Abstract
Aims
Takotsubo syndrome (TTS) is characterized by transient left ventricular dysfunction with symptoms and electrocardiographic changes mimicking acute myocardial infarction (AMI). The objective of this study was to evaluate in-hospital death and hospital readmission in patients with TTS and to compare outcomes to patients with AMI.
Methods and results
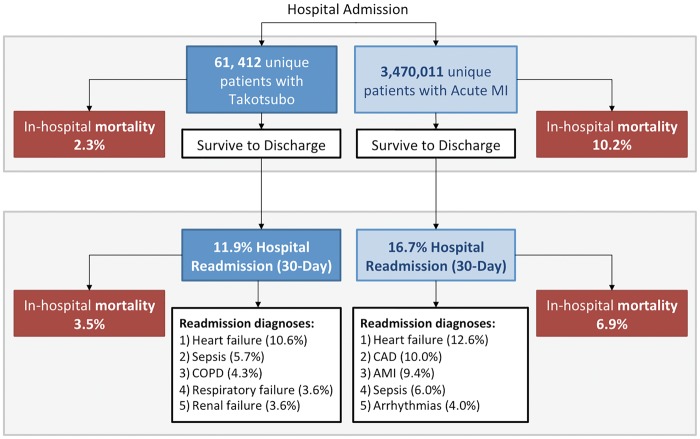
Patients diagnosed with TTS and AMI were identified using the United States Nationwide Readmission Database from 2010 to 2014. In-hospital outcomes for the index admission, and rates and causes of 30 day readmissions were compared between TTS patients and AMI patients without TTS. Sixty-one thousand, four hundred, and twelve patients with TTS and 3 470 011 patients with AMI without TTS were identified. Patients with TTS were younger, more often women (89% vs. 41%), and less likely to have cardiovascular risk factors than AMI patients. Mortality during the index admission was lower in TTS compared with AMI (2.3% vs. 10.2%, P < 0.0001). Cardiogenic shock occurred at the same frequency (5.7%) with TTS or AMI. Among TTS survivors, 7132 patients (11.9%) were readmitted within 30 days, and mortality associated with readmission was 3.5%. The most common reason for readmission after TTS was heart failure (HF; 10.6% of readmissions).
Conclusion
Takotsubo syndrome is associated with substantial morbidity and mortality. Although outcomes are more favourable than AMI, approximately 2% of patients died in hospital and approximately 12% of survivors were readmitted within 30 days; HF was the most frequent indication for rehospitalization. Careful outpatient follow-up of TTS patients may be warranted to avoid readmissions.
Keywords: Acute coronary syndrome, Hospital readmission, Myocardial infarction, Readmission, Takotsubo syndrome
Introduction
Takotsubo syndrome (TTS) is characterized by transient left ventricular dysfunction with symptoms and electrocardiographic changes mimicking acute myocardial infarction (AMI).1 Takotsubo syndrome predominantly affects post-menopausal women and often occurs following an emotional stressor. Classically patients with TTS have non-obstructive coronary artery disease (CAD) at angiography with apical left ventricular ballooning and hypokinesis.2 With increasing awareness over the past decade, TTS is now recognized as an entity that is distinct from AMI and is believed to account for nearly 2% of patients who present with suspected acute coronary syndrome.3–5 Although there is increasing recognition of TTS in clinical practice, few large cohorts with TTS are available for analysis and outcomes have not been fully characterized. Furthermore, despite the transient nature of left ventricular (LV) dysfunction in TTS, in-hospital mortality of Medicare beneficiaries age ≥65 years with TTS has been reported at 1.3%, and 1 year mortality was 6.9%.6 Additionally, among Medicare eligible adults with TTS, 11–16% were readmitted at 30 days. Rates of hospital readmission from a more representative sample of the population with TTS have not been reported.
The objectives of this study were to evaluate rates of in-hospital death and cardiogenic shock, and the frequency and causes of hospital readmission in patients with TTS using a large administrative database of United States hospital admissions. To provide context for these figures, we also compared outcomes to patients with AMI without TTS.
Methods
Study population
Adults ≥18 years old admitted to hospitals between 2010 and 2014 with a diagnosis of TTS were identified from the United States (US) Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project’s (HCUP) Nationwide Readmission Database (NRD), a national administrative database of hospital discharge-level data. The most recent year of data from the NRD includes 22 states and represents 51.2% of the USA population and 49.3% of all USA hospitalizations.7 Diagnosis of TTS was identified based on a primary or non-primary International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9) diagnosis code 429.83 (TTS) in patients who also underwent diagnostic coronary angiography (ICD-9 procedure code 37.22, 37.23, 88.55, 88.56, and 88.57). In order to increase specificity, we also required that patients who were diagnosed with TTS based on this ICD-9 code did not undergo coronary revascularization with percutaneous coronary intervention (PCI; ICD-9 procedure codes 36.01, 36.02, 36.05, 36.07, 36.09, or 00.66) or coronary artery bypass grafting (CABG; ICD-9 procedure codes 36.10, 36.11, 36.12, 36.13, 36.14, 36.15, 36.16, 36.17, and 36.19), consistent with methods previously described.4,6 Patients with AMI were identified using ICD-9 diagnosis codes for acute ST-segment elevation myocardial infarction (STEMI) (ICD-9 diagnosis codes 410.01–410.61, 410.81, and 410.91) and non-ST-segment elevation myocardial infarction (NSTEMI) (ICD-9 diagnosis code 410.71) in the absence of an ICD-9 diagnosis code for TTS. For patients with >1 admission with TTS during a calendar year, only the first hospitalization was included in the final analysis. Similarly, in the cohort of patients with AMI, only the first hospitalization for AMI in the calendar year was included in the final analysis.
The primary study outcome was 30 day all-cause hospital readmission, determined based on methodology described by HCUP.8 Among patients with multiple readmissions within 30 days of the index hospital discharge, only the first readmission was included for analysis. Patients discharged in the month of December during each year were excluded from the analysis due to incomplete follow-up data from the calendar-year files as per AHRQ guidelines.8
Clinical characteristics, hospital readmission, and outcomes
Demographics and comorbidities during the index hospitalization were defined by relevant ICD-9 diagnosis codes and AHRQ Elixhauser comorbidities. Principal Clinical Classifications Software diagnosis codes, AHRQ-defined groups of related ICD-9 diagnosis codes, were used to identify the indications for hospital readmission. Hospital length of stay and in-hospital mortality were also evaluated.
Sensitivity and subgroup analyses
Sensitivity analyses were performed to determine in-hospital mortality in patients with and without TTS who were assigned an ICD-9 diagnosis code for STEMI or NSTEMI. Given the possibility that diagnoses of AMI without TTS may have included significant numbers of patients with medical comorbidities precluding invasive management, a sensitivity analysis was performed excluding AMI patients who did not undergo coronary angiography during the index hospitalization. Subgroup analyses were also performed comparing outcomes of patients with a primary discharge diagnosis of TTS with patients who were assigned a diagnosis of TTS in a non-primary (secondary) position.
Statistical analysis
Continuous variables were reported as means with the standard error of measurement (SEM). Categorical variables were reported as percentages. Multivariable logistic regression models were generated to estimate odds of mortality and 30 day readmission, adjusted for patient demographics and medical comorbidities. Models included age, sex, hypertension, hyperlipidaemia, diabetes mellitus, chronic kidney disease, end stage renal disease, peripheral vascular disease, prior PCI, prior CABG, heart failure (HF), valvular disease, atrial fibrillation, malignancy, anaemia, chronic lung disease, hypothyroidism, obesity, rheumatoid arthritis, depression, psychosis, alcohol abuse, drug abuse, ST-segment elevation myocardial infarction diagnosis during hospital admission, and cardiogenic shock. In all analyses, sampling weights were applied to determine national incidence estimates.9 Statistical analyses were performed using SPSS 20 (IBM SPSS Statistics, Armonk, NY, USA) and SAS 9.4 (SAS Institute, Cary, NC, USA). Statistical tests are two-sided and P-values <0.05 were considered to be statistically significant. The NRD is a publicly available, de-identified dataset, and the study was exempt from institutional board review.
Results
Study population
Among 70 496 201 hospitalizations recorded in the NRD from 2010 to 2014, there were 52 811 hospitalizations with an ICD-9 diagnosis code for TTS. A total of 28 053 met inclusion criteria for a new diagnosis of TTS based on the use of diagnostic coronary angiography without coronary revascularization during the hospital admission. Among these admissions, 27 989 unique patients were identified. After excluding 2449 patients with hospital discharge in December of any calendar year, 25 540 unique patients hospitalized with TTS met full study inclusion criteria. After applying sampling weights, this represents 61 412 hospitalizations for TTS nationally between 2010 and 2014 (Supplementary material online, Figure S1). During the same 5 year period, after excluding December hospitalizations and applying sampling weights, there were 3 470 011 unique patients hospitalized with AMI.
Baseline characteristics of patients with TTS and those with AMI are shown in Table 1. Patients with TTS were younger, more likely to be women, and more likely to have a history of depression, psychosis, alcohol or drug abuse, hypothyroidism, rheumatoid arthritis and collagen vascular disease, and chronic pulmonary disease than patients with AMI. Cardiovascular risk factors and established cardiovascular disease were less frequent among patients with TTS in comparison to those with AMI.
Table 1.
Baseline characteristics of patients with takotsubo syndrome and acute myocardial infarction
| Takotsubo syndrome (n = 61 412) | Acute myocardial infarction (n = 3 470 011) | P-value | |
|---|---|---|---|
| Age, years (SEM) | 66.70 (0.11) | 68.91 (0.06) | <0.0001 |
| Female sex | 54 574 (88.9%) | 1 431 530 (41.3%) | <0.0001 |
| Primary payer | <0.0001 | ||
| Medicare | 37 738 (61.5%) | 2 189 310 (63.3%) | |
| Medicaid | 4025 (6.6%) | 228 819 (6.6%) | |
| Private insurance | 15 337 (25%) | 743 449 (21.5%) | |
| Self-pay | 2310 (3.8%) | 172 677 (5%) | |
| No charge | 249 (0.4%) | 19 919 (0.6%) | |
| Other | 1661 (2.7%) | 106 975 (3.1%) | |
| Hypertension | 41 305 (67.3%) | 2 543 908 (73.3%) | <0.0001 |
| Hyperlipidaemia | 29 971 (48.8%) | 1 949 458 (56.2%) | <0.0001 |
| Diabetes mellitus | 13 209 (21.5%) | 1 258 814 (36.3%) | <0.0001 |
| CKD | 5025 (8.2%) | 804 692 (23.2%) | <0.0001 |
| ESRD | 652 (1.1%) | 154 784 (4.5%) | <0.0001 |
| Peripheral vascular disorders | 4512 (7.3%) | 453 477 (13.1%) | <0.0001 |
| Prior PCI | 2979 (4.9%) | 447 607 (12.9%) | <0.0001 |
| Prior CABG | 932 (1.5%) | 298 232 (8.6%) | <0.0001 |
| Heart failure | 6293 (10.2%) | 394 235 (11.4%) | <0.0001 |
| Valvular heart disease | 1573 (2.6%) | 130 332 (3.8%) | <0.0001 |
| Atrial fibrillation | 8691 (14.2%) | 717 017 (20.7%) | <0.0001 |
| Malignancy | 2224 (3.6%) | 142 283 (4.1%) | 0.0021 |
| Anaemia | 9744 (15.9%) | 727 370 (21%) | <0.0001 |
| Chronic pulmonary disease | 17 674 (28.8%) | 797 999 (23%) | <0.0001 |
| Hypothyroidism | 10 581 (17.2%) | 404 197 (11.6%) | <0.0001 |
| Obesity | 6519 (10.6%) | 489 146 (14.1%) | <0.0001 |
| Rheumatoid arthritis/collagen vascular disease | 2678 (4.4%) | 94 115 (2.7%) | <0.0001 |
| Depression | 9667 (15.7%) | 290 827 (8.4%) | <0.0001 |
| Psychosis | 3006 (4.9%) | 104 251 (3%) | <0.0001 |
| Alcohol abuse | 2590 (4.2%) | 122 449 (3.5%) | <0.0001 |
| Drug abuse | 2348 (3.8%) | 89 806 (2.6%) | <0.0001 |
| Hospital urban-rural designation | <0.0001 | ||
| Large metropolitan area (>1 million residents) | 31 810 (51.8%) | 1 787 022 (51.5%) | |
| Small metropolitan area (<1 million residents) | 25 950 (42.3%) | 1 355 669 (39.1%) | |
| Micropolitan area | 3441 (5.6%) | 267 469 (7.7%) | |
| Rural area | 211 (0.3%) | 59 850 (1.7%) |
CKD, chronic kidney disease; ESRD, end stage renal disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
In patients with TTS, 26 711 (43.5%) patients were also assigned a diagnosis code for AMI. Among these, 5972 (22.3%) were coded as STEMI and 20 738 were coded as NSTEMI (77.6%). Among patients with AMI, STEMI, and NSTEMI were reported in 28.1% and 71.9% of cases, respectively.
A total of 25 572 patients (42%) were assigned a primary discharge diagnosis of TTS and 35 840 (58%) had TTS coded as a non-primary (secondary) diagnosis. Patients with a diagnosis of TTS in a non-primary position were more likely to be men and had a greater burden of cardiovascular and non-cardiovascular risk factors and comorbidities (Supplementary material online, Table S1). Among patients with a TTS coded in a non-primary position, the most common primary discharge diagnoses were AMI (50%), septicaemia (5.5%), adult respiratory failure (5%), and HF (4.6%).
In-hospital outcomes
A total of 1432 patients (2.3%) with TTS died during the index hospitalization. Among patients with TTS, cardiogenic shock [adjusted OR [aOR] 7.23, 95% confidence interval (CI) 5.63–9.32], STEMI (aOR 2.94, 95% CI 2.32–3.72), malignancy (aOR 2.32, 95% CI 1.60–3.36), HF (aOR 2.21, 95% CI 1.64-2.99), kidney disease (aOR 1.74, 95% CI 1.26–2.41), peripheral arterial disease (aOR 1.592, 95% CI 1.15—2.204), and atrial fibrillation (aOR 1.50, 95% CI 1.15–1.96) were independently associated with in-hospital mortality. When stratified by age and sex, men >50 years of age had the highest in-hospital mortality (4.6%), greater than that of women ≤50 years (1.1%), women >50 years (2.2%), and men ≤ 50 years (1.5%); (P < 0.001 for all comparisons). In contrast to outcomes of TTS, a total of 355 444 patients with AMI without TTS (10.2%) died during the index admission (P < 0.0001). Cardiogenic shock occurred with a similar frequency (5.7%) in patients with TTS and AMI. Cardiovascular complications, including cardiac arrest (2.7% vs. 4.4%, P < 0.0001) and complete heart block (0.9% vs. 1.2%, P = 0.0001) were less common during the index admission among patients with TTS in comparison to patients with AMI (Table 2).
Table 2.
Outcomes during index hospitalization
| Takotsubo syndrome (n = 61 412) | Acute myocardial infarction (n = 3 470 011) | P-value | |
|---|---|---|---|
| Cardiogenic shock | 3497 (5.7%) | 197 817 (5.7%) | 0.973 |
| Cardiac arrest | 1672 (2.7%) | 151 033 (4.4%) | <0.0001 |
| Ventricular fibrillation | 893 (1.5%) | 98 406 (2.8%) | <0.0001 |
| Complete heart block | 557 (0.9%) | 42 869 (1.2%) | 0.0001 |
| Death | 1432 (2.3%) | 355 444 (10.2%) | <0.0001 |
Among 5972 TTS patients and 973 485 AMI patients with coding for STEMI, 406 TTS patients (6.8%) and 134 583 AMI patients (13.8%) died during the index hospitalization (P < 0.0001). Among 20 736 TTS patients and 2 496 527 AMI patients with coding for NSTEMI, 315 TTS patients (1.5%) and 220 861 AMI patients (8.8%) died during the index hospitalization (P < 0.0001). In a sensitivity analysis including 2 001 830 patients with AMI who underwent coronary angiography (58% of all AMI), mortality remained significantly lower for patients with TTS than for those with AMI (2.3% vs. 3.6%, P < 0.0001). Among patients with a diagnosis of TTS, in-hospital outcomes differed based on the position of the discharge diagnosis code for TTS (Supplementary material online, Table S1).
Incidence, causes, predictors, and outcomes of 30 day hospital readmission
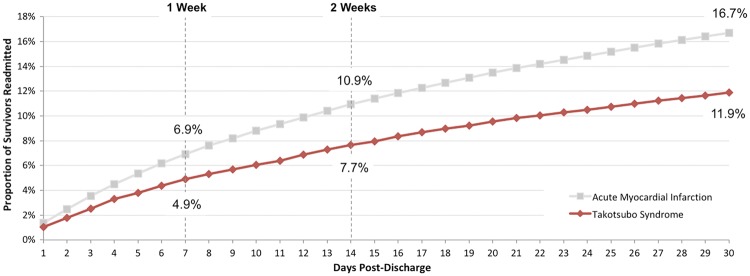
Among 59 980 patients with TTS who were discharged alive, 30 day readmission occurred in 7132 patients (11.9%). Among survivors of TTS readmitted to the hospital within 30 days, 64.5% of readmissions occurred within the first 2 weeks following the index hospital discharge (Figure 1). Indications for hospital readmission are shown in Table 3. Among TTS survivors, age, malignancy, peripheral vascular disorders, chronic lung disease, HF, drug abuse, and anaemia were independently associated with 30 day hospital readmission. Other clinical characteristics associated with 30 day hospital readmission among TTS are shown in Table 4. In comparison to TTS, among 3 114 567 AMI survivors, 520 082 patients were readmitted within 30 days (16.7%) (P < 0.001) (Figure 2). Heart failure was the most common indication for readmission in patients with TTS and AMI. The risk of death during readmission after TTS was 3.5% (n = 249), lower than that after AMI (6.9%, n = 35 799, P < 0.001).
Figure 1.
Proportion of patients with takotsubo syndrome and acute myocardial infarction with hospital readmission over time.
Table 3.
Primary indication for hospital readmission at 30 days
| Takotsubo syndrome | Acute myocardial infarction | ||
|---|---|---|---|
| Congestive heart failure | 758 (10.6%) | Congestive heart failure | 67 342 (12.9%) |
| Septicaemia | 405 (5.7%) | Coronary atherosclerosis and other heart disease | 51 858 (10%) |
| Chronic obstructive pulmonary disease and bronchiectasis | 305 (4.3%) | Acute myocardial infarction | 48 638 (9.4%) |
| Respiratory failure; insufficiency; arrest (adult) | 258 (3.6%) | Septicaemia | 31 107 (6%) |
| Acute and unspecified renal failure | 258 (3.6%) | Cardiac dysrhythmias | 20 939 (4%) |
| Cardiac dysrhythmias | 255 (3.6%) | Non-specific chest pain | 17 955 (3.5%) |
| Pneumonia | 245 (3.4%) | Complications of surgical procedures or medical care | 17 901 (3.4%) |
| Other and ill-defined heart disease | 244 (3.4%) | Pneumonia | 16 178 (3.1%) |
| Non-specific chest pain | 240 (3.4%) | Complication of device; implant or graft | 14 634 (2.8%) |
| Complications of surgical procedures or medical care | 237 (3.3%) | Acute and unspecified renal failure | 14 225 (2.7%) |
| Acute cerebrovascular disease | 200 (2.8%) | Respiratory failure; insufficiency; arrest (adult) | 12 930 (2.5%) |
| Coronary atherosclerosis and other heart disease | 166 (2.3%) | Gastrointestinal haemorrhage | 12 898 (2.5%) |
Indications for hospital readmission were determined by primary clinical classification software codes for each hospitalization.
Table 4.
Clinical characteristics associated with 30 day hospital readmission among survivors of takotsubo syndrome
| aOR (95% CI)a | |
|---|---|
| Malignancy | 1.88 (1.51–2.33) |
| End stage renal disease | 1.66 (1.15–2.39) |
| Chronic lung disease | 1.52 (1.38–1.68) |
| Drug abuse | 1.49 (1.20–1.86) |
| Peripheral vascular disorders | 1.48 (1.26–1.75) |
| Anaemia | 1.44 (1.29–1.62) |
| Congestive heart failure | 1.43 (1.23–1.67) |
| Atrial fibrillation | 1.39 (1.22–1.58) |
| Chronic kidney disease | 1.39 (1.18–1.63) |
| Diabetes | 1.31 (1.18–1.47) |
| Prior PCI | 1.31 (1.06–1.61) |
| Depression | 1.30 (1.141–1.47) |
| Age | 1.01 (1.01–1.01) |
| Hyperlipidaemia | 0.76 (0.69–0.83) |
The odds ratios for the model were adjusted for patient demographics and medical comorbidities including age, sex, hypertension, hyperlipidaemia, diabetes mellitus, chronic kidney disease, end stage renal disease, peripheral vascular disease, prior percutaneous coronary intervention, prior coronary artery bypass grafting, heart failure, valvular disease, atrial fibrillation, malignancy, anaemia, chronic lung disease, hypothyroidism, obesity, rheumatoid arthritis, depression, psychosis, alcohol abuse, and drug abuse.
Figure 2.
In-hospital outcomes and 30 day hospital readmissions in patients with takotsubo syndrome compared with acute myocardial infarction.
Discussion
Readmission within 30 days after TTS was common in this large national database, occurring in 12% of TTS survivors. Mortality during the index TTS hospitalization was 2.3%. Among patients with TTS who were readmitted within 30 days, 3.5% died during the first rehospitalization. Two-thirds of the hospital readmissions within 30 days of TTS occurred in the first 2 weeks post-discharge, highlighting the need for careful follow-up in these vulnerable patients. Rates of hospital readmission among survivors of TTS in the present analysis are consistent with data from prior analyses of smaller TTS cohorts.6,10–12
This is the first analysis of 30 day hospital readmissions in a representative sample of patients age ≥18 years with TTS compared with AMI, and one of the largest analyses of patients with TTS in the USA. We identified HF, sepsis, and chronic obstructive pulmonary disease as the most common indications for rehospitalization among patients with recent TTS. The most common cardiovascular indications for readmission were HF (10.6%) and cardiac dysrhythmias (3.6%). The burden of readmissions for HF in patients with TTS is a novel finding. In a recent cross-sectional study evaluating patients 1–3 years after a presentation with TTS, multimodality imaging demonstrated long-term functional and structural impairments of the left ventricle associated with HF phenotypes, despite normal left ventricular ejection fraction.13 The present analysis provides a clinical context for these mechanistic findings.
The reported incidence of TTS in the USA has increased three-fold in recent years, largely due to improved recognition and diagnosis.14 In prior analyses of TTS, overall mortality ranged from 1% to 6%, consistent with our results.2,3,14–20 In contrast, studies reporting lower rates of mortality associated with TTS have been small, single centre studies with few participants.21–26 In the present analysis of TTS patients, predictors of mortality included age, presentation with STEMI, cardiogenic shock, and underlying comorbidities including malignancy, and congestive HF. When stratified by sex, men had higher in-hospital mortality than women. These findings are consistent with prior reports that suggest a poorer prognosis associated with TTS in men, largely driven by higher rates of in-hospital complications including cardiogenic shock and respiratory failure.2,6,14 It remains unclear whether these differences in outcomes can be attributed to sex-specific pathophysiology, delay in diagnosis, or other factors.
The current analysis also includes patients with a diagnosis of primary and secondary TTS. A subgroup analysis demonstrated lower observed in-hospital mortality of patients with a primary diagnosis of TTS in comparison to those with a TTS diagnosis in a non-primary position. This likely reflects a higher acuity of illness among patients with secondary TTS occurring in the setting of acute non-cardiac medical conditions and comorbidities. The finding of higher mortality rates in secondary TTS is comparable to results from prior cohorts.3,6,14
In this study, 89% patients with TTS were women, and 91% of women were age ≥50 years, likely representing a predominantly post-menopausal cohort. Patients with TTS had a lower incidence of underlying cardiovascular risk factors and established cardiovascular disease than patients with AMI. In contrast, TTS patients were more likely to have psychiatric disease diagnoses in comparison to patients with AMI, a finding consistent with data from the International Takotsubo registry (InterTAK).2 In addition, patients with TTS were more likely to suffer from non-cardiac comorbidities, including pulmonary disease, rheumatoid arthritis and collagen vascular disease, and hypothyroidism. The significance of these diagnoses in the pathogenesis of TTS is uncertain.
Although the acute presentation of TTS often closely mimics acute MI, the subsequent clinical course may be more related to that of acute HF. Prior studies of large US cohorts hospitalized for acute decompensated HF have demonstrated in-hospital mortality of 3.1% and frequent 30 day readmission rates of 19–23%.27,28 These rates are comparable to our takotsubo cohort. In the present analysis, we demonstrated a greater than four-fold higher short-term mortality in AMI patients when compared to patients with TTS. Even after excluding AMI patients who did not undergo coronary angiography at the time of hospitalization, differences in mortality were attenuated but remained significantly higher in AMI in comparison to TTS. These findings contrast with data from the SWEDEHEART registry, which reported similar early mortality in patients with TTS and AMI.15 The discrepancy may be attributed to population differences between the USA and Sweden. In comparison to this study, the Swedish registry included a smaller AMI cohort with a significantly lower burden of cardiovascular comorbidities and lower mortality rates than observed in the present analysis.
Although outcomes of TTS appear favourable when compared with acute MI, patients with TTS have a substantial risk of in-hospital death and 30 day readmission among survivors. Thus, TTS is associated with morbidity and mortality in thousands of patients in the USA each year. Prospective investigations of patients with TTS are required to improve understanding of the pathophysiology and mechanisms of death in these patients, and ultimately, to determine optimal prevention and management strategies.
Limitations
Data from the NRD are derived from ICD-9 diagnosis and procedure codes recorded in state administrative databases and may be subject to errors in coding and/or reporting bias. To combat this concern, we used an accepted definition of TTS that requires diagnostic coronary angiography in the absence of coronary revascularization. This approach enhances the specificity of the diagnosis by excluding patients with a diagnosis code for TTS that was merely carried forward from a prior encounter. However, some patients coded as having TTS may truly have had MI with non-obstructive coronary artery disease (MINOCA) and not TTS. We have no information about potential triggers of TTS or the features used by clinicians to make the diagnosis. In many cases, in-hospital diagnoses of TTS are presumed to be provisional, pending confirmation of recovery of left ventricular wall motion. Takotsubo syndrome may be under-reported or missed in some cases, particularly if echocardiography or left ventriculography are not performed early after presentation. Similarly, we cannot confirm the readmission diagnoses because chart review was not feasible. Nearly half of patients who met the definition of TTS in this study also had a diagnosis code for AMI assigned during the index hospitalization. Codes for AMI were present in a primary position in 50% of patients with non-primary diagnosis of TTS. However, AMI is a rare cause of TTS, and the presence of an AMI code in the primary position in patients with a non-primary TTS diagnosis likely reflects an error in coding rather than a concurrent AMI diagnosis in the vast majority of cases, as has been described previously in a large Medicare cohort.6
Discrete clinical data, including the results of diagnostic coronary angiography, left ventricular systolic function, and left ventricular wall motion abnormalities were not captured in this administrative dataset. Therefore, we were unable to characterize the anatomical variant of TTS in our cohort. In-hospital and discharge medications were not recorded and could not be determined. Out-of-hospital mortality could not be determined from this dataset and readmission data were limited to 30 days. Due to limitations of the NRD datasets, longer-term rates of hospital readmission could not be determined across calendar years.
Conclusions
Takotsubo syndrome is associated with substantial morbidity and mortality. Although outcomes are more favourable than AMI, more than 2% of patients died in-hospital and nearly 12% of survivors were readmitted within 30 days with mortality of 3.5% during readmission. Two-third of hospital readmissions in patients with TTS were due to HF, sepsis, chronic obstructive pulmonary disease, respiratory failure, or renal failure. Careful outpatient follow-up of TTS patients may be warranted to avoid HF and hospital readmissions.
Funding
This work was supported by the NIH National Heart, Lung, and Blood Institute under award [T32HL098129 to N.R.S.] and American Heart Association [16SFRN28730004 to A.H.]. Additional support was provided through a generous donation by family of the late Dr. Grace Griffenberg.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Bybee KA, Prasad A.. Stress-related cardiomyopathy syndromes. Circulation 2008;118:397–409. [DOI] [PubMed] [Google Scholar]
- 2. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M. et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 3. Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR. et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- 4. Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL.. Prevalence of takotsubo cardiomyopathy in the United States. Am Heart J 2012;164:66–71.e1. [DOI] [PubMed] [Google Scholar]
- 5. Brinjikji W, El-Sayed AM, Salka S.. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the national inpatient sample 2008 to 2009. Am Heart J 2012;164:215–221. [DOI] [PubMed] [Google Scholar]
- 6. Murugiah K, Wang Y, Desai NR, Spatz ES, Nuti SV, Dreyer RP. et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among Medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail 2016;4:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steiner C, Elixhauser A, Schnaier J.. The healthcare cost and utilization project: an overview. Eff Clin Pract 2002;5:143–151. [PubMed] [Google Scholar]
- 8. Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. Methodological Issues when Studying Readmissions and Revisits Using Hospital Adminstrative Data. 2011. HCUP Methods Series Report # 2011-01. U.S. Agency for Healthcare Research and Quality Available from: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp (4 January 2018).
- 9. Yoon F, Sheng M, Jiang HJ, Steiner CA, Barrett ML, Calculating Nationwide Readmissions Database (NRD) Variances. 2017. HCUP Methods Series Report # 2017-01. U.S. Agency for Healthcare Research and Quality. Available from: http://www.hcup- us.ahrq.gov/reports/methods/methods.jsp (4 January 2018).
- 10. Nayeri A, Bhatia N, Xu M, Farber-Eger E, Blair M, McPherson J. et al. Prognostic significance of early rehospitalization after takotsubo cardiomyopathy. Am J Cardiol 2017;119:1572–1575. [DOI] [PubMed] [Google Scholar]
- 11. Yayehd K, N’da NW, Belle L, Bataille V, Hanssen M, Leddet P. et al. Management of Takotsubo cardiomyopathy in non-academic hospitals in France: the Observational French SyndromEs of TakoTsubo (OFSETT) study. Arch Cardiovasc Dis 2016;109:4–12. [DOI] [PubMed] [Google Scholar]
- 12. Parodi G, Bellandi B, Del Pace S, Barchielli A, Zampini L, Velluzzi S. et al. Natural history of tako-tsubo cardiomyopathy. Chest 2011;139:887–892. [DOI] [PubMed] [Google Scholar]
- 13. Scally C, Rudd A, Mezincescu A, Wilson H, Srivanasan J, Horgan G. et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation 2018;137:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S.. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J 2016;172:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redfors B, Vedad R, Angeras O, Ramunddal T, Petursson P, Haraldsson I. et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int J Cardiol 2015;185:282–289. [DOI] [PubMed] [Google Scholar]
- 16. Singh K, Carson K, Shah R, Sawhney G, Singh B, Parsaik A. et al. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol 2014;113:1420–1428. [DOI] [PubMed] [Google Scholar]
- 17. Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C. et al. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 2016;18:650.. [DOI] [PubMed] [Google Scholar]
- 18. Nunez-Gil IJ, Almendro-Delia M, Andres M, Sionis A, Martin A, Bastante T. et al. Secondary forms of Takotsubo cardiomyopathy: a whole different prognosis. Eur Heart J Acute Cardiovasc Care 2016;5:308–316. [DOI] [PubMed] [Google Scholar]
- 19. Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN. et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–341. [DOI] [PubMed] [Google Scholar]
- 20. Zalewska-Adamiec M, Bachorzewska-Gajewska H, Tomaszuk-Kazberuk A, Nowak K, Drozdowski P, Bychowski J. et al. Takotsubo cardiomyopathy: serious early complications and two-year mortality—a 101 case study. Neth Heart J 2016;24:511.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cacciotti L, Passaseo I, Marazzi G, Camastra G, Campolongo G, Beni S. et al. Observational study on Takotsubo-like cardiomyopathy: clinical features, diagnosis, prognosis and follow-up. BMJ Open 2012;2:e001165. doi:10.1136/bmjopen-2012- 001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glaveckaite S, Serpytis P, Peciuraite D, Puronaite R, Valeviciene N.. Clinical features and three-year outcomes of Takotsubo (stress) cardiomyopathy: observational data from one center. Hellenic J Cardiol 2016;57:428–434. [DOI] [PubMed] [Google Scholar]
- 23. Nunez-Gil IJ, Molina M, Bernardo E, Ibanez B, Ruiz-Mateos B, Garcia-Rubira JC. et al. Tako-tsubo syndrome and heart failure: long-term follow-up. Rev Esp Cardiol (Engl Ed) 2012;65:996–1002. [DOI] [PubMed] [Google Scholar]
- 24. Santoro F, Tarantino N, Ferraretti A, Ieva R, Musaico F, Guastafierro F. et al. Serum interleukin 6 and 10 levels in takotsubo cardiomyopathy: increased admission levels may predict adverse events at follow-up. Atherosclerosis 2016;254:28–34. [DOI] [PubMed] [Google Scholar]
- 25. Song BG, Hahn JY, Cho SJ, Park YH, Choi SM, Park JH. et al. Clinical characteristics, ballooning pattern, and long-term prognosis of transient left ventricular ballooning syndrome. Heart Lung 2010;39:188–195. [DOI] [PubMed] [Google Scholar]
- 26. Vriz O, Brosolo G, Martina S, Pertoldi F, Citro R, Mos L. et al. In-hospital and long-term mortality in takotsubo cardiomyopathy: a community hospital experience. J Community Hosp Intern Med Perspect 2016;6:31082.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arora S, Patel P, Lahewala S, Patel N, Patel NJ, Thakore K. et al. Etiologies, trends, and predictors of 30-day readmission in patients with heart failure. Am J Cardiol 2017;119:760–769. [DOI] [PubMed] [Google Scholar]
- 28. Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS. et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail 2010;3:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.