Abstract
Importance
Familial chilblain lupus is a monogenic autosomal dominant form of cutaneous lupus erythematosus that in most cases is caused by mutations in the 3 prime repair exonuclease 1 (TREX1). Familial chilblain lupus presents in early childhood with cold-induced painful erythematous infiltrates leading to mutilation and is associated with systemic involvement illustrated by an elevated type I interferon (IFN) signature in the skin and blood. Effective treatment is currently not available.
Objectives
To evaluate the clinical response to the Janus kinase inhibitor baricitinib in familial chilblain lupus and assess the effect of cold on patient fibroblasts.
Design, Setting, and Participants
In this case series, 3 patients with familial chilblain lupus due to TREX1 mutation underwent treatment with baricitinib for 3 months.
Interventions
Doses of baricitinib, 4 mg, were administered daily for 3 months.
Main Outcomes and Measures
Reduction of cutaneous lupus lesions was measured by the revised cutaneous lupus area and severity index, pain due to skin and joint involvement was assessed by visual analog scale, type I IFN signature in blood was determined by polymerase chain reaction, and the in vitro response of fibroblasts to cold exposure was analyzed.
Results
All 3 patients (2 women and 1 man; mean [SD] age, 51 [24] years) showed a significant improvement of cutaneous lupus lesions with suppression of systemic type I IFN activation. One patient had a complete remission regarding pain and, in 2 patients, pain associated with joint inflammation was partially reduced. No severe adverse reactions were reported. Exposure of patient fibroblasts to cold induced a stress response and enhanced senescence along with induction of IFN-stimulated gene in vitro.
Conclusions and Relevance
These findings demonstrate the therapeutic efficacy of Janus kinase inhibition in a monogenic form of lupus among 3 patients and provide mechanistic insight into the process of disease exacerbation by cold in TREX1-deficient cells. This finding may be relevant to other type I IFN–mediated disorders and implicates Janus kinase inhibition as a potential therapeutic option also for multifactorial cutaneous lupus erythematosus.
This case series evaluates the clinical response to the Janus kinase inhibitor baricitinib in 3 individuals with familial chilblain lupus and assesses the effect of cold on fibroblasts.
Key Points
Question
Is inhibition of the Janus kinase signaling pathway a therapeutic option in familial chilblain lupus?
Findings
In this case series study, 3 patients with familial chilblain lupus caused by mutations in the 3 prime repair exonuclease 1 (TREX1) responded to treatment with baricitinib with improvement of cutaneous chilblain lesions, relief of pain, and reduction of type I interferon signature. Cold, the main trigger factor of cutaneous disease, induced a stress response in patient fibroblasts, providing insight into pathogenesis of cutaneous disease.
Meaning
Janus kinase inhibition may represent a novel therapeutic approach for cutaneous manifestations of monogenic and multifactorial lupus erythematosus.
Introduction
Familial chilblain lupus (FCL) is an autosomal dominant form of cutaneous lupus erythematosus caused by mutations in the 3 prime repair exonuclease 1 (TREX1 [OMIM 606609]). Familial chilblain lupus presents in early childhood with cold-induced bluish-red infiltrates on acral locations that tend to ulcerate and may be associated with signs of systemic lupus erythematosus.1,2,3,4 TREX1 is a cytosolic DNase anchored in the outer nuclear membrane that safeguards the cell against innate immune activation by degrading short DNA metabolites derived from the nucleus that leak into the cytosol.1 In TREX1-deficient cells, self-DNA accumulates in the cytosol and stimulates the cyclic guanosine monophosphate (cGAS)–adenosine monophosphate synthase–stimulator of interferon (STING) pathway, leading to induction of type I interferons (IFNs).1,2,5 Inappropriate activation of chronic type I IFN can break immune tolerance and promote autoimmunity. In keeping with this finding, patients with FCL exhibit constitutive upregulation of IFN-stimulated genes and proteins in blood and skin.4
The effector functions of type I IFN are mediated by the INF-α and IFN-β receptor (IFNAR), which signals via the Janus kinase (JAK) and signal transducer and activators of transcription pathway.6 The JAK family comprises the receptor-associated tyrosine kinases JAK1, JAK2, and JAK3, which activate signal cascades downstream of type I cytokine receptors, and tyrosine kinase 2, which activates signal cascades downstream of type I and type II cytokine receptors.6 The IFNAR receptor signals through JAK1 and tyrosine kinase 2.6 Janus kinase inhibitors are small molecules inhibiting JAK activation. The first available JAK inhibitor, ruxolitinib, was approved for the treatment of myelodysplastic disorders and inhibits mainly JAK1 and JAK2, while baricitinib, which was recently approved in Europe for the treatment of rheumatoid arthritis, similarly impairs JAK1 and JAK2 signaling. Tofacitinib is also approved for the treatment of rheumatoid arthritis and inhibits JAK3, but also JAK1 and, to a lesser extent, JAK2.6 The therapeutic potential of JAK inhibition was recently demonstrated in patients with type I IFN–mediated vasculopathy due to activating TMEM173 mutations using ruxolitinib and tofacitinib,7,8 while baricitinib was shown to be effective in patients with chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature.9 We therefore investigated the therapeutic potential of baricitinib in patients with FCL.
Methods
Patients
All patients received a diagnosis of FCL with onset in early childhood. Patient 1 (TREX1 D18N, heterozygous) is a woman in her 20s with FCL.10,11 Patient 2 (TREX1 H195Q, heterozygous) is a man in his 70s, and patient 3 (TREX1 H195Q, heterozygous) is a woman in her 50s.12 Primary human fibroblasts were derived from skin biopsies of lesional skin from the hand of patients 1 and 2 (patient 3 did not wish to provide fibroblasts), who provided written informed consent. Control samples were obtained from skin excised during plastic surgery in age-matched individuals. All 3 patients received treatment with the JAK 1/2 inhibitor baricitinib. The study was performed in accordance with the Declaration of Helsinki13 and was approved by the ethics committee of the Medical Faculty, Technical University Dresden.
Cell Culture and Cold Exposure
Cells were cultured in Dulbecco Modified Eagle medium supplemented with 10% fetal calf serum, 4mM of l-glutamine, 1% penicillin and streptomycin sulfate, and 1mM of sodium pyruvate. In all experiments, passage-matched fibroblasts of patients 1 and 2 and controls were used. For cold exposure, cells were cultivated at 25°C for 3 or 5 days followed by a rewarming phase at 37°C.
Measurement of Reactive Oxygen Species
For detection of reactive oxygen species (ROS), cells were treated with the cell-permeable fluorogenic indicator dye dihydrorhodamine 123 (DHR 123, Molecular Probes, 1 μg/mL) in Dulbecco Modified Eagle medium without phenol red. After incubation for 15 minutes at 37°C, ROS-induced fluorescence was measured using a Tecan microplate reader (excitation at 488 nm, emission at 530 nm).
β-Galactosidase Staining
At the indicated time points after cultivation in complete growth medium, fibroblasts were stained using the Senescence β-Galactosidase Staining Kit (Cell Signaling) and analyzed by light microscopy.
Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted from peripheral blood mononuclear cells isolated by Ficoll density gradient centrifugation using the RNeasy Mini Kit (Qiagen) followed by DNase I digestion. Gene expression of the IFN-stimulated genes (IFI27, IFI44, IFI44L, IFIT1, ISG15, RSAD2, and SIGLEC1) was determined by quantitative real time reverse transcription–polymerase chain reaction using Taqman Universal PCR Master Mix (Applied Biosystems) on an ABI7300 and normalized to HPRT and GAPDH messenger RNA expression. Target genes were analyzed using predesigned TaqMan probes. Assays were calibrated using a calibrator cDNA calibrator. Interferon scores were calculated as previously described.14
Statistical Analysis
Data are representative of at least 3 independent experiments. Statistical analysis was performed using GraphPad Prism, version 6 (GraphPad Software). Normal distribution of data was tested using the Shapiro Wilk test. In normally distributed data, a 2-tailed t test was used, otherwise a Mann-Whitney test was used for comparison of 2 groups. P < .05 was considered statistically significant.
Results
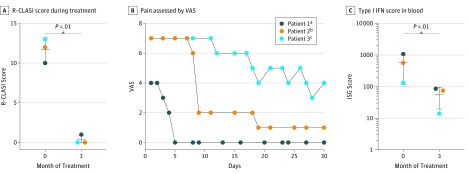
In 3 adults with FCL with painful and mutilating erythematous ulcerative skin infiltrates on acral locations as well as arthritis,2,4 we initiated treatment with the JAK 1/2 inhibitor baricitinib during the winter season.6 All patients had previously been treated with topical corticosteroids and hydroxychloroquine sulfate, with unsatisfactory results. Corticosteroids and hydroxychloroquine had been stopped at least 6 weeks before administration of baricitinib at a daily dose of 4 mg. Treatment with baricitinib significantly improved cutaneous lesions as assessed by the revised cutaneous lupus area and severity index15 after 3 months (Figure 1A). Patients who usually experienced multiple disease flares during the winter period reported reduced episodes as well as improved perfusion of digits during treatment (Figure 2). In addition, patients described complete or partial relief of skin and joint pain as measured on a visual analog scale (where 0 indicated no pain and 10 indicated the most severe pain; patient 1: Spearman ρ = –0.7970; P < .001; and patient 2: Spearman ρ = –0.9550; P < .001; Figure 1B). During treatment with baricitinib, the IFN signature in blood calculated from the messenger RNA expression levels of the IFN-stimulated genes IFI27, IFI44, IFI44L, IFIT1, ISG15, RSAD2, and SIGLEC1 was significantly suppressed after 3 months (Figure 1C), mirroring the therapeutic effect and underpinning the central role of type I IFN in the pathogenesis of disease. Treatment with baricitinib was well tolerated and no severe adverse effects occurred. Two patients experienced repeated mild upper respiratory tract infections during the winter season. In addition to a marked improvement of all aspects of disease symptoms, all 3 patients also reported that previously used topical corticosteroids and immunosuppressive therapies such as hydroxychloroquine had been less effective and did not induce comparable remission of cutaneous lesions and pain.
Figure 1. Clinical Response to Janus Kinase Inhibition in Familial Chilblain Lupus.
A, Mean (SD) revised cutaneous lupus area and severity index (R-CLASI) activity during treatment. B, Pain assessed by the visual analog scale (VAS) (where 0 indicates no pain and 10 indicates most severe pain). C, Mean (SD) type I interferon (IFN) score in blood before and after 3 months of baricitinib treatment measured by quantitative reverse transcription–polymerase chain reaction of IFN-stimulated genes (ISGs). aSpearman ρ = –0.7970; P < .001. bSpearman ρ = –0.9550; P < .001. cSpearman ρ = –0.9260; P < .001.
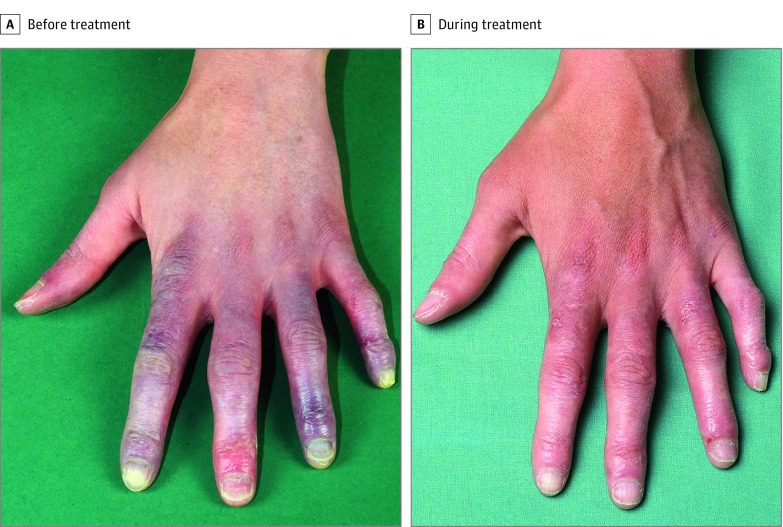
Figure 2. Representative Clinical Images Before and During 3 Months of Treatment With Baricitinib.
A, Bluish-red discoloration, painful erythematous infiltrates, and erosions on the hand of a patient with familial chilblain lupus. B, Clinical improvement during treatment with baricitinib.
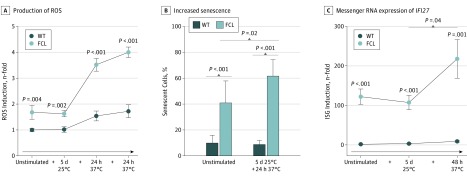
To investigate the influence of the clinical trigger factor cold on patient cells in vitro, we exposed primary fibroblasts generated from patients with FCL to low temperature. Cultivation of patient fibroblasts for 5 days at 25°C led to an enhanced formation of ROS on rewarming compared with wild-type fibroblasts from controls, indicating an enhanced stress response in TREX1-deficient cells (Figure 3A). Oxidative stress is a well-known trigger of DNA damage, suggesting that cold-induced ROS formation might further activate DNA damage signaling. One consequence of the DNA damage response is cellular senescence. As previously shown, TREX1-deficient fibroblasts exhibited an increased number of senescent cells under basal condition (Figure 3B). Unlike in wild-type cells, exposure to cold led to a significant increase in senescence in TREX1-deficient patient cells (Figure 3B). In addition, the cold-induced stress response in patient cells was associated with enhanced expression of the IFN-stimulated gene IFI27 (Figure 3C).
Figure 3. Cold-Induced Stress Response and Induction of Interferon-Stimulated Genes (ISGs).
A, Mean (SEM) production of reactive oxygen species (ROS) in fibroblasts exposed to 25°C for 5 days simulating cold with a rewarming phase at 37°C for 24 hours or without a rewarming phase at 37°C for 48 hours. B, Mean (SD) increased senescence as shown by staining for β-galactosidase activity after cold exposure for 5 days followed by 24 hours of rewarming. C, Mean (SEM) messenger RNA expression of IFI27 after exposure to cold (wild-type [WT], n = 3; familial chilblain lupus [FCL], n = 2; Aicardi-Goutières-Syndrome [AGS], n = 2). Mann-Whitney test or unpaired t test.
Discussion
These 3 cases demonstrated a good clinical response to baricitinib in patients with FCL due to TREX1 mutation. The pathologic characteristics of cutaneous disease in patients with TREX1-deficient FCL are driven mainly by type I IFN, which is illustrated by constitutive upregulation of IFN-stimulated genes and proteins in the blood and skin of patients.4 This finding is supported by studies in TREX1-deficent mice that develop lethal systemic autoimmunity. Moreover, concomitant genetic inactivation of the type I IFN receptor was shown to fully rescue the Trex1−/− phenotype.2
In this study, baricitinib was chosen for treatment from among the JAK inhibitors currently available for clinical use because of its highly specific inhibitory effect on the IFNAR receptor. The responsiveness of cutaneous lesions to baricitinib further underpins the pathogenic role of type I IFN in FCL.
In contrast to the effect of baricitinib treatment on cutaneous symptoms, pain associated with arthritis and skin lesions was not completely resolved. Type I IFNs can initiate an inflammatory cascade, with activation of additional cytokines that might be involved in joint inflammation. Future efforts are therefore necessary to find suitable combinations with anti-inflammatory drugs and to test other JAK inhibitors for possible superior effects on arthritis. Furthermore, long-term observations are needed to identify the minimal dose of baricitinib required to limit cutaneous disease and prevent possible infectious adverse effects associated with suppression of type I IFN.6
Despite proficiency in DNA damage repair, TREX1-deficient fibroblasts exhibit chronic activation of DNA damage signaling due to exhaustion of the single-stranded DNA–binding capacity of the DNA replication and DNA repair proteins RPA and Rad51.1 The ensuing enhanced formation of DNA metabolites along with activation of the DNA damage response contributes to the constitutively enhanced expression of IFN-stimulated genes in patient cells. Given that chilblain lupus is triggered by cold, we investigated the cellular response to cold in fibroblasts derived from patient skin. Exposure to cold led to an increase in oxidative stress that was accompanied by activation of type I IFN, suggesting that cold-induced cellular stress might act as a trigger that switches the cell from an immunologically activated state to an inflammatory state. In addition, other cold-induced effects such as reduced acral perfusion due to vasoconstriction may contribute to cold-induced disease flares in patients with FCL. To interfere with cold-induced disease flares, JAK inhibition might therefore be particularly recommended during the winter season in patients with FCL.
Limitations
A limitation of this study is the small number of patients included.
Conclusions
Our findings demonstrate the therapeutic efficacy of the JAK inhibitor baricitinib in 3 patients with FCL, a monogenic form of lupus, and provide mechanistic insight into the process of disease exacerbation by cold in TREX1-deficient cells. This finding implicates JAK inhibition as a potentially valuable therapeutic approach for monogenic type I interferonopathies and multifactorial type I IFN–driven forms of lupus erythematosus.16,17
References
- 1.Wolf C, Rapp A, Berndt N, et al. . RPA and Rad51 constitute a cell intrinsic mechanism to protect the cytosol from self DNA. Nat Commun. 2016;7:11752. doi: 10.1038/ncomms11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee-Kirsch MA. The type I interferonopathies. Annu Rev Med. 2017;68:297-315. doi: 10.1146/annurev-med-050715-104506 [DOI] [PubMed] [Google Scholar]
- 3.Rice G, Newman WG, Dean J, et al. . Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;80(4):811-815. doi: 10.1086/513443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peschke K, Friebe F, Zimmermann N, et al. . Deregulated type I IFN response in TREX1-associated familial chilblain lupus. J Invest Dermatol. 2014;134(5):1456-1459. doi: 10.1038/jid.2013.496 [DOI] [PubMed] [Google Scholar]
- 5.Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med. 2018;215(5):1287-1299. doi: 10.1084/jem.20180139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843-862. doi: 10.1038/nrd.2017.201 [DOI] [PubMed] [Google Scholar]
- 7.Frémond ML, Rodero MP, Jeremiah N, et al. . Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol. 2016;138(6):1752-1755. doi: 10.1016/j.jaci.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 8.König N, Fiehn C, Wolf C, et al. . Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis. 2017;76(2):468-472. doi: 10.1136/annrheumdis-2016-209841 [DOI] [PubMed] [Google Scholar]
- 9.Sanchez GAM, Reinhardt A, Ramsey S, et al. . JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018;128(7):3041-3052. doi: 10.1172/JCI98814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee-Kirsch MA, Gong M, Schulz H, et al. . Familial chilblain lupus, a monogenic form of cutaneous lupus erythematosus, maps to chromosome 3p. Am J Hum Genet. 2006;79(4):731-737. doi: 10.1086/507848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günther C, Meurer M, Stein A, Viehweg A, Lee-Kirsch MA. Familial chilblain lupus—a monogenic form of cutaneous lupus erythematosus due to a heterozygous mutation in TREX1. Dermatology. 2009;219(2):162-166. doi: 10.1159/000222430 [DOI] [PubMed] [Google Scholar]
- 12.Günther C, Berndt N, Wolf C, Lee-Kirsch MA. Familial chilblain lupus due to a novel mutation in the exonuclease III domain of 3′ repair exonuclease 1 (TREX1). JAMA Dermatol. 2015;151(4):426-431. doi: 10.1001/jamadermatol.2014.3438 [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Tüngler V, König N, Günther C, et al. . Response to: ‘JAK inhibition in STING-associated interferonopathy’ by Crow et al. Ann Rheum Dis. 2016;75(12):e76. doi: 10.1136/annrheumdis-2016-210565 [DOI] [PubMed] [Google Scholar]
- 15.Kuhn A, Meuth AM, Bein D, et al. . Revised Cutaneous Lupus Erythematosus Disease Area and Severity Index (RCLASI): a modified outcome instrument for cutaneous lupus erythematosus [published correction appears in Br J Dermatol. 2010;163(4):898]. Br J Dermatol. 2010;163(1):83-92. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DJ, Furie RA, Tanaka Y, et al. . Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222-231. doi: 10.1016/S0140-6736(18)31363-1 [DOI] [PubMed] [Google Scholar]
- 17.Wenzel J, van Holt N, Maier J, Vonnahme M, Bieber T, Wolf D. JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J Invest Dermatol. 2016;136(6):1281-1283. doi: 10.1016/j.jid.2016.02.015 [DOI] [PubMed] [Google Scholar]