Abstract
Purpose
IL-10 is a cytokine known to inhibit inflammatory cytokines. To determine its role in the pathogenesis of systemic lupus erythematosus (SLE), the presence of anti-IL-10 antibody is required to be examined. Although antibodies against cytokines are known to be present in SLE, no studies have determined the role of IL-10, particularly in Japanese patients. We assayed anti-IL-10 antibody in SLE and examined the clinical significance.
Patients and methods
We performed a retrospective study of 80 Japanese patients with SLE. Sixteen scleroderma patients, 19 rheumatoid arthritis (RA) patients, 23 Behcet’s disease patients, and 23 healthy subjects were selected as control groups. Clinical information was abstracted from medical records. Anti-IL-10 antibody level was determined with an ELISA.
Results
With the cutoff established as serum absorbance +2 SDs (OD 0.729) in healthy subjects, we defined any sample above this cutoff as anti-IL-10 antibody-positive. Fourteen patients with SLE (17.5%) were found to be anti-IL-10 antibody positive. Absorbance was significantly higher in serum from patients with SLE and RA than in healthy individuals. In SLE, patients with low complement values were significantly more common in the antibody-positive group. Serum IgG levels were significantly higher in the antibody-positive group. In multivariable analysis, high level of serum IgG is associated with anti-IL-10 antibody positive.
Conclusion
The present study found that anti-IL-10 antibody is present in SLE and related to clinical parameters. These results suggest that the presence of anti-IL-10 antibody was associated with high level of serum IgG, but is not associated with disease activity in patients with SLE.
Keywords: anti-IL-10 antibody, IL-10, systemic lupus erythematosus, autoantibody
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of antinuclear and other autoantibodies. Moreover, previous studies1–4 demonstrate that cytokines contribute to various manifestations of SLE.
IL-10 is a cytokine known to inhibit inflammatory cytokines.5–7 In an SLE mouse model, IL-10 has been reported to inhibit nephritis, arthritis, and neurological symptoms. Therefore, low levels of IL-10 may be associated with active SLE.8,9 On the contrary, some studies report high levels of IL-10 in SLE patients.10–13 This may be attributed not only to enhanced production of IL-10 but also to the positive feedback of anti-IL-10 antibodies or the effect of anti-IL-10 receptor antibodies.
Low levels of IL-10 suggest decreased production of IL-10 and the presence of anti-IL-10 antibody. Antibodies against cytokines have been shown to be involved in various autoimmune manifestations. Antibodies against granulocyte-macrophage colony-stimulating factor have been reported in pulmonary alveolar proteinosis.14 Antibodies against erythropoietin receptors have been reported in patients with pure red-cell aplasia.15 SLE can develop in association with all of the above pathologies. Patients with SLE have higher levels of anti-erythropoietin receptor antibodies compared with healthy individuals.16 Furthermore, some patients with SLE are positive for anti-thrombopoietin receptor antibody, which are significantly correlated with thrombocytopenia.17
Therefore, to determine the role of IL-10 in the pathogenesis of SLE, the presence of anti-IL-10 antibody is required to be examined. Although antibodies against cytokines are known to be present in SLE, there have been few studies that have determined the presence of anti-IL-10 antibody, especially no studies in Japanese patients.
Patients and methods
Patients
We performed a retrospective study of Japanese patients with SLE. We selected 80 SLE patients who visited St. Mari-anna University Hospital from 2013 through 2018. Sixteen scleroderma patients, 19 rheumatoid arthritis (RA) patients, 23 Behcet’s disease patients, and 23 healthy subjects were selected as control group as well as healthy volunteers. The details of selection are shown in Figure 1.
Figure 1.
Selection of patients.
Notes: Eighty-four patients provided informed consent and provided blood samples. Eighty patients met SLE classification criteria.
Abbreviation: SLE, systemic lupus erythematosus.
All patients met SLE classification criteria (American College of Rheumatology, ACR 1997), RA classification criteria (ACR/European League Against Rheumatism, EULAR 2010), scleroderma classification criteria (ACR/EULAR 2013), or Behcet’s disease diagnostic criteria (Japanese Ministry of Health, Labor and Welfare). All participating subjects gave their written informed consent, and this study was conducted in accordance with the Declaration of Helsinki. The present study was approved by the institutional review board of the St. Marianna University School of Medicine Hospital.
Data collection
The following information was abstracted from medical records: sex, age, disease duration, physical symptoms, physical findings, laboratory findings, and treatment. For laboratory findings, we examined data for urine, leukocytes, platelets, creatine kinase, immunoglobulin, and various antibodies. These were also collected from medical records. SLE disease activity was assessed using the SLE disease activity index (SLEDAI). SLEDAI was evaluated from the blood sample.
Antibody assays
Anti-IL-10 antibody level was determined with an ELISA. A 96-well ELISA plate was precoated with recombinant human IL-10 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) diluted to 1 µg/mL with carbonate buffer and incubated overnight at 4°C.
Next, the plate was washed six times with PBS-tween, 100 µL blocking buffer (Block Ace Power) was added to each well, and the plate was incubated at room temperature for 2 hours. The plate was washed again with PBS-tween six times, 50 µL serum diluted to 1/1,500 was added to each well, and the plate was incubated again for 1 hour. Finally, the plate was washed six more times with PBS-tween, peroxidase-conjugated AffiniPure goat anti-human IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) diluted to 1/5,000 was added as a secondary antibody, and the plate was left standing at 4°C for 1 hour.
After washing the plate six times, 50 µL of 1 M H2SO4 was added to color each well, and absorbance was measured at 450 nm using a Microplate Reader iMark (Bio-Rad Laboratories Inc., Hercules, CA, USA). All ELISA reactions were represented according to the following formula:
The cutoff value for anti-IL-10 antibody was defined as the OD mean + 2 SDs in healthy individuals (23 samples). To exclude the possibility of nonspecific reactions in the ELISA method, an inhibition study was performed.
Statistical analysis
Data were analyzed with the Mann–Whitney U test and Fisher’s exact test using Graph Pad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA), and multivariable analysis using JMP (SAS Institute Inc., Cary, NC, USA).
Results
Baseline patient characteristics
Table 1 shows patient characteristics. The 80 SLE patients had a mean age of 44.7±13.81 years and a male:female ratio of 1:9. Mean disease duration and mean SLEDAI scores were 11.5±7.9 years and 6.6±7.5, respectively.
Table 1.
Clinical characteristics of SLE patients
| Clinical characteristics of SLE patients | Result (%) |
|---|---|
| Age (years) | 44.7±13.81 |
| Sex ratio (M/F) | 1:9 |
| Duration (years) | 11.5±7.9 |
| SLEDAI | 6.6±7.5 |
| NPSLE | 2 (3%) |
| Visual disturbance | 1 (1%) |
| Arthritis | 8 (10%) |
| Myositis | 2 (3%) |
| Urinary casts | 15 (19%) |
| Hematuria | 9 (11%) |
| Proteinuria | 18 (23%) |
| New rash | 11 (14%) |
| Alopecia | 4 (5%) |
| Mucosal ulcers | 8 (10%) |
| Pleurisy/pericarditis | 7 (9%) |
| Low complementa | 46 (58%) |
| IgG (mg/dL) | 1,501±563.7 |
| Increased DNA bindingb | 39 (49%) |
| Fever | 10 (13%) |
| Thrombocytopenia | 7 (9%) |
| Leukopenia | 12 (15%) |
| Medication | |
| Prednisolone dosage (mg) | 10.9±10.9 |
| Mycophenolate mofetil | 12 (15%) |
| Tacrolimus | 8 (10%) |
| Cyclosporine | 3 (4%) |
| Azathioprine | 6 (8%) |
| Cyclophosphamide | 2 (3%) |
Note:
C3 <65 mg/dL or C4 <13 mg/dL or CH50 <30 mg/dL;
>2.5% binding in Farr assay or above normal range for laboratory testing.
Abbreviations: IgG, immunoglobulin G; NPSLE, neuropsychiatric SLE; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
Organ lesions consisted of neuropsychiatric SLE in two patients (3%) and visual disturbance in one patient. Arthritis was observed in eight patients.
Among all patients who demonstrated findings of nephritis, proteinuria of ≥500 mg/day was observed in 18 patients. Lupus enteritis was not observed in any patient.
In serological testing, low complement was observed in 46 patients (58%). The mean level of IgG was 1,501±563.7 mg/dL. A total of 39 patients were positive for anti-DNA antibodies or anti-ds-DNA antibodies. Leukopenia and thrombocytopenia were observed in 12 and 7 patients, respectively. Mean prednisolone dosage was 10.9±10.9 mg. Mycophenolate mofetil, tacrolimus, cyclosporine, azathioprine, and cyclophosphamide were used in 12, 8, 3, 6, and 2 patients, respectively.
Experimental results
Fourteen patients with SLE (17.5%) were found to be anti-IL-10 antibody positive. One patient with scleroderma (6.25%) and one patient with RA (5.26%) were anti-IL-10 antibody positive, but no Behcet’s disease patients were anti-IL-10 antibody positive (Figure 2).
Figure 2.
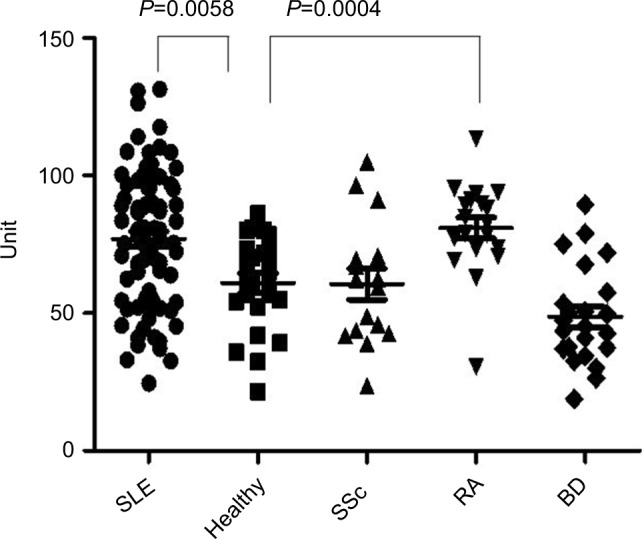
Results of the cytokine assay.
Notes: Serum levels of anti-IL-10 antibody in patients with SLE (n=80), normal controls (n=23), SSc (n=16), RA (n=19), and BD (n=23). Horizontal lines represent the mean. Absorbance was significantly higher in SLE (P=0.0058) and RA (P=0.0004) than in healthy subjects. Data were analyzed with the Mann–Whitney U test.
Abbreviations: BD, Behcet’s disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic scleroderma.
Absorbance was significantly higher in serum from patients with SLE and RA than in healthy individuals.
Table 2 shows comparisons of data between patients with SLE who were positive and negative for anti-IL-10 antibody. In serological testing, the two groups did not differ significantly in positive results for anti-DNA or anti-ds-DNA antibody. However, patients with low complement were significantly more common in the antibody-positive group. Specifically, the antibody-positive group had a significantly high percentage of patients with low C3.
Table 2.
Comparison of SLE patients
| Characteristic | Anti-IL-10 Ab positive (n=14) | Anti-IL-10 Ab negative (n=66) | P-value |
|---|---|---|---|
| Age (years) | 37.7±14.0 | 46.2±13.4 | 0.033 |
| Age at onset (years) | 26.8±14.0 | 34.9±14.2 | 0.066 |
| Duration (years) | 10.9±7.6 | 11.6±8.0 | NS |
| SLEDAI | 6.7±4.8 | 6.6±8.0 | NS |
| NPSLE | 0 (0%) | 2 (3%) | NS |
| Arthritis | 1 (7%) | 7 (11%) | NS |
| Urinary casts | 3 (21%) | 12 (18%) | NS |
| Hematuria | 2 (14%) | 7 (11%) | NS |
| Proteinuria | 3 (21%) | 15 (23%) | NS |
| New rash | 3 (21%) | 8 (12%) | NS |
| Alopecia | 1 (7%) | 3 (5%) | NS |
| Mucosal ulcers | 1 (7%) | 7 (11%) | NS |
| Pleurisy/pericarditis | 1 (7%) | 6 (9%) | NS |
| Low complement | 12 (86%) | 34 (52%) | 0.018 |
| Low C3 | 10 (76%) | 26 (41%) | 0.031 |
| Low C4 | 10 (76%) | 31 (51%) | NS |
| Low CH50 | 3 (100%) | 19 (58%) | NS |
| IgG | 2,019.6±744.3 | 1,391.8±455.0 | 0.001 |
| Increased DNA binding | 7 (50%) | 32 (48%) | NS |
| Fever | 2 (14%) | 8 (12%) | NS |
| Thrombocytopenia | 1 (7%) | 6 (9%) | NS |
| Leukopenia | 2 (14%) | 1 (15.9) | NS |
Notes: Data were analyzed with the Mann–Whitney U test and Fisher’s exact test. Significant differences were observed in low complement and increased serum IgG.
Abbreviations: IgG, immunoglobulin G; NPSLE, neuropsychiatric SLE; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
Serum IgG levels were also significantly higher in the antibody-positive group. Age was significantly lower in the antibody-positive group, but the groups showed no difference in disease duration. The two groups did not differ significantly in SLEDAI score. No differences were observed between the two groups in neuropsychiatric SLE, nephritis, arthritis, scleroderma, or general symptoms.
We analyzed univariate/multivariable analysis to determine a risk factor of anti-IL-10 antibody-positive patients with SLE. The results are shown in Table 3. In multivariable analysis, high level of serum IgG is associated with anti-IL-10 antibody positive.
Table 3.
Multivariate analysis for risk factors of anti-IL10 antibody with SLE
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age (years) | 0.951 | 0.906–0.998 | 0.031 | 0.925 | 0.864–0.990 | 0.0118 |
| IgG (mg/dL) | 1.002 | 1.000–1.003 | <0.001 | 1.002 | 1.001–1.003 | 0.0003 |
| Low C3 | 0.131 | 0.027–0.643 | 0.004 | 0.268 | 0.041–1.741 | 0.1449 |
Notes: Data were analyzed with univariate/multivariable analysis. High level of serum IgG is associated with anti-IL-10 antibody positive.
Abbreviations: IgG, immunoglobulin G; Low C3, C3 >65 mg/dL (less than reference value); SLE, systemic lupus erythematosus.
Discussion
In the present study, patients with SLE had significantly higher levels of anti-IL-10 antibody compared with healthy controls. A variety of anti-cytokine antibodies have been identified in SLE. The present study found that anti-IL-10 antibody is also present in SLE.
Anti-IL-10 antibody-positive SLE patients had signifi-cantly higher IgG values than anti-IL-10 antibody-negative patients. In this study, it has been found that the presence of anti-IL-10 antibody is associated with high level of serum IgG, but is not associated with disease activity in patients with SLE. The present article is the first to report that anti-IL-10 antibody is present in some patients with SLE.
Several studies have reported on the genetic polymorphisms of IL-10.18–20
Therefore, a certain number of patients with SLE have a genetic polymorphism of IL-10, which may be involved in the disease process of SLE. Even if anti-IL-10 antibody has to be produced postnatally, the same phenomenon may occur if IL-10 is neutralized and decreases.
In the present study, nephritis, arthritis, and neurological lesions were presumed to be associated with IL-10.8,9 However, no differences were observed between groups in any of these pathologies. Despite the absence of significant differences in symptoms and overall disease activity as represented by SLEDAI scores, anti-IL-10 antibody may be partially involved in the disease process of SLE in terms of IgG levels and complement titer. Patients with RA reportedly have low serum levels of IL-10.21 In addition, patients with advanced RA have even lower serum levels of IL-10 than early RA.21 Since this study indicates the presence of anti-IL-10 antibody in RA patients, this antibody may neutralize IL-10, resulting in low IL-10 values.
The present study is limited by the small sample size. Therefore, further study with larger numbers is necessary to analyze new subgroups and the function of anti-IL-10 antibody as a biomarker. Although the present study found the presence of anti-IL-10 antibodies, whether they functionally block IL-10 is undetermined.
Further examination of anti-IL-10 receptor antibody and the IL-10 family of cytokines may elucidate the role of IL-10 in SLE.
Conclusion
The present study found that anti-IL-10 antibody is present in SLE and related to high level of serum IgG. These results suggest that anti-IL-10 antibody may be involved in aberrant B-cell response. Further study with larger number of samples is needed.
Acknowledgments
We gratefully acknowledge the helpful discussions of the members of our institution.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hooks JJ, Jordan GW, Cupps T, Moutsopoulos HM, Fauci AS, Notkins AL. Multiple interferons in the circulation of patients with systemic lupus erythematosus and vasculitis. Arthritis Rheum. 1982;25(4):396–400. doi: 10.1002/art.1780250406. [DOI] [PubMed] [Google Scholar]
- 2.Linker-Israeli M, Bakke AC, Quismorio FP, Horwitz DA. Correction of interleukin-2 production in patients with systemic lupus erythematosus by removal of spontaneously activated suppressor cells. J Clin Invest. 1985;75(2):762–768. doi: 10.1172/JCI111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llorente L, Richaud-Patin Y, Fior R, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren’s syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37(11):1647–1655. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 4.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147(1):117–123. [PubMed] [Google Scholar]
- 5.Trifunović J, Miller L, Debeljak Ž, Horvat V, Debeljak Z. Pathologic patterns of interleukin 10 expression-a review. Biochem Med (Zagreb) 2015;25(1):36–48. doi: 10.11613/BM.2015.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 7.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Z, Bahtiyar G, Zhang N, et al. IL-10 regulates murine lupus. J Immunol. 2002;169(4):2148–2155. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita T. Regulatory B cell and autoimmune disease. Nihon Rinsho Meneki Gakkai Kaishi. 2010;33(5):234–241. doi: 10.2177/jsci.33.234. [DOI] [PubMed] [Google Scholar]
- 10.Yao Y, Wang JB, Xin MM, et al. Balance between inflammatory and regulatory cytokines in systemic lupus erythematosus. Genetics Mol Res. 2016;15(2) doi: 10.4238/gmr.15027626. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara E, Gourley MF, Lee S, Klinman DK. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39(3):379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 12.Chun HY, Chung JW, Kim HA, et al. Cytokine IL-6 and IL-10 as biomark-ers in systemic lupus erythematosus. J Clin Immunol. 2007;27(5):461–466. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 13.Godsell J, Rudloff I, Kandane-Rathnayake R, et al. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep. 2016;6(1):34604. doi: 10.1038/srep34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonfield TL, Russell D, Burgess S, Malur A, Kavuru MS, Thomassen MJ. Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. Am J Respir Cell Mol Biol. 2002;27(4):481–486. doi: 10.1165/rcmb.2002-0023OC. [DOI] [PubMed] [Google Scholar]
- 15.Peschle C, Marmont AM, Marone G, Genovese A, Sasso GF, Condorelli M. Pure red cell aplasia: studies on an IgG serum inhibitor neutralizing erythropoietin. Br J Haematol. 1975;30(4):411–417. doi: 10.1111/j.1365-2141.1975.tb01855.x. [DOI] [PubMed] [Google Scholar]
- 16.Luo XY, Yang MH, Peng P, et al. Anti-erythropoietin receptor antibodies in systemic lupus erythematosus patients with anemia. Lupus. 2013;22(2):121–127. doi: 10.1177/0961203312463980. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana M, Okazaki Y, Kajihara M, et al. Autoantibody to c-Mpl (thrombopoietin receptor) in systemic lupus erythematosus: relationship to thrombocytopenia with megakaryocytic hypoplasia. Arthritis Rheum. 2002;46(8):2148–2159. doi: 10.1002/art.10420. [DOI] [PubMed] [Google Scholar]
- 18.Guzowski D, Chandrasekaran A, Gawel C, et al. Analysis of single nucleotide polymorphisms in the promoter region of interleukin-10 by denaturing high-performance liquid chromatography. J Biomol Tech. 2005;16(2):154–166. [PMC free article] [PubMed] [Google Scholar]
- 19.Hirankarn N, Wongpiyabovorn J, Hanvivatvong O, et al. The synergistic effect of Fc gamma receptor IIa and interleukin-10 genes on the risk to develop systemic lupus erythematosus in Thai population. Tissue Antigens. 2006;68(5):399–406. doi: 10.1111/j.1399-0039.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosado S, Rua-Figueroa I, Vargas JA, et al. Interleukin-10 promoter polymorphisms in patients with systemic lupus erythematosus from the Canary Islands. Int J Immunogenet. 2008;35(3):235–242. doi: 10.1111/j.1744-313X.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 21.Avrămescu C, Vere CC, Mărgăritescu C, Turculeanu A, Bălăşoiu M, Rogoz S. Cytokinin panel in rheumatoid arthritis and correlation with histological patterns of synovitis – active type of disease. Rom J Morphol Embryol. 2005;46(2):87–92. [PubMed] [Google Scholar]


