care—indicating that there is further room for improvement.
Research has shown that having discussions with cancer patients about their prognosis is associated with more realistic patient expectations of life expectancy (25). Unfortunately, many patients with metastatic cancers, and also their physicians, do not have accurate estimates of their prognosis (26–28). The discrepancy between patient understanding and actual prognosis may be one of the driving factors behind the underutilization of hospice care. Among a sample of over 5000 Medicare beneficiaries, less than 1% of patients reported having end-of-life conversations with physicians (29). It is possible that providing reimbursement to providers for having discussions regarding end-of-life care will help to reduce aggressive care at end of life, increase hospice enrollment in an appropriate time frame, and improve the dying process for patients and their families. These discussions provide an opportunity to reframe the thought process regarding cancer at end of life, which helps patients and families understand that cessation of aggressive care and enrollment in hospice do not signify capitulation in the “battle” with cancer (30). Further, in some circumstances it may not be clinically apparent that patients are close to end of life. Better prognostic tools may be needed to help clinicians identify patients who no longer benefit from active treatment.
This study did find relatively high uses of some aggressive care measures at the end of life. Specifically, more than half of patients were admitted to the hospital within the last 30 days of life, and one-third died in the hospital. The proximity of patients’ receipt of hospital-based care to the day of death was also striking. We found an increasing percentage of patients who received emergency department visits, hospital admission, and intensive care in the last days of life, with more than 90% of invasive procedures on day of death related to life-sustaining measures. Together, these findings portray an overall picture where physicians indeed are stopping cancer-directed therapy at the end of life, but a proportion of patients continue to receive hospital-based care, which can likely be reduced with even higher uses of hospice enrollment, symptom management, palliative care, and clarification of patient goals at the end of life (20). Prior studies have shown that patients often prefer to spend their last days of life at home, and dying in the hospital is often not in accord with their wishes (3).
A potential limitation of this study is that cause of death was not available, though this is not unique to our study examining end-of-life care on a large scale (10,20). We attempted to minimize this limitation with inclusion criteria requiring both a cancer diagnosis and a diagnosis of metastasis within 12 months of death. This is a similar but more stringent approach than that used in prior studies using Medicare data (10), but the sensitivity and specificity of this approach compared with only using a cancer diagnosis is unknown. Diagnostic codes in claims data may have limited validity when inferring cancer stage (31). Another limitation is a possibility that a small number of patients could have received hospice care outside of their insurance coverage, resulting in undercapture of hospice use in our data. Additionally, we did not have information on patient sociodemographic characteristics or treatment provider characteristics, and we did not directly elicit patient preferences regarding their goals of care at the end of life.
There are also several strengths of this study. The data source (HIRD) provides near real-time data, which allowed for examination of care in a contemporary cohort of patients through the end of 2014. Another strength of the study is the inclusion of patients from across 14 states representing approximately 20% of the population age 18 to 64 years, which enhances the generalizability of results from this study.
There is a low use of chemotherapy within the last 14 days of life for patients younger than age 65 years with incurable cancers across the United States, and 54.4% to 59.6% of patients receive hospice care at least three days before death. However, more than half of patients receive hospital-based care at the end of life, and one-third die in the hospital instead of at home. These results demonstrate an opportunity for continued improvements in the provision of high-value, patient-centered care at the end of life for younger cancer patients.
Funding
This work was supported by North Carolina Translational and Clinical Sciences Institute Grant #550KR101503 (Dr. Ronald Chen, principal investigator, Dr. Aaron Falchook, co-principal investigator).
Dr. Dusetzina is supported by the National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 Program and the North Carolina Translational and Clinical Sciences Institute (UL1TR001111).
Notes
The funders had no role in the the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
These data were presented at the annual meeting of the American Society of Clinical Oncology (ASCO), Chicago, Illinois, and were specifically highlighted in ASCO’s Press Program on June 6, 2016.
Supplementary Material
References
- 1. Wright AA, Zhang B, Ray A et al. , Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tucker-Seeley RD, Abel GA, Uno H et al. , Financial hardship and the intensity of medical care received near death. Psychooncology. 2015;24(5):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright AA, Keating NL, Ayanian JZ et al. , Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Quality Forum Measure #0210. http://www.qualityforum.org/QPS/0210 Accessed August 4, 2016.
- 5.National Quality Forum Measure #0213. http://www.qualityforum.org/QPS/0213 Accessed August 4, 2016.
- 6.National Quality Forum Measure #0211. http://www.qualityforum.org/QPS/0211 Accessed August 4, 2016.
- 7.National Quality Forum Measure #0216. http://www.qualityforum.org/QPS/0216 Accessed August 4, 2016.
- 8.National Quality Forum Measure #0215. http://www.qualityforum.org/QPS/0215 Accessed August 4, 2016.
- 9. Guadagnolo BA, Liao KP, Elting L et al. , Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bekelman JE, Halpern SD, Blankart CR et al. , Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315(3):272–283. [DOI] [PubMed] [Google Scholar]
- 11. Bekelman JE, Sylwestrzak G, Barron J et al. , Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA. 2014;312(23):2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 13. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 14. Little RJA. Direct standardization: A tool for teaching linear models for unbalanced data. Am Stat. 1982;36(1):38–43. [Google Scholar]
- 15. Spiegelman D, Hertzmark E.. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 16. Schnipper LE, Smith TJ, Raghavan D et al. , American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724. [DOI] [PubMed] [Google Scholar]
- 17. Sohal DP, Mangu PB, Khorana AA et al. , Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(23):2784–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basch E, Loblaw DA, Oliver TK et al. , Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32(30):3436–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teno JM, Gozalo PL, Bynum JP et al. , Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obermeyer Z, Makar M, Abujaber S et al. , Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gawande A. Quantity and quality of life: Duties of care in life-limiting illness. JAMA. 2016;315(3):267–269. [DOI] [PubMed] [Google Scholar]
- 22. Rubin R. Improving the quality of life at the end of life. JAMA. 2015;313(21):2110–2112. [DOI] [PubMed] [Google Scholar]
- 23. Greer JA, Pirl WF, Jackson VA et al. , Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30(4):394–400. [DOI] [PubMed] [Google Scholar]
- 24. Stuver SO, McNiff K, Fraile B et al. , Novel data sharing between a comprehensive cancer center and a private payer to better understand care at the end of life. J Pain Symptom Manage. 2016;52(2):161–169. [DOI] [PubMed] [Google Scholar]
- 25. Enzinger AC, Zhang B, Schrag D et al. , Outcomes of prognostic disclosure: Associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu PH, Landrum MB, Weeks JC et al. , Physicians' propensity to discuss prognosis is associated with patients' awareness of prognosis for metastatic cancers. J Palliat Med. 2014;17(6):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weeks JC, Catalano PJ, Cronin A et al. , Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clément-Duchêne C, Carnin C, Guillemin F et al. , How accurate are physicians in the prediction of patient survival in advanced lung cancer? Oncologist. 2010;15(7):782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keary S, Moorman SM.. Patient-physician end-of-life discussions in the routine care of Medicare beneficiaries. J Aging Health. 2015;27(6):983–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ellis LM, Blanke CD, Roach N.. Losing “Losing the Battle With Cancer.” JAMA Oncol. 2015;1(1):13–14. [DOI] [PubMed] [Google Scholar]
- 31. Chawla N, Yabroff KR, Mariotto A et al. , Limited validity of diagnosis codes in Medicare claims for identifying cancer metastases and inferring stage. Ann Epidemiol. 2014;24(9):666–672, e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.