Abstract
Xanthogranulomatous pyelonephritis (XGP) is an uncommon and distinct type of chronic infective pyelonephritis causing destruction of the kidney, severely affecting the renal function. The perinephric adipose tissue and peritoneum is not uncommonly involved. The study was undertaken to decipher the clinicopathologic spectrum of XGP. Forty cases of XGP were diagnosed on histopathology over a period of 13 years (2005–2017). Relevant clinical details and radiological findings were recorded from the case files. Out of a total of 40 cases, 26 were female and 14 were male with a mean age of 39.5 ± 13.6 years. Flank pain was the most common presenting symptom. All the patients had unilateral disease and underwent nephrectomy for a nonfunctional kidney. Gross examination showed enlarged kidney with replacement of cortico-medullary tissue by yellow nodular areas of fatty tissue and dilatation of the pelvicalyceal system. Thirty-six (90%) cases had nephrolithiasis. Histologically, the characteristic feature was the existence of lipid-laden foamy macrophages. Renal parenchymal involvement was diffuse in majority (31, 77.5%). Two (5.0%) of the patients had coexisting carcinoma in the same kidney. Histopathologic examination gives the definitive diagnosis of XGP which relies on the characteristic morphology. Surgical intervention in the form of nephrectomy is the treatment of choice and offers good treatment outcomes.
Keywords: Nephrectomy, pyelonephritis, xanthogranulomatous
Introduction
Xanthogranulomatous pyelonephritis (XGP) is a rare type of chronic infective pyelonephritis being increasingly recognized as an important cause of renal morbidity worldwide. Schlagenhaufer in 1916 first described XGP, which now globally accounts between 0.6% and 1% of all pyelonephritis cases.[1] It causes renal parenchymal destruction which compromises renal function and involvement of perinephric adipose tissue and peritoneum quite often. It affects all ages, with predilection for middle-aged females and the elderly. The main cause of XGP is chronic obstruction and infection.[2] The condition is usually unilateral and results in a nonfunctioning kidney.[3]
Substantial accumulation of lipid-laden macrophages causes yellow nodules in the renal parenchyma often visible on gross examination. Renal parenchymal destruction may be accompanied by fibrosis and the inflammatory process can extend beyond the renal territory. Hydronephrosis and renal calculi are often observed. XGP usually appears as kidney enlargement akin to a neoplasm and hence the nomenclatures of pseudo tumor/inflammatory tumor.[3] The clinical manifestations are varied and radiological imaging too simulates other inflammatory and neoplastic renal disorders.[4] Surgical intervention in the form of nephrectomy is the only definitive treatment.[5]
We intend to present our 13 years' retrospective data on XGP including the diverse clinical and histopathological features.
Methods
The present study is a retrospective study comprising 40 cases of XGP which were diagnosed on the basis of histopathology findings during a period of 13 years (2005–2017). The study was conducted in the department of pathology, in a tertiary care hospital. Ethical clearance was not taken considering the retrospective nature of the study. Specimens received were total nephrectomy in 38 (95.0%) cases and partial nephrectomy in 2 (5.0%) cases from the urology department of our hospital. Written informed consent was taken from all the patients before nephrectomy. Clinical and radiological details were obtained from the patients' file records.
The nephrectomy specimens were fixed in 10% buffered formalin and appropriately grossed as per the standard protocol for tissue processing and paraffin embedding. Sections were cut at 3-μm thickness and stained with hematoxylin and eosin stain. Special stains like periodic acid Schiff and Ziehl Neelsen were done wherever required.
Results
The patients ranged from 22 to 75 years of age (mean age: 39.5 ± 13.6 years). Of 40 cases, 26 were female and 14 were male with male-to-female ratio of 1:1.9. Five (12.5%) patients were on antihypertensive medication. All the patients had unilateral disease with predilection for the right kidney (25/40 cases). Flank pain was the commonest presenting symptom, seen in 37 (92.5%) cases followed by fever in 25 (62.5%) cases and dysuria in 19 (47.5%) patients. Renal angle tenderness was elicited in 16 (40.0%) cases and a lump was palpable in 12 (30%) cases on per abdomen examination. Ultrasound examination was done in all the cases. Computed tomography (CT) scan was available in 36 cases. Contrast enhanced computed tomography (CECT)/Intravenous pyelogram (IVP) [Figure 1]/Diethylene triamine penta acetic acid (DTPA) were done to assess the functional status and nonfunctioning/poorly functioning kidney was detected in all the cases.
Figure 1.
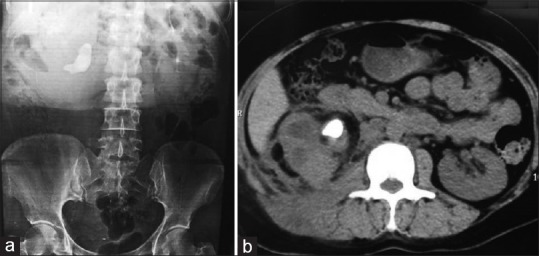
(a) Intravenous pyelogram shows right staghorn calculus with nonfunctioning kidney while left renal function is normal and (b) axial non-contrast computed tomography scan image shows areas of low attenuation, parenchymal inflammation with staghorn calculus at pelvis
On ultrasound – KUB, an enlarged kidney with disappearance of its normal architecture and inhomogeneous echogenicity was seen in all the cases. Dilated pelvicalyceal system, large amorphous central echogenicity, multiple fluid-filled masses, and contracture of the renal pelvis were visualized in most of the cases. In addition, renal space occupying lesion (SOL) was suggested in two cases.
CT scans available in 36 cases showed areas of low attenuation, parenchymal inflammation, hydronephrosis with expansion of renal calyces, and perinephric fat accumulation in all the cases. Contracted renal pelvis was seen in 32 cases while perinephric/peripelvic/post-ureteric stranding was observed in 30 cases. Thickening of renal fascias was noted. Obstruction caused by calculi in renal pelvis was seen in 36 (90%) cases, of which 24 cases had staghorn calculi. One case had a ureteric calculus. Note was made of the renal SOLs in two cases suspected to be neoplastic.
Urine culture reports were available in 34 cases and growth observed were of Escherichia coli (15), Proteus mirabilis (8), and Klebsiella pneumoniae (2). The culture was sterile in nine cases.
The diagnoses on the basis of CECT/IVP/DTPA scans were nonfunctioning kidney secondary to pyelonephritis in 22 (55.0%) cases, nonfunctioning kidney with pyonephrosis in 14 (35.0%), and perinephric abscess and renal SOL suspected to be tumor in 2 (5.0%) cases each.
Gross examination showed enlarged kidney in all the cases. Perinephric fat was increased in 34 (85.0%) cases. Renal capsule was adherent and external surface showed scarring in 32 (80%) cases. Perinephric fat showed gray brown to gray yellow necrotic areas in two (5.0%) cases. On cut section, dilatation of pelvicalyceal system was noted in all the cases with loss of cortico-medullary distinction and replacement of renal parenchyma by multiple yellow fatty nodular areas [Figure 2]. Necrotic areas with gray tan to gray yellow areas (variegated appearance) were seen. In addition, multiple pus-filled abscesses were seen in eight (20%) cases. Two (5.0%) cases showed focal gray tan areas of hemorrhage. In two (5.0%) cases, renal tumors were seen. One measured 6.0 cm in diameter and involved the upper pole of the kidney with gray yellow to gray tan cut section. The other tumor measured 2 × 2 × 1.5 cm and involved the renal pelvis. This tumor was friable, with focal papillae. Nephrolithiasis was seen in 14 (35.0%) cases on gross examination. In rest of the cases, the stones were handed over directly to the patients' attendants postoperatively. Attached ureter was dilated with ragged inner surface in eight (20.0%) cases. One case had a nephrocutaneous fistula where in addition to the nephrectomy specimen, fistulectomy specimen was also received.
Figure 2.
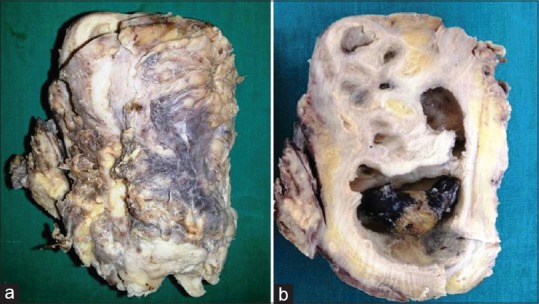
Gross photographs: (a) enlarged kidney with adherent capsule and (b) cut section shows dilated pelvicalyceal system with impacted stone, areas of fibrosis, and multiple yellow areas
On microscopy, representative sections examined showed granulomatous mixed inflammatory cell infiltrate. Sheets/Aggregates/Nodules of xanthomatous histiocytes with an admixture of neutrophils, lymphocytes, and plasma cells in variable numbers were seen [Figure 3]. Multinucleated histiocytic giant cells were noteworthy. Areas of fibrosis were also seen. In addition, lymphoid aggregates with germinal center formation (21, 52.5% cases), cholesterol clefts (19, 47.5% cases) [Figure 4], areas of hemorrhage and necrosis (11, 27.5% cases), foci of calcification (8, 20% cases), and cholesterol granulomas (5, 12.5% cases) were noted. Abscess formation walled off by chronic granulation tissue was observed in 10 (25.0%) cases.
Figure 3.
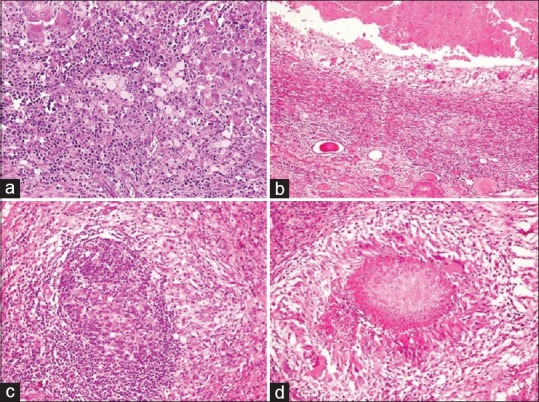
Histopathology sections in xanthogranulomatous pyelonephritis: (a) renal parenchyma with plentiful xanthoma cells admixed with lymphoplasmacytic cells and giant cells (H and E, ×400), (b) abscess formation (H and E, ×200), (c) lymphoid follicle (H and E, ×400), and (d) granuloma formation (H and E, ×400)
Figure 4.
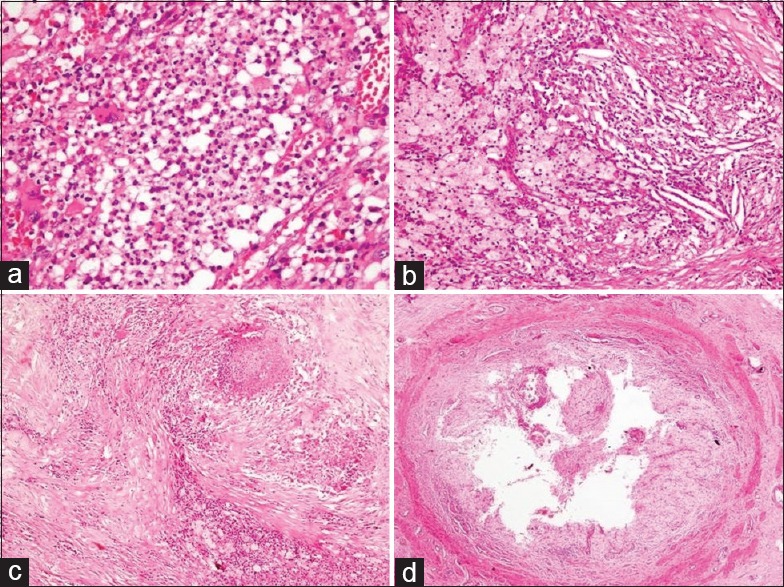
Histopathology sections in xanthogranulomatous pyelonephritis: (a) neutrophilic collections (H and E, ×400), (b) cholesterol clefts, xanthoma cells, and mixed inflammation (H and E, ×200), (c) fibrosis, patchy areas of necrosis and histiocytes (H and E, ×100), and (d) severe ureteritis with complete replacement of urothelial lining by foam cells (H and E, ×40)
Renal parenchymal involvement in XGP was diffuse in 31 (77.5%) cases and focal in 9 (22.5%) cases. The inflammation was seen extending into perirenal fat in three (7.5%) cases. Background showed changes of chronic pyelonephritis characterized by periglomerular fibrosis, tubular atrophy and thyroidization, interstitial fibrosis, and chronic inflammation. Features of chronic ureteritis in form of partly ulcerated transitional lining, mural fibrosis, and mild-to-moderate lymphomononuclear cell infiltrate were noted in 11 (27.5%) cases. Sections from renal blood vessels were largely unremarkable barring changes of hyaline arteriosclerosis noted in nine (22.5%) cases. In one case, changes of diabetic nodular glomerulosclerosis were seen in addition to XGP where the glomerular deposits were positive on periodic acid Schiff staining.
In two (5.0%) cases, with renal tumors detected in the same kidney, one was conventional renal cell carcinoma (RCC) (Fuhrman nuclear grade 2) composed of cells arranged in nests, acini, and focally as tubules [Figure 5]. The cells were uniform with clear cytoplasm, prominent cell membranes, round to oval nuclei, and tiny nucleoli. Sections from the adjacent renal parenchyma showed features of XGP. Other case showed papillary urothelial carcinoma, low grade with changes of diffuse XGP in the adjoining renal parenchyma. Overlying capsule, perinephric fat, ureter, and renal vessels were free of tumor in both the cases.
Figure 5.
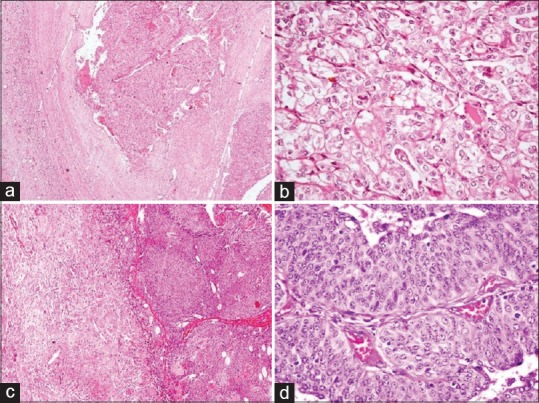
(a and b) Xanthogranulomatous pyelonephritis with renal cell carcinoma: (a) tumor arranged in nests with adjoining renal parenchyma showing xanthogranulomatous pyelonephritis (H and E, ×40) and (b) tumor cell nests separated by thin septae (H and E, ×400), (c and d) xanthogranulomatous pyelonephritis with papillary urothelial carcinoma: (c) papillary tumor with adjacent xanthogranulomatous pyelonephritis (H and E, ×100) and (d) true papillae with fibrovascular cores and lined by layers of neoplastic urothelial cells (H and E, ×400)
Histopathological diagnoses rendered were XGP in 23 (57.5%) cases, XGP with pyonephrosis in 10 (25.0%), XGP with perinephric abscess in 3 (7.5%); XGP with diabetic nodular glomerulosclerosis, XGP with chronic nonspecific fistula, renal cell carcinoma (clear cell type) in a background of XGP, and papillary urothelial carcinoma, low grade with XGP in 1 (2.5%) case each.
Discussion
XGP is an uncommon variant of chronic pyelonephritis with 1.4 cases per 100,000 population being recorded per year.[6] Three forms of XGP, diffuse, focal, and segmental, are recognized.[7] Renal parenchyma destruction by granulomas, abscesses, and lipid-laden macrophages is observed.[6,8] It is seen essentially in all age groups, but most frequently presents in middle aged to elderly patients. The mean age varies from 45 to 55.2 years.
XGP has a female predilection.[9] In our study too, female preponderance was seen with a male-to-female ratio of 1:1.9 and mean age of presentation was 39.5 years. The lesion is generally unilateral and more often involves the right kidney.[10] Bilateral lesions, although rare, are on record and invariably have a fatal outcome.[6] Twenty five of total 40 cases had involvement of the right kidney in our study with none of the cases having bilateral involvement.
Long-term genitourinary obstruction and recurrent urinary tract infection are nearly quintessential for development of XGP.[1,8] The exact pathology remains an unfolded mystery. Nephrolithiasis, most often with the staghorn-type calculus, is not a prerequisite, nevertheless remains a well-established predisposition for XGP.[11] In our series, nephrolithiasis leading to obstructive lesions was present in 36 (90%) cases. Urinary tract infection especially with P. mirabilis and E. coli, ureteropelvic junction syndrome, ureteropelvic duplication, severe vesicoureteral reflux, chronic interstitial nephritis, diabetes mellitus, rheumatoid arthritis, cirrhosis, obesity, metabolic syndrome, and immunocompromised states are all believed to be the predisposing factors.[1,8,12,13] Alterations in lipid metabolism and transport, arteriovenous occlusions, lymphatic obstruction, hemorrhage, and necrosis of pericalyceal fat are other plausible factors. Association with renal cell carcinoma, transitional cell carcinoma of the renal pelvis, and squamous cell carcinoma has been described.[14,15,16,17]
Clinical symptomatology of XGP is often nonspecific with fever, flank pain, palpable mass, malaise, anorexia, and weight loss being commonly observed.[8,18] Dysuria, frequency, pyuria, or hematuria may be experienced. Abscess formation (paranephric and psoas), fistula formation (renocutaneous and renocolonic), and sepsis are known complications.[19,20,21,22] In the present study, pyonephrosis and perinephric abscess were seen in 10 (25.0%) cases and 3 (7.5%) patients, respectively, and one case had nephrocutaneous fistula. Few patients may develop hypertension or hepatomegaly. Ischemic colitis and emphysematous pyelonephritis are reported albeit extremely rare.[23,24] All the patients were symptomatic in the current study and the most common symptom was flank or abdominal pain followed by fever and dysuria. Hematological and biochemical alterations like anemia, leukocytosis, raised Erythrocyte sedimentation rate (ESR), proteinuria, high fasting blood glucose, azotemia, elevated alkaline phosphatase, raised aspartate aminotransferase, and hypoalbuminemia can all occur which are again not specific for XGP.[8]
Radiologically, ultrasound examination in diffuse XGP reveals kidney enlargement, disappearance of its normal architecture, large amorphous central echogenicity, multiple fluid-filled masses, hydronephrosis, and contracted pelvis.[4,9,25] Ultrasonography findings in focal forms of XGP are nonspecific. IVP and DTPA renal scans are informative on the renal functional status. A fairly reliable radiologic diagnosis of XGP can be made on CT. Three stages of diffuse XGP,[26] stage I (nephric XGP – confined to renal parenchyma), stage II (perinephric XGP – involvement of perirenal space, Gerota's fascia), and stage III (paranephric XGP – involvement of pararenal space, retroperitoneal structures), have been described.
XGP is a diagnostic dilemma preoperatively as the clinical and radiologic findings imitate both benign and malignant lesions.[27,28] Histomorphology is pathognomonic with diffuse granulomatous inflammatory cell infiltrate.[8] There is an admixture of lipid-laden foamy macrophages, neutrophils, lymphocytes, plasma cells, and giant cells. In addition, cholesterol crystals, fibrosis, a variable degree of renal tubular atrophy, tubular dilatation, focal squamous metaplasia of the urothelium, microabscesses, lymphoid aggregates with germinal center formation, and spindle cell proliferation can be observed. In our study, similar findings were noted and diffuse renal parenchymal involvement was seen in majority (31, 77.5%).
The histomorphologic differentials include renal cell carcinoma with sarcomatoid features, leiomyosarcoma, Wilm's tumor, lymphoma, malakoplakia, megalocytic interstitial nephritis, pyelonephritis, tuberculosis, and perinephric abscess.[2,3,8,29] The benign entities, malakoplakia and megalocytic interstitial nephritis, can be excluded by observing cytoplasmic periodic acid Schiff-positive, diastase-resistant material in the histiocytes. Michaelis–Gutmann bodies are characteristically seen in malakoplakia. A critical evaluation of sections reveals epithelial and atypical spindle cell component in sarcomatoid RCC and interlacing bundles of spindled tumor cells with blunted nuclei and ample eosinophilic cytoplasm in leiomyosarcoma. Mitotic activity is seen in both. Lipid-laden xanthomatous cells of XGP may mimic the clear cells of clear-cell RCC. Exuberant foamy histiocytes may also be seen in papillary RCC. The xanthomatous cells have a foamy cytoplasm compared with the more cleared cytoplasm of tumoral clear cells. Immunohistochemistry (IHC) may be helpful in certain cases. Sarcomatoid RCC shows at least focal positivity for cytokeratin and epithelial membrane antigen. Leiomyosarcoma is diffusely positive for desmin and smooth muscle actin. Clear-cell and papillary RCC show good positivity for CD10 and epithelial membrane antigen. The xanthomatous cells and macrophages in XGP show positive cytoplasmic staining for lysozyme, diffuse positivity for CD68, and vimentin. IHC is extremely useful in cases with coexistence of XGP and tumors which is a rarity. Two of our patients had tumors, RCC and urothelial carcinoma concomitant with XGP in the same kidney.
Conclusions
XGP is a rare variant of chronic pyelonephritis with progressive loss of renal parenchyma resulting in a nonfunctioning kidney. Histopathologic examination gives the definitive diagnosis which relies on the characteristic morphology. The treatment of choice is nephrectomy for the more common diffuse forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siddappa S, Ramprasad K, Muddegowda MK. Xanthogranulomatous pyelonephritis: A retrospective review of 16 cases. Korean J Urol. 2011;52:421–4. doi: 10.4111/kju.2011.52.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lintong PM, Durry M. Xanthogranulomatous pyelonephritis. MOJ Clin Med Case Rep. 2015;2:1–3. [Google Scholar]
- 3.Das DP, Pal DK. Co-existing malakoplakia and xanthogranulomatous pyelonephritis of kidney: Two different spectrum of same disease process. Urol Ann. 2016;8:252–4. doi: 10.4103/0974-7796.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loffroy R, Guiu B, Watfa J, Michel F, Cercueil JP, Krausé D, et al. Xanthogranulomatous pyelonephritis in adults: Clinical and radiological findings in diffuse and focal forms. Clin Radiol. 2007;62:884–90. doi: 10.1016/j.crad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Addison B, Zargar H, Lilic N, Merrilees D, Rice M. Analysis of 35 cases of xanthogranulomatous pyelonephritis. ANZ J Surg. 2015;85:150–3. doi: 10.1111/ans.12581. [DOI] [PubMed] [Google Scholar]
- 6.Tsai KH, Lai MY, Shen SH, Yang AH, Su NW, Ng YY, et al. Bilateral xanthogranulomatous pyelonephritis. J Chin Med Assoc. 2008;71:310–4. doi: 10.1016/S1726-4901(08)70128-X. [DOI] [PubMed] [Google Scholar]
- 7.Chandanwale SS. Xanthogranulomatous pyelonephritis: Unusual clinical presentation: A Case report with literature review. J Family Med Prim Care. 2013;2:396–8. doi: 10.4103/2249-4863.123942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Parwani AV. Xanthogranulomatous pyelonephritis. Arch Pathol Lab Med. 2011;135:671–4. doi: 10.5858/2009-0769-RSR.1. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ghazo MA, Ghalayini IF, Matalka II, Al-Kaisi NS, Khader YS. Xanthogranulomatous pyelonephritis: Analysis of 18 cases. Asian J Surg. 2006;29:257–61. doi: 10.1016/S1015-9584(09)60099-3. [DOI] [PubMed] [Google Scholar]
- 10.Nandedkar SS, Malukani K, Sakhi P. Xanthogranulomatous pyelonephritis masquerading as a tumor in an infant. Indian J Urol. 2014;30:354–6. doi: 10.4103/0970-1591.134238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow J, Kabani R, Lithgow K, Sarna MA. Xanthogranulomatous pyelonephritis presenting as acute pleuritic chest pain: A case report. J Med Case Rep. 2017;11:101. doi: 10.1186/s13256-017-1277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshino T, Moriyama H. Case of the diffuse form of xanthogranulomatous pyelonephritis. Case Rep Urol 2013. 2013:936035. doi: 10.1155/2013/936035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tüysüz G, Tayfun F, Canpolat F, Zeytun H, Goya C, Keleş AN, et al. A case of xanthogranulomatous pyelonephritis mimicking wilms tumor. Turk J Pediatr. 2015;57:409–12. [PubMed] [Google Scholar]
- 14.Smith RD, Khoubehi B, Chandra A, Glass JM, Mee AD. Xanthogranulomatous pyelonephritis and renal malignancy: Unusual fellows in the renal bed. BJU Int. 2000;86:558–9. doi: 10.1046/j.1464-410x.2000.00817.x. [DOI] [PubMed] [Google Scholar]
- 15.Tseng CW, Chen WN, Juang GD, Hwang TI. Staghorn calculi and xanthogranulomatous pyelonephritis associated with transitional cell carcinoma. Urol Sci. 2015;26:69–71. [Google Scholar]
- 16.Mardi K, Kaushal V, Sharma V. Rare coexistence of keratinizing squamous cell carcinoma with xanthogranulomatous pyelonephritis in the same kidney: Report of two cases. J Cancer Res Ther. 2010;6:339–41. doi: 10.4103/0973-1482.73351. [DOI] [PubMed] [Google Scholar]
- 17.Fallatah A, Tarakji M, Amuesi J. Xanthogranulomatous pyelonephritis: A retrospective study of 10 cases and review of the literature. Saudi J Kidney Dis Transpl. 2001;12:520–4. [PubMed] [Google Scholar]
- 18.Leoni FA, Kinleiner P, Revol M, Zaya A, Odicio A. Xanthogranulomatous pyelonephritis: Review of 10 cases. Arch Esp Urol. 2009;62:259–71. doi: 10.4321/s0004-06142009000400001. [DOI] [PubMed] [Google Scholar]
- 19.Puthenveetil RT, Baishya D, Barua S, Sarma D. Unusual case of nephrocutaneous fistula – Our experience. Asian J Urol. 2016;3:56–8. doi: 10.1016/j.ajur.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alazab R, Ghawanmeh HM, Abushamma F, Ababneh O, Al-Karasneh AI. Spontaneous nephrocutaneous fistula: Rare complication of xanthogranulomatous pyelonephritis. Urol Case Rep. 2017;11:44–6. doi: 10.1016/j.eucr.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghoz HM, Williams M, Perepletchikov A, James N, Babeir AA. An unusual presentation of xanthogranulomatous pyelonephritis: Psoas abscess with reno-colic fistula. Oxf Med Case Reports 2016. 2016:150–3. doi: 10.1093/omcr/omw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott U, Busch M, Steiner T, Schubert J, Gröne E, Gröne HJ, et al. Renal failure, sepsis, and bilateral kidney tumors. Xanthogranulomatous pyelonephritis. Kidney Int. 2008;73:787–8. doi: 10.1038/sj.ki.5002778. [DOI] [PubMed] [Google Scholar]
- 23.Wen YK, Chen ML. Xanthogranulomatous pyelonephritis complicated by emphysematous pyelonephritis in a hemodialysis patient. Clin Nephrol. 2007;68:422–7. doi: 10.5414/cnp68422. [DOI] [PubMed] [Google Scholar]
- 24.Su YJ, Lai YC, Chou CY, Chang WH. Ischemic colitis secondary to xanthogranulomatous pyelonephritis. Int J Infect Dis. 2009;13:e89–91. doi: 10.1016/j.ijid.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Craig WD, Wagner BJ, Travis MD. Pyelonephritis: Radiologic-pathologic review. Radiographics. 2008;28:255–77. doi: 10.1148/rg.281075171. [DOI] [PubMed] [Google Scholar]
- 26.Khalid S, Zaheer S, Zaheer S, Ahmad I, Mohd Khalid. Xanthogranulomatous pyelonephritis: Rare presentation of a rare disease. South Asian J Cancer. 2013;2:4. doi: 10.4103/2278-330X.105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorzos I, Moutzouris V, Petraki C, Katsou G. Xanthogranulomatous pyelonephritis – The “great imitator” justifies its name. Scand J Urol Nephrol. 2002;36:74–6. doi: 10.1080/003655902317259418. [DOI] [PubMed] [Google Scholar]
- 28.AlDarrab RM, AlAkrash HS, AlKhateeb SS, AlBqami NM. A case report of a xanthogranulomatous pyelonephritis case mimicking the recurrence of renal cell carcinoma after partial nephrectomy. Urol Ann. 2015;7:524–6. doi: 10.4103/0974-7796.164857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dwivedi US, Goyal NK, Saxena V, Acharya RL, Trivedi S, Singh PB, et al. Xanthogranulomatous pyelonephritis: Our experience with review of published reports. ANZ J Surg. 2006;76:1007–9. doi: 10.1111/j.1445-2197.2006.03919.x. [DOI] [PubMed] [Google Scholar]


