Abstract
Aims
Randomized clinical trials investigating a possible outcome effect of remote monitoring in patients with implantable defibrillators have shown conflicting results. This study analyses the information flow and workflow details from the IN-TIME study and discusses whether differences of message content, information speed and completeness, and workflow may contribute to the heterogeneous results.
Methods and results
IN-TIME randomized 664 patients with an implantable cardioverter/defibrillator indication to daily remote monitoring vs. control. After 12 months, a composite clinical score and all-cause mortality were improved in the remote monitoring arm. Messages were received on 83.1% of out-of-hospital days. Daily transmissions were interrupted 2.3 times per patient-year for more than 3 days. During 1 year, absolute transmission success declined by 3.3%. Information on medical events was available after 1 day (3 days) in 83.1% (94.3%) of the cases. On all working days, a central monitoring unit informed investigators of protocol defined events. Investigators contacted patients with a median delay of 1 day and arranged follow-ups, the majority of which took place within 1 week of the event being available.
Conclusion
Only limited data on the information flow and workflow have been published from other studies which failed to improve outcome. However, a comparison of those data to IN-TIME suggest that the ability to see a patient early after clinical events may be inferior to the set-up in IN-TIME. These differences may be responsible for the heterogeneity found in clinical effectiveness of remote monitoring concepts.
Keywords: Telemedicine, Defibrillator, implantatble, Heart failure
Introduction
Remote monitoring (RM) of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) has become widely available and is recommended by the HRS/EHRA expert consensus panels from 2008 on.1,2 State-of-the-art RM technology allows automatic transmission of data related to device function, arrhythmias, and other physiological parameters without active participation of the patient.2–9 Remote monitoring enables safe reduction of the in-person follow-up (FU) burden for patient and clinician convenience and a substantial shortening of the delay between a clinically actionable event detected by the device and the clinical reaction.2,4,6,10
The potential influence of RM on patient outcomes has been investigated in randomized controlled trials (RCTs) employing a variety of RM technologies in various healthcare models.11–13 To date, only the IN-TIME trial8 demonstrated a significant impact of implant-based RM on hard clinical endpoints, a composite clinical score for heart failure patients14 (primary endpoint of the study) and mortality. In eight other RCTs included in a dedicated meta-analysis (2015)15 and in three recent trials (2016–17),16–18 RM failed to improve patient outcomes. However, a pooled patient-level analysis of the TRUST,4 ECOST,19 and IN-TIME8 results (2010–14) with the same RM system, confirmed the benefit reported in the IN-TIME and suggested that it was driven by the prevention of heart failure exacerbation.20 The question arises why the results with this particular RM system differ from those of other systems.
It is generally plausible that a clinical effect of RM can be conferred only if relevant information on the patient’s medical status is received in time and if it leads to important therapeutic changes.21 The present article reports details of the information flow (content and information speed) and workflow in the IN-TIME trial. Its aim is to discuss whether a difference in these aspects across studies may be responsible for different clinical outcomes, with IN-TIME being an ‘outlier’ not because of chance or a faulty analysis or interpretation, but due to differences in study set-up and RM technology.
Methods
IN-TIME study design and results
The IN-TIME study design and main results have been published elsewhere.8,11 In brief, the study enrolled 716 patients with chronic heart failure, New York Heart Association (NYHA) Class II/III symptoms, ejection fraction ≤35%, no permanent atrial fibrillation (AF), and an indication for dual-chamber ICD or CRT-D treatment. After a run-in phase of 1 month, patients were randomly assigned to either automatic, daily RM in addition to optimal care (n = 333) or optimal care without RM (n = 331). After 12 months of FU, the prevalence of a worsened composite clinical score, combining all-cause death, overnight hospitalization for heart failure, increase in NYHA class, and worsening in patient’s global self-assessment,14 was 18.9% in the RM group vs. 27.2% in the control group (P = 0.013).8 The Kaplan–Meier estimate of all-cause mortality was 3.4% vs. 8.7% (P = 0.004).
The proprietary Biotronik Home Monitoring® (HM) technology (Biotronik SE & Co. KG, Berlin, Germany) was used. Everyday, the implanted devices sent data wirelessly to the Home Monitoring Service Center (HMSC) via a patient device situated at the patient’s bedside. The only patient activity required was to connect once the patient device to a power line. The kind of transmitted data is summarized in Table 1. The HMSC processes incoming data and posts them as trend graphs and tables on a secure website for treating physicians. Furthermore, it sends e-mails (‘HM-alerts’) when criteria, which can be adapted online for individual patient, are met. In IN-TIME, HM data were transmitted irrespective of the randomization group but were concealed for control patients until study completion. Four patients (0.6%) with poor data transmission during the run-in phase were excluded from randomization.
Table 1.
Data received in the Home Monitoring Service Center on a daily basis
| Trend data |
|---|
| Bradycardia and CRT |
| Pacing statistics for all pacing channels |
| Atrioventricular conduction statistics |
| Percentage of CRT pacing |
| HF monitor and physiological parameters |
| Mean atrial heart rate |
| Mean ventricular heart rate |
| Mean ventricular heart rate at rest |
| Mean number of VES per hour |
| Atrial heart rate variability |
| Patient activity in hours per day |
| Thoracic impedancea |
| Atrial tachyarrhythmia |
| Atrial burden (percent of the day in AF) |
| Mean and maximum ventricular rate during AF |
| SVT episode (counter) |
| Mode switch (counter) |
| Ventricular tachyarrhythmia |
| VT1, VT2, and VF zone detected (counter) |
| ATP and shock therapies, started/successful (counters) |
| Ineffective shock with maximum energy (counter) |
| Lead related |
| RA, RV, and LV shock impedance |
| RA amplitude (mean) |
| RV, LV amplitude (minimum) |
| RV, LVa pacing threshold |
| Technical |
| Actual device programming setting |
| Battery and technical status |
| IEGM episode (typically one per day) |
| VT1, VT2, VF, or SVT episode |
| Atrial monitoring episode |
| Periodic IEGM |
All data are transmitted on a daily basis and displayed as trends or listings.
AF, atrial fibrillation; ATP, antitachycardia pacing; CRT, cardiac resynchronization therapy; HF, heart failure; IEGM, intracardiac electrogram; LV, left ventricular; RA, right atrial; RV, right ventricular; SVT, supraventricular tachyarrhythmia; VES, ventricular extrasystoles; VF, ventricular fibrillation; VT1/VT2, slow/fast ventricular tachycardia.
Available in one-third of implanted study devices.
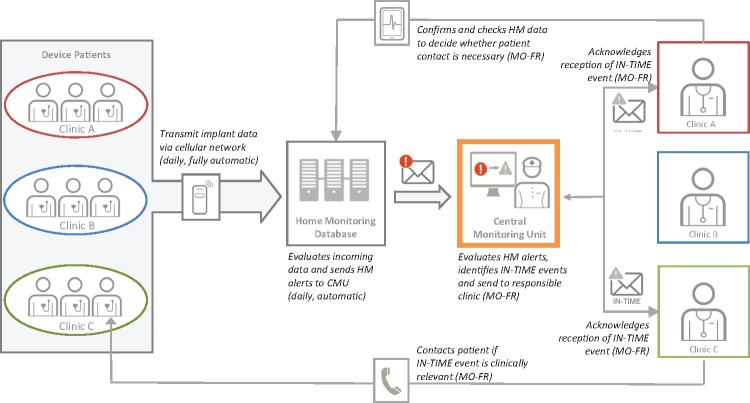
Home Monitoring information flow in IN-TIME is summarized in Figure 1. In contrast to normal HM routines, HM-alerts from the HMSC were sent to a central monitoring unit (CMU) located at an investigational site in Leipzig.8 The CMU had standard operating procedures which defined study specific alerts, mostly based on HM-alerts (Table 2). Study nurses, supported by local physicians, screened all patients’ data during normal office working hours (Mondays–Fridays), alerted sites and required confirmation of alert receipt. Investigators could ask for modification of alerts for specific patients. The CMU was established to tailor the alerts specifically for the study, to enhance sites’ awareness of alerts, and to record alerts and receipt confirmations systematically. The CMU staff did not assist in diagnostic or therapeutic decisions, except that they checked intracardiac electrogram snapshots for suspicious content.
Figure 1.
Sketch of the remote monitoring information flow and interaction between parties in IN-TIME. CMU, clinical monitoring unit; HM, Home Monitoring.
Table 2.
Alerts sent to investigational sites by the central monitoring unit in IN-TIME: definitions and occurrencesa
| Alert type | Device/HMSC: alert setting | CMU activity in a patient with HMSC alert | CMU: condition for SOP compatible event | Number of alerts (number of patients) |
|---|---|---|---|---|
| Atrial tachyarrhythmia | 109 (65) | |||
| First onset of AF for >30 s in patients with no prior documented AF | Device: IEGM for atrial monitoring episode | Check IEGM for atrial monitoring episode in patients with no prior documented AF | IEGM shows true AF | 31 (31) |
| HMSC: sends alert | ||||
| Long atrial arrhythmia episode (>6 h) with high-ventricular rate (>120 b.p.m.) | HMSC: alert for atrial burden >6 h with ventricular rate >120 b.p.m. | Check IEGM | Ventricular rate >120 b.p.m. | None |
| Daily AF burden >50% on 7 consecutive days after a period of 4 weeks with burden <25% for patients with known AF | HMSC: alert for atrial burden >6 h | Check trend graph | Atrial burden ≥50% on 7 consecutive days | 20 (16) |
| Atrial arrhythmia in the VT zone | HMSC: VT zone detected | Check IEGM | Suspicious IEGM | 1 (1) |
| First onset of AF for >30 s in patients with prior documented AF | Device: IEGM for atrial monitoring episode | Check IEGM for atrial monitoring episode | Not predefined | 22 (22) |
| HMSC: alert for atrial monitoring episode | ||||
| Atrial episode | HMSC: alert for atrial monitoring episode | Check IEGM for atrial monitoring episode | Not predefined | 22 (14) |
| Atrial burden >50% for more or less than 1 week | HMSC: alert for atrial burden ≥6 h | Check trend graph | Not predefined | 13 (8) |
| Ventricular tachyarrhythmia or ICD shock | 56 (42) | |||
| ≥3 VT2/VF episodes over 48 h | HMSC: alerts for VT2 and VF detection | Check IEGM for appropriateness and count true VT/VF episodes in 48 h | ≥3 episodes within 48 h | 7 (6) |
| First ICD shock in spontaneous episode | HMSC: alert for VT and VF detections | Check if the episode was induced or spontaneous | Spontaneous episodes with shock | 22 (22) |
| First occurrence of slow VT (<150 b.p.m.) | HMSC: alert for VT1 (monitoring zone) | Check IEGM if true VT | True VT <150 b.p.m. | 8 (8) |
| VT/VF detection with suspicious IEGM (e.g. sensing issue or inappropriate detection) | HMSC: alert for VT and VF detections | Check IEGM | Suspicious IEGM | 2 (2) |
| <3 VT/VF episodes over 48 h | HMSC: alert for VT and VF detections | Check IEGM | Not predefined | 18 (14) |
| Ineffective ICD shock | HMSC: ineffective shock | Check IEGM | Not predefined | 2 (2) |
| VT and low CRT pacing | HMSC: alert for VT and low CRT | Check IEGM | Not predefined | 1 (1) |
| Low percentage of CRT pacing | 91 (35) | |||
| CRT pacing <80% over 48 h | HMSC: alert for CRT pacing <80% | Check CRT pacing on the day before | CRT pacing <80% on two consecutive days | 81 (30) |
| CRT pacing <80% combined with IEGM | HMSC: alert for CRT pacing <80% | Check IEGM | Suspicious IEGM | 3 (3) |
| CRT pacing <80% over 24 h | HMSC: alert for CRT pacing <80% | Check trend graph | Not predefined | 8 (7) |
| CRT pacing <80% and ventricular or atrial arrhythmia | HMSC: alert for CRT pacing <80% | Check IEGM | Not predefined | 2 (2) |
| Ventricular extrasystoles | 54 (46) | |||
| Visible upward trend in VES/h over 7 days | Not programmable | Check trend graph weekly | Visible trend | 39 (34) |
| VES >110 per hour for more than 10 days | Not programmable | Check trend graph | Not predefined | 15 (15) |
| Physiological trends | 2 (2) | |||
| Visible downward trend in patient activity over 7 days | Not programmable | Check rend graph weekly | Visible trend | 1 (1) |
| Rising mean heart rate | Not programmable | Check trend graph | Not predefined | 1 (1) |
| Lead related measurements and IEGMs | 98 (58) | |||
| Suspicious IEGMb | HMSC: alert for any new IEGM | Check IEGM | Suspicious IEGM | 25 (18) |
| RA amplitude | HMSC: programmable | Check trend graph | <0.5 mV | 13 (9) |
| RV amplitude | HMSC: programmable | Check trend graph | <2.0 mV | 22 (18) |
| RV pacing threshold | HMSC: programmable | Check trend graph | Safety margin <1 V | 11 (10) |
| RV impedance | HMSC: programmable | Check trend graph | <250 or >1500 Ohm | 1 (1) |
| LV amplitude | HMSC: programmable | Check trend graph | <2.0 mV | None |
| LV impedance | HMSC: programmable | Check trend graph | <250 or >1500 Ohm | 2 (2) |
| LV pacing threshold | HMSC: programmable | Check trend graph | Safety margin <1 V | 12 (8) |
| Shock impedance | HMSC: programmable | Check trend graph | <30 or >125 Ohm | 13 ( 5) |
| Gaps in data transmission | 818 (241) | |||
| Missing HM messages for 3 days | HMSC: alert for missing messages for 3 days | Check if the patient is known to be on holidays or in hospital | If the patient is not known to be absent | 818 (241) |
| Technical alerts | None | |||
| Elective replacement indicator | HMSC: programmable | None | ||
| Technical alert | HMSC: programmable | None | ||
Sums of numbers of alerts do not match completely because few alerts are contained in more than one category, and three alerts contained no description of the content.
CMU, central monitoring unit; ICD, implantable cardioverter-defibrillator; HM, Home Monitoring; SOP, standard operating procedure defined in the study protocol; VT, ventricular tachycardia; AF, atrial fibrillation; CRT, cardiac resynchronization therapy; IEGM, intracardiac electrogram; LV, left ventricular; RA, right atrial; RV, right ventricular; VES, ventricular extrasystoles; VF, ventricular fibrillation; VT1/VT2, slow/fast ventricular tachycardia.
During the randomized period in the RM group.
T-wave oversensing, far-field atrial sensing of ventricular activity, or other suspected sensing problem.
If the investigators considered the content of the alert clinically relevant, they were supposed to contact the patient and conduct a structured interview on the patient’s overall condition, weight change, and drug compliance. After gaps in RM transmission, this interview was also conducted to confirm the patient’s status after a period without monitoring data. Per patient-year of FU, the CMU sent 4.0 alerts to the sites, the sites contacted the patient 2.1 times, and 0.3 additional FUs or contacts to other physicians were arranged.8 Investigators were suggested to react according to current guidelines but the therapeutic consequences were not followed.
Data analysis
The present analysis of IN-TIME data aims at evaluating the transmission performance of HM, describing the CMU performance, and estimating delays from alerts to FU visits. The following aspects are addressed as follows:
Establishing HM transmission: the time from post-implant hospital discharge to first HM transmission described by the Kaplan–Meier method, in all patients with successful implantation including some not who were not randomized at 1 month.
Transmission performance: the number of days with HM message divided by the total days between randomization and study termination. Also the length of transmission gaps was analysed.
Decline of transmission during the study: a linear fit of the share of patients with a HM message as a function of time after randomization, weighted with the number of patients in the study.
Delay from an event until the information is received in the HMSC: an estimation based on the distribution of the time to the next successful transmission for all days between randomization and study termination in patients randomized to RM.
Working time compliance of the CMU: we estimated whether the CMU worked on every working day. For this, we selected a period of 2 years when most patients were included in the study and calculated the mean number of CMU alerts per day and the number of 7 days of the week (Mondays, Tuesdays, …, Sundays) without any alert. The Poisson-distribution predicts an expected number of days without any alert from the mean number of alerts per day, assuming that alerts were independent of each other. If the CMU did not comply with their working time rules, a higher than predicted number of days without alerts would be expected.
Delay from alert to patient contact and follow-up: the delay from a HM alert to a patient contact was recorded in the study documentation. However, the delay from a HM alert to related FU visits was not available because the study data do not allow an assignment of FUs to alerts. We, therefore, estimated alert-to-FU delays without any a priori assumptions on relations between FUs and alerts by a mathematical model. It used as inputs 1222 alerts and 1289 FUs captured on case report forms or evident in the HMSC as dates of reprogramming in 280 patients who had alerts in the RM group. For each patient, we calculated the set of time intervals between each alert and each FU. We show the relative number of FUs per day in the 8 weeks after the alert, as mean and standard deviation, normalized to the FU rate between 14 and 100 days after the alert.
Statistical methods
Home Monitoring performances had non-normal distributions and were compared with the Mann–Whitney U-test. We used a linear regression model to calculate the decline of HM transmission after randomization. A P-value <0.05 was considered statistically significant. The analysis was conducted with the R 3.3 statistical software (R Development Core Team, Vienna, Austria).
Results
Transmission performance of home monitoring
Of 702 patients discharged from hospital with a study device implanted, 41.6% had HM transmission established already 1 day after discharge, 68.8% on Day 3, and 95.5% on Day 30.
In the RM group, HM messages were received on 82.2% of all patient-days, or on 83.1% out-of-hospital days after randomization. Patient-individually, the median percentage of days with a message was higher in the RM group [87.8%, interquartile range (IQR): 78.6–93.4%] than in the control group (85.4%, IQR: 71.8–91.8%) (P = 0.003). In the RM group, 79.9% of the patients transmitted messages on 75% or more of all study days.
There were 29.5 transmission interruptions per patient-year in the RM group with an average length of 2.1 days, but only 2.3 interruptions per year were longer than 3 days. In the control group, the total number of interruptions was similar (29.2 per patient-year), but interruptions longer than 3 days were more frequent (2.8 per patient-year).
A decline of transmission performance during the study was observed in both randomization groups (Figure 2). The linear fit line fell by an absolute of 3.3%, from 84.7% (randomization) to 81.4% (365 days), in the RM group, and by 10.1%, from 83.4% to 73.3%, in the control group.
Figure 2.
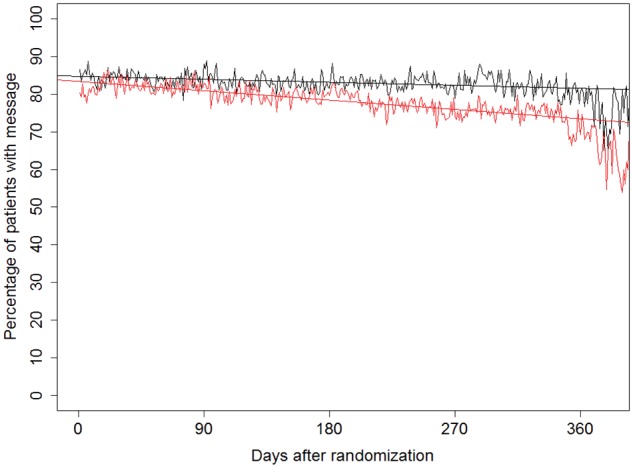
Percentage of patients with home monitoring data transmission on any given day between randomization and study termination. Black line denotes the RM group and red line denotes the control group. The straight lines are the linear fits. The linear regression model shows that the decline was statistically significant in both groups (P < 0.001).
The estimated delay between a medical event and the information being received in the HMSC was 1 day in 83.1% of all cases. On Days 2 and 3, 91.2% and 94.3% of all events were available, respectively.
Definition and occurrence of alerts sent by the central monitoring unit
Table 2 summarizes CMU alerts. For example, the first AF episode was reported in 31 patients without history of AF. Twenty alerts were sent for daily AF burden ≥50% on 7 consecutive days. A first ICD shock triggered by spontaneous ventricular tachyarrhythmia was reported in 22 patients, whereas arrhythmia ‘storm’ (≥3 ventricular episodes over 48 h) led to seven alerts. Low percentage of CRT pacing over two consecutive days was reported 83 times. Beyond alerts predefined in the study protocol, the CMU sent self-initiatively notifications for a first AF episode in patients with known AF history and for some other conditions (Table 2).
Working time compliance of the central monitoring unit
In a period of 104 weeks between 1 July 2008 and 28 June 2010, on average 113 patients (between 73 and 140) were followed by the CMU. For these patients, the CMU sent 938 alerts, or 1.29 alerts per day. No alerts were sent on 12.5% of all Mondays (13/104), on 30.0% of all Tuesdays–Fridays (Tuesdays 29/104, Wednesdays 30/104, Thursdays 31/104, and Fridays 35/104), and on 85.1% of all Saturdays and Sundays (Saturdays 86/104 and Sundays 91/104) in this period. The data indicate that the CMU did not work during most weekends, as defined per study protocol, and that a backlog of alerts remained for Mondays. The Poisson-distribution predicts no alerts on 27.5% of all days, which is close to the result for Tuesdays–Fridays, indicating good working time compliance.
Delay from alert to patient contact and follow-up (estimated)
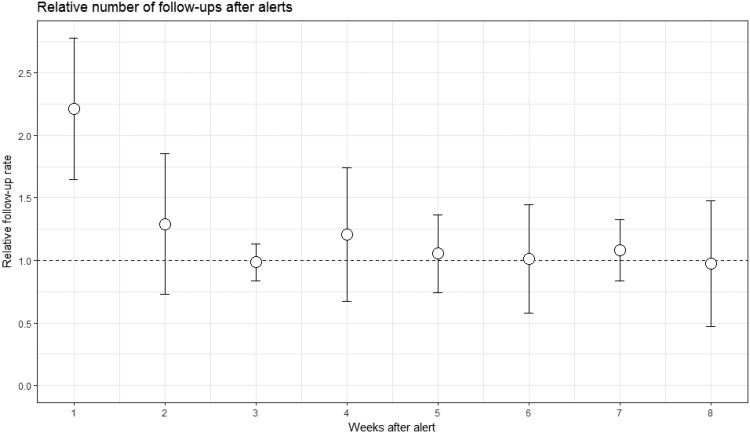
Patients were contacted after a median delay of 1 day (IQR 0–6 days). Figure 3 shows that the number of FUs is clearly increased by a factor of two in the week after the alert, but only very slightly in the second week, suggesting that FUs, which were conducted as a consequence of HM information took place in most cases within a week of the alert. A sketch of some performance figures is given in Figure 4.
Figure 3.
Increase of follow-ups after alerts. Based on 1222 alerts and 1289 follow-ups in 280 patients who had alerts in the RM group. We calculated the set of time intervals between each alert and all follow-ups following in the same patient. We show the relative number of follow-ups per day in the 8 weeks after the alert, as mean and standard deviation, normalized to the follow-up rate between 14 and 100 days after the alert. Note that the number of follow-ups is increased in the week after the alert, but not later. This suggests that most follow-ups resulting as a consequence of home monitoring information took place within a week of the alert.
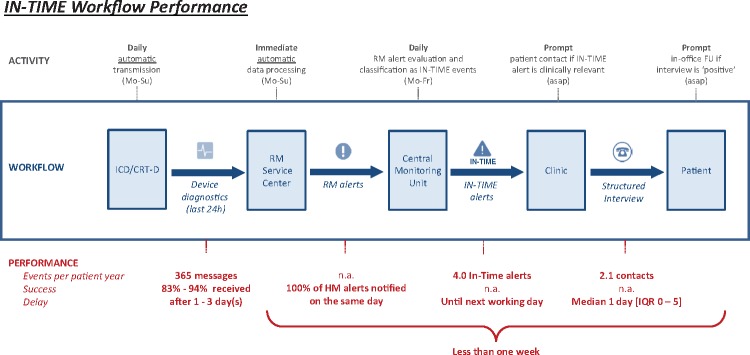
Figure 4.
Sketch of the IN-TIME information- and workflow with some performance characteristics. HM, Home Monitoring; Mo, Monday; Fr, Friday; Su, Sunday; FU, follow-up; asap, as soon as possible; n.a., not available; IQR, interquartile range.
Discussion
To understand whether differences in clinical outcomes of implant-based RM in different studies can be attributed to differences in study set-up, we analysed the information flow and workflow in the IN-TIME trial. Three main possible sources of heterogeneity between studies will be discussed: content of RM messages, information speed and completeness, and workflow in response to RM messages.
Content of remote monitoring messages
In the IN-TIME study, a multitude of different triggers was used to generate alerts. When treating physicians examined the patients’ data, they had access to up-to-date data trends for most variables stored in the device statistics, including arrhythmia burden, heart rates, patient activity, intracardiac electrograms, and pacemaker timing statistics. These data may help with the decision if an alert, mostly derived from a single variable, is appropriate. The possibility to modify the alert criteria online without reprogramming the implanted device may reduce inappropriate alerts and increase the physician’s willingness to act.
In other studies, the number of possible alerts was seemingly limited by study design (thoracic impedance only12) or by the RM system (e.g. five alerts available: thoracic impedance, atrial burden, ventricular rate during AF, high number of shocks per episode, and all therapies exhausted).22,23 It is unclear from the publications of these studies which up-to-date data were available when an alert was received to review the patient’s condition and decide about the clinical need for a patient contact. At least in one study, a patient contact and a manual data download was required after automatic alerts to allow for RM data review.12 In REM-HF, no alerts were programmed but a complete data set was checked after every transmission (scheduled once per week).13
Speed and completeness
The results on HM transmission in IN-TIME show that HM is suited for long-term monitoring in consecutive unselected ICD/CRT-D recipients. Within 1 month of implantation, 95% of all patients had successfully sent a first message. Less than 1% of patients were excluded from the study due to inability to establish HM transmission. Only 3.3% of surviving patients in the RM group who sent messages in the beginning failed to do so after 1 year. The fact that messages were still received on more than 70% of all patient-days in control group patients after being left alone for 1 year suggests that the transmission is robust. However, the early reaction to interruptions of the transmission, which is only possible with frequent scheduled transmission, exerts a significant effect on transmission performance.
In REM-HF, 58%, 66%, and 62% of the patients successfully transmitted 75% or more of the scheduled weekly messages, at 6, 12, and 24 months, respectively.13 The corresponding figure from IN-TIME is 80%; this is higher, but the main difference is that messages were scheduled daily, not weekly. Since no alerts were transmitted in REM-HF, changes in the patient’s status were detected only in the next scheduled transmission.
In the CONNECT study, 180 of 575 clinical events resulted in a transmitted alert (31%).6 The devices used in this study could transmit each type of alert only once between FUs. Other studies reported a better rate of alerts transmission: 76% (OptiLink)16 and 88% (MORE-CARE).17
In the RM system used in IN-TIME, alerts are not generated in the device but in the service centre. Because most alerts are based on a comparison between the recent and the previous transmission, alerts will not be lost even if the transmission on that day fails. About 83% of all events detected in the RM group were available in the HMSC within 1 day, and approximately 94% were available within 3 days. The median delay until the contact to the patient—if a contact took place—was 1 day. The delay between the events and their availability in the RM service centre has not been reported from other trials. The median delay between alerts and their reviewing 1.4 days in EVOLVO7 and 3 days in MORE-CARE.24
Workflow
The IN-TIME study protocol required checking of the transmitted HM data in the CMU on all working days. Although the chain from medical event to clinical action cannot be reconstructed exactly, the data indicate that the CMU fulfilled its duties. It worked on all days between Mondays and Fridays, with stand-in for holidays and sick leave. The vast majority of CMU alerts were compliant with the predefined rules, but some went beyond, such as the first AF episode in patients with known history of AF. Whether this was by oversight or on purpose, the information can be medically relevant. The feedback to clinical sites on transmission gaps exerted a significant effect on transmission performance, as evidenced by better transmission rate in the RM group vs. the control group by absolute 8.1% late in the study.
The analysis of the temporal relationship between alerts and FUs indicated that most FUs took place within a week of the alert. The information speed and completeness (of alerts) is an obvious requirement for this result, but it is per se not sufficient without a daily check of the RM data. We are not aware of publications of workflow details from other studies, such as estimates of a delay from alert to FU.
Influence of remote monitoring on outcome
With the presented data, we cannot prove that the difference between the results of IN-TIME and other studies has its cause in different study set-ups or the RM system characteristics. However, RM can influence outcome only through early appraisal of relevant medical information by the physician. We have shown that in IN-TIME, a considerable list of alerts was used; that the vast majority of alerts was available within 1 day, together with a current set of diagnostic data comparable to that in the device’s memory; and that the investigators managed to see most patients within a week of an event, if they decided that this was indicated. Comparable results are not (yet?) reported for other studies, so we are not able to describe differences with the required precision. However, several performance characteristics reported here are inherently connected to the technology of the RM system used in IN-TIME, especially the ability to transmit the complete data set daily.
A recent analysis of several clinical endpoints from the IN-TIME and ECOST studies has suggested that daily HM improves only heart failure events.20 The IN-TIME study was the first study to demonstrate clinical benefit of implant-based RM, but it has been overseen that it was also the first study to use a heart-failure specific primary endpoint.8,14
If one observes new onset AF, asymptomatic ventricular tachycardia, increasing frequency of ventricular extrasystoles, or decreasing percentage of CRT pacing in a heart failure patient, we believe that it is very plausible that a deterioration of the patient’s clinical status can be prevented if the patient is seen within a few days. The lack of an appropriate alert trigger, a failure to transmit the alert and accompanying data or a significant lag before transmission, the inability to judge the patient’s status from RM data, or the failure to contact the patient without delay may be the reason for the failure to improve clinical outcome. Too many of these details are unknown for too many trials to dismiss IN-TIME as an outlier.
The observation that the clinical benefit of RM is restricted to heart failure events suggests that it may be reasonable to establish a monitoring unit especially for heart failure patients, possibly in co-operation between device and heart failure clinics. IN-TIME had no more than 140 patients under HM surveillance at any time, thus, even medium size implanting centres may establish an efficient monitoring team.
Conclusion
The difference between the IN-TIME result and other outcome studies may be caused by differences in content of transmitted data, speed and completeness of transmission, and workflow to contact the patient when needed. Both for clinical routine and for future studies, we suggest to establish processes to assure a high transmission compliance in the long term, to use a wide array of medical data to trigger alerts and to judge if a patient contact is needed, and to establish processes allowing to see the patient within less than 1 week after a medically relevant event. Publications of work- and information flow details from other studies—whether successful or not—would be valuable to inform such planning.
Acknowledgements
The authors would like to thank Dejan Danilovic for critical reading and editorial assistance.
Funding
The study was funded by Biotronik SE & Co. KG, Berlin, Germany. The funder contributed to the study design but had not role in data collection. D.H. was head of the Clinical Monitoring Unit. J.S. was the responsible biostatistician of the present results.
Conflict of interest: C.G. reports minor travel support and lecture fees from Biotronik. C.S. reports grants from BIOTRONIK. J.C.N. reports grants from the Novo Nordisk Foundation. S.P.H. reports personal fees from Pfizer/BMS. J.S. is an employee of Biotronik. T.L. reports personal fees from Biotronik. G.H. received research grants through the Heart Centre Leipzig from Abbott and Boston Scientific. And all other authors have nothing to disclose.
References
- 1. Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE.. HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA). Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace 2008;10:707–725. [DOI] [PubMed] [Google Scholar]
- 2. Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, McLean RC, Mittal S, Morichelli L, Patton KK, Raitt MH, Pietro Ricci R, Rickard J, Schoenfeld MH, Serwer GA, Shea J, Varosy P, Verma A, Yu C-M.. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015;12:e69–e100. [DOI] [PubMed] [Google Scholar]
- 3. Burri H, Senouf D.. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace 2009;11:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C.. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325–332. [DOI] [PubMed] [Google Scholar]
- 5. Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP.. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–2367. [DOI] [PubMed] [Google Scholar]
- 6. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH.. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57:1181–1189. [DOI] [PubMed] [Google Scholar]
- 7. Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S, Marzegalli M.. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125:2985–2992. [DOI] [PubMed] [Google Scholar]
- 8. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Søgaard P.. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 9. Nägele H, Lipoldová J, Oswald H, Klein G, Elvan A, Vester E, Bauer W, Bondke H, Reif S, Daub C, Menzel F, Schrader J, Zach G.. Home monitoring of implantable cardioverter-defibrillators: interpretation reliability of the second-generation “IEGM Online” system. Europace 2015;17:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hindricks G, Elsner C, Piorkowski C, Taborsky M, Geller JC, Schumacher B, Bytesnik J, Kottkamp H.. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial. Eur Heart J 2014;35:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arya A, Block M, Kautzner J, Lewalter T, Mörtel H, Sack S, Schumacher B, Søgaard P, Taborsky M, Husser D, Hindricks G.. Influence of Home Monitoring on the clinical status of heart failure patients: design and rationale of the IN-TIME study. Eur J Heart Fail 2008;10:1143–1148. [DOI] [PubMed] [Google Scholar]
- 12. Brachmann J, Böhm M, Rybak K, Klein G, Butter C, Klemm H, Schomburg R, Siebermair J, Israel C, Sinha A-M, Drexler H.. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF Study (Optimization of Heart Failure Management using OptiVol Fluid Status Monitoring and CareLink). Eur J Heart Fail 2011;13:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan JM, Dimitrov BD, Gill J, Kitt S, Ng GA, McComb JM, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Yao GL, Cowie MR.. Rationale and study design of the REM-HF study: remote management of heart failure using implanted devices and formalized follow-up procedures. Eur J Heart Fail 2014;16:1039–1045. [DOI] [PubMed] [Google Scholar]
- 14. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–182. [DOI] [PubMed] [Google Scholar]
- 15. Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DH, Roberts-Thomson KC, Young GD, Sanders P, Ganesan AN.. Remote monitoring of implantable cardioverter-defibrillators: a systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol 2015;65:2591–2600. [DOI] [PubMed] [Google Scholar]
- 16. Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, Bimmel D, Käab S, Huegl B, Brachmann J.. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016;37:3154–3163. [DOI] [PubMed] [Google Scholar]
- 17. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, Clementy N, Amori V, Mangoni di S. Stefano L, Burri H.. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail 2017;19:416–425. [DOI] [PubMed] [Google Scholar]
- 18. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Harris S, Cowie MR; Results of the REM-HF study. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017;38:2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida J-S, Aliot E, Boursier M, Bizeau O, Kacet S.. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon-Moreau L, Proff J, Gerds TA, Anker SD, Torp-Pedersen C.. Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomised controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J 2017;38:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anker SD, Koehler F, Abraham WT.. Telemedicine and remote management of patients with heart failure. Lancet 2011;378:731–739. [DOI] [PubMed] [Google Scholar]
- 22. Marzegalli M, Landolina M, Lunati M, Perego GB, Pappone A, Guenzati G, Campana C, Frigerio M, Parati G, Curnis A, Colangelo I, Valsecchi S.. Design of the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study to assess the ability of remote monitoring to treat and triage patients more effectively. Trials 2009;10:42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burri H, Quesada A, Ricci RP, Boriani G, Davinelli M, Favale S, Da Costa A, Kautzner J, Moser R, Navarro X, Santini M.. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) study: rationale and design. Am Heart J 2010;160:42–48. [DOI] [PubMed] [Google Scholar]
- 24. Boriani G, Da Costa A, Ricci RP, Quesada A, Favale S, Iacopino S, Romeo F, Risi A, Mangoni di S Stefano L, Navarro X, Biffi M, Santini M, Burri H.. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res 2013;15:e167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S.. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65:2601–2610. [DOI] [PubMed] [Google Scholar]