This case report study describes 2 patients with locally advanced primary melanomas and the use of reflectance confocal microscopy for imaging of cutaneous involvement and disease monitoring.
Key Points
Question
Is it possible to monitor locally advanced primary melanomas and cutaneous metastasis treated with systemic immunotherapy using reflectance confocal microscopy?
Findings
In this case report study of 2 patients with locally advanced primary melanomas, reflectance confocal microscopy was found to correlate well with the clinical and histopathologic findings, thus showing a possible role in the treatment monitoring of these patients.
Meaning
Reflectance confocal microscopy is potentially a novel, noninvasive tool for imaging and monitoring of cutaneous involvement of advanced melanomas treated systemically.
Abstract
Importance
Melanoma incidence and the use of systemic treatments for it are rising. Current treatment monitoring uses clinical examination and radiologic examinations; however, cutaneous involvement and cutaneous metastasis may not be well visualized. Reflectance confocal microscopy (RCM) is a US Food and Drug Administration–approved, noninvasive technology that enables visualization of the skin with quasihistological resolution.
Objective
To evaluate the feasibility of using RCM to monitor advanced melanomas treated with immunotherapy.
Design, Setting, and Participants
This case report study took place from March 2017 to June 2018 and included 2 patients with locally advanced melanoma who were not candidates for surgery or were not willing to have surgery and who were started on an immunotherapy regimen at a tertiary care cancer hospital.
Main Outcomes and Measures
Clinical and RCM findings correlated with histopathology.
Results
In the patients, locally advanced melanoma with cutaneous involvement was treated with immunotherapy (pembrolizumab in 1 patient and an ipilimumab-nivolumab combination in the other) with resulting clearance of the lesions. Use of RCM showed the disappearance of clear melanoma features seen at baseline; these findings correlated with histopathology. The response was not seen with radiologic images, such as magnetic resonance imaging and computed tomography.
Conclusions and Relevance
Although RCM will not replace larger field imaging (such as magnetic resonance imaging, positron emission tomography, and computed tomography) in the management and follow-up of melanoma or other tumors, for imaging of cutaneous involvement and disease monitoring, RCM holds promise as a novel noninvasive technique.
Introduction
As the incidence of melanoma rises, the number of patients receiving systemic therapies for the management of locally advanced or metastatic disease is predicted to increase.1,2 Cases that are not amenable to surgery may be managed by radiation therapy or with systemic therapies. With improved response rates to targeted and immune checkpoint inhibitors, a need for imaging to evaluate response of cutaneous disease is being sought.3 To date, treatment monitoring has been completed by clinical or radiologic examinations; however, these may fail to detect changes in cutaneous and subcutaneous disease. Reflectance confocal microscopy (RCM) is noninvasive, provides semihistological cellular resolution images, and is a promising tool for the evaluation of cutaneous lesions in patients receiving systemic therapies for melanoma. Reflectance confocal microscopy enables the bedside visualization of skin pathology up to the superficial dermis (250 μm) without performing a biopsy. It was initially used as a diagnostic tool owing to its high sensitivity and specificity for melanoma and nonmelanoma skin cancers.4,5 Recently, RCM has been used to define tumor margins and monitor response to different therapies.6,7,8,9
Report of Cases
This study took place from March 2017 to June 2018 and was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. The 2 patients who were included in this study had locally advanced or regional lymph node metastatic disease, were not candidates for surgery or were not willing to have surgery, and were treated with systemic immune checkpoint blockade therapy. They provided written informed consent for RCM and biopsies. Staging was completed according to the American Joint Committee on Cancer’s Cancer Staging Manual, 8th Edition. Using a handheld confocal device (VivaScope 3000, Caliber Imaging & Diagnostics) to facilitate RCM, the primary tumoral sites were evaluated for melanoma-specific criteria: pagetoid cells in the superficial layers of the epidermis (presence of cells with a dark nucleus and bright cytoplasm, at least twice the size of keratinocytes), atypical nucleated cells at the dermoepidermal junction or superficial dermis (presence of dendritic or roundish cells with a dark nucleus and bright cytoplasm, at least twice the size of keratinocytes), and nests (oval to round bright aggregate with well-defined borders, composed of clustered cells, frequently large and highly refractive; nests can be dense, sparse, or cerebriform).10,11 Biopsies were then obtained from the RCM-evaluated areas.
Case 1
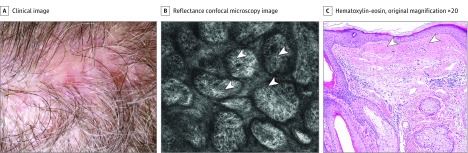
A patient (age, 70s) was referred for evaluation of a melanoma on the scalp. On physical examination, black nodules with multiple satellite lesions were noted (Figure 1A). Biopsy showed a nonulcerated, superficial spreading melanoma with Breslow thickness of 3.6 mm and mitotic index of 10 mitoses/mm2 (Figure 1B). Satellite lesions also proved to be melanoma. Reflectance confocal microscopy was used to visualize the extent of the cutaneous involvement. Multiple large, nucleated, hyperreflective cells were seen at the dermoepidermal junction around biopsy sites (Figure 1C). In addition, dense dermal nests were appreciated on RCM at the superficial dermis and surrounding the central mass (Figure 1D). Positron emission tomography (PET) and computed tomography (CT) did not demonstrate distant metastatic disease. Magnetic resonance imaging (MRI) of the brain was unremarkable and was not able to evidence any scalp lesions.
Figure 1. Case 1 Examination.
A, A pigmented tumor on the scalp of the patient (age, 70s) (crusted area represents biopsy site). B, Histopathology showing atypical melanocytes (yellow arrowheads) going deep into the dermis. C, Reflectance confocal microscopy image (750 × 750 μm) showing atypical, round, large, nucleated cells at the dermoepidermal junction (white arrowheads) into a disarranged dermal papilla. D, Reflectance confocal microscopy image (750 × 750 μm) showing dense nests in the superficial dermis (yellow arrowheads).
The patient was diagnosed with a stage IIIB melanoma (T3aN1cM0), and after thorough discussion of treatment options, and owing to the extent of the disease, pembrolizumab every 3 weeks was initiated as neoadjuvant therapy. We discussed with the patient that it would not be possible to assess disease response radiographically and that clinical assessment may be aided by using RCM. After 3 doses of pembrolizumab, there was evident clearing of the cutaneous involvement on both visual and dermoscopic inspection (Figure 2A). Reevaluation with RCM showed only fibrosis and no evidence of residual dermal disease, and all melanoma-specific criteria had disappeared (Figure 2B). Histopathological examination showed subtle fibrosis, lymphangiectasia, sparse chronic inflammation, rare melanophages consistent with features of regression, and no residual tumor, which confirmed the RCM findings (Figure 2C).
Figure 2. Clinical, Confocal, and Histopathologic Features of Case 1 After Treatment.
A, Total clearance of the lesion on the patient after 3 rounds of treatment with pembrolizumab. B, No atypical cells and fibrosis are seen (white arrowheads). C, Histopathology image of the superficial dermis showing an increase in the number of collagen, concordant with fibrosis (white arrowheads). No atypical cells or nests are seen.
Case 2
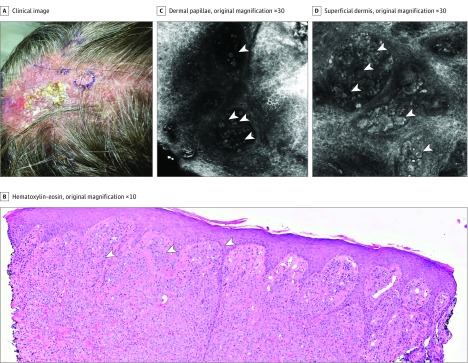
A patient (age, 60s) with history of cutaneous psoriasis and arthritis treated with TNFα inhibitors was referred for evaluation of a recently diagnosed amelanotic melanoma of the scalp. At physical examination, an ill-defined, extensive, pink-to-tan tumor was seen (Figure 3A). Biopsy showed superficial spreading melanoma of the scalp with Breslow thickness of 2.9 mm, mitotic index 2/mm2, and no ulceration; surrounding in-transit metastases were present (Figure 3B). The use of PET and CT showed pathologic axillary, supraclavicular, and mediastinal lymph nodes; an MRI of the brain was negative for metastases but showed a superficial plaque-type T1 hypointensity within the scalp among the midline vertex, which corresponded to the known tumor. The patient was diagnosed with a stage IIIC melanoma (T3aN3cM0) and, owing to the unresectable primary tumor, was started on neoadjuvant combined immunotherapy with ipilimumab and nivolumab. Prior to systemic therapy, RCM images of the biopsy site showed evident pleomorphic, large cells in the epidermis and dermis that formed dense and sparse nests—all clear melanoma features (Figure 3C and D). After 3 doses of combined therapy over 10 weeks, there was evident regression of the scalp lesions clinically and dermoscopically, though pigment remained in focal areas (Figure 4A). The RCM evaluation of the scalp areas showed no evidence of melanoma, with preserved cutaneous architecture and only melanophages (plump cells) and fibrosis (Figure 4B). Biopsies obtained confirmed RCM findings with no residual melanoma (Figure 4C).
Figure 3. Case 2 Examination.
A, An infiltrated amelanotic verrucous plaque on the scalp of the patient (age, 60s). B, Histopathology showing nests of atypical melanocytes invading the dermal papillae and going deep into the dermis (white arrowheads). C, Reflectance confocal microscopy image (750 × 750 μm) showing round, single nucleated atypical cells in the dermal papillae (white arrowheads). D, Reflectance confocal microscopy image (750 × 750 μm) showing dense and sparse nests in the superficial dermis (white arrowheads).
Figure 4. Clinical, Confocal, and Histopathologic Features of Case 2 After Treatment.
A, A smooth scalp surface with clearance of the scalp lesion on the patient after 3 months of treatment with ipilimumab and nivolumab. B, Fibrosis (white arrowheads) and plump nonnucleated melanophages (yellow arrowheads) are seen. C, Histopathology image showing fibrosis (white arrowheads) and melanin-laden melanophages (yellow arrowheads).
Discussion
In the present study, we describe 2 patients with locally advanced primary melanomas in which a complete remission was achieved after systemic immunotherapy with clinical-site resolution, RCM resolution, and histopathologic resolution confirmation. The most commonly used means to evaluate response to systemic therapy are either invasive (ie, tissue biopsies) or are limited to imaging techniques that do not achieve resolution at the cellular level and are suboptimal for evaluation of cutaneous involvement (ie, Response Evaluation Criteria in Solid Tumors criteria: CT, MRI, ultrasound).12 Emerging imaging techniques such as RCM provide value by offering both resolution and sensitivity when evaluating the skin in vivo beyond traditional imaging techniques. Because RCM offers cellular resolution of skin structures noninvasively, it can be used to track cutaneous involvement earlier than traditional imaging modalities.
In this study, we used a handheld device that allows for navigation of large and convex or concave areas but with a limited field of view. However, because we are imaging for melanoma-specific criteria rather than architecture, this shouldn’t be a limitation. Wide-probe devices enable evaluation of overall architecture of melanocytic lesions but may fail to detect subtle areas because they are limited to 8 × 8 mm and cannot be used in complex anatomic locations, including head, neck, and genitalia.
Limitations
The depth of RCM imaging is limited in vivo to about 250 μm, which is roughly at the level of the papillary dermis. Therefore, deep subcutaneous disease and dermal metastasis may not be visualized with this technique. We acknowledge the depth limitation and discourage performing confocal monitoring in the absence of evident simultaneous epidermal disease or to consider a false-negative result if confocal fails to demonstrate disease despite the presence of palpable tumor. Nevertheless, in our experience, this is not common, and confocal monitoring may even help guide biopsies in large melanomas. Multimodal images (such as when using RCM and optical coherence tomography) may overcome this limitation by simultaneously showing cellular details (with RCM) and depth information (with optical coherence tomography).13 Also, inflammation and necrosis may obscure visualization under RCM, so timing of imaging needs to be considered. The presence of melanophages (plump cells) under confocal may cause confusion with melanoma cells; however, the absence of nuclei in the former and presence of nuclei in the latter are key features to distinguish between both cell types (Figures 2 and 4). In some instances, it may be difficult to differentiate them under RCM.
Additionally, the RCM finding of dense nests (clusters) is more commonly associated with benign melanocytic lesions. In one study,6 dense nests were seen in 100% of nevi vs 65% of melanomas. We believe the finding of dense nests in the absence of other melanoma-specific criteria is reassuring, but the finding of dense nests in association of melanoma-specific confocal features (such as those seen in Figure 1C) suggests the diagnosis of melanoma with dermal component.
Conclusions
Although RCM will not replace larger field imaging (such as MRI, PET, and CT) in the management of melanoma or other tumors, for imaging of cutaneous involvement and disease monitoring, RCM holds promise as a novel and noninvasive technique. In addition, RCM may be helpful in assessing response of cutaneous melanoma metastases that have been injected with oncolytic viral therapy, an emerging area given the recent US Food and Drug Administration approval of talimogene laherparepvec.14
References
- 1.Karimkhani C, Green AC, Nijsten T, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134-140. doi: 10.1111/bjd.15510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies—update 2017. Eur J Cancer. 2017;83:247-257. doi: 10.1016/j.ejca.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 4.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127(12):2759-2765. doi: 10.1038/sj.jid.5700993 [DOI] [PubMed] [Google Scholar]
- 5.Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51(6):923-930. doi: 10.1016/j.jaad.2004.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Pellacani G, Cesinaro AM, Seidenari S. In vivo assessment of melanocytic nests in nevi and melanomas by reflectance confocal microscopy. Mod Pathol. 2005;18(4):469-474. doi: 10.1038/modpathol.3800330 [DOI] [PubMed] [Google Scholar]
- 7.Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57(4):644-658. doi: 10.1016/j.jaad.2007.05.044 [DOI] [PubMed] [Google Scholar]
- 8.Yélamos O, Cordova M, Blank N, et al. Correlation of handheld reflectance confocal microscopy with radial video mosaicing for margin mapping of lentigo maligna and lentigo maligna melanoma. JAMA Dermatol. 2017;153(12):1278-1284. doi: 10.1001/jamadermatol.2017.3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sierra H, Yélamos O, Cordova M, Chen CJ, Rajadhyaksha M. Reflectance confocal microscopy-guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22(8):1-13. doi: 10.1117/1.JBO.22.8.085005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61(2):216-229. doi: 10.1016/j.jaad.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 11.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132(10):2386-2394. doi: 10.1038/jid.2012.172 [DOI] [PubMed] [Google Scholar]
- 12.Humphreys TR, Shah K, Wysong A, Lexa F, MacFarlane D. The role of imaging in the management of patients with nonmelanoma skin cancer: when is imaging necessary? J Am Acad Dermatol. 2017;76(4):591-607. doi: 10.1016/j.jaad.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22(7):76006. doi: 10.1117/1.JBO.22.7.076006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780-2788. doi: 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]