Abstract
Background
Quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG) are powerful phytochemicals that have been shown to exhibit disease prevention and health promotion properties. However, there may exist transformations between Qr, IQ, and QG in vivo. And the pharmacokinetic profiles of Qr, IQ, and QG have not been systematically compared. The pharmacokinetics study would be helpful to better understand the pharmacological actions of them.
Methods
Herein, we developed a reliable HPLC-MS method to compare the pharmacokinetics of Qr, IQ, and QG after separate (50 mg/kg) oral administration of them in rats, using puerarin as internal standard. The detection was performed using negative selected ion monitoring. This method was validated in terms of selectivity, linearity, precision, accuracy, extraction recovery, matrix effect, and stability; and shows reliabilities in monitoring the pharmacokinetic behaviors of these three compounds.
Results
Our results showed that after separate oral administration of Qr, IQ, and QG, all of the compounds could be detected in plasma. In addition, QG could be detected in the Qr group; Qr and QG could be measured in the IQ group; and Qr could be found in rat plasma after 1.5 h of QG administration. Moreover, the AUC0−t of Qr in the; Qr group (2,590.5 ± 987.9 mg/L*min), IQ group (2,212.7 ± 914.1 mg/L*min), and QG group (3,505.7 ± 1,565.0 mg/L*min) was larger than the AUC0−t of QG in the; Qr group (1,550.0 ± 454.2 mg/L*min), IQ group (669.3 ± 188.3 mg/L*min), and QG group (962.7 ± 602.3 mg/L*min). The AUC0−t of IQ was the lowest among all groups.
Discussion
Quercetin, IQ, and QG can all be absorbed into plasma. A mutual transformation exists between Qr and QG, and IQ can be metabolized into Qr and QG in SD rats. These results would provide a meaningful basis for understanding the pharmacological actions of these three compounds.
Keywords: Pharmacokinetic, Biotransformation, Quercetin, Isoquercitrin, Quercetin-3-O-β-D-glucuronide
Introduction
Flavonols with the quercetin structure are found in many medicinal plants (Sun, Simonyi & Sun, 2002) and have attracted significant attention as powerful phytochemicals for disease prevention and health promotion (Fraga, Celep & Galleano, 2009; Murakami, Ashida & Terao, 2008). Quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG), representatives of quercetin flavonols, are present in many herbal plants, such as Cichorium glundulosum Boiss et Hout (Ding et al., 2014), Nelumbo nucifera (Kitanov, 1988), and Hypericum hirsutum (Kashiwada et al., 2005). Qr is one of the most widely used dietary flavonoids and its anti-atherosclerotic, lipid-modulating, and anti-carcinogenic effects have been extensively studied (Aguirre et al., 2014; Terao, Kawai & Murota, 2008; Youdim, Shukitt-Hale & Joseph, 2004). IQ, the glycoside form of Qr, has attracted increasing attention owing to its activity against cardiovascular disorders, diabetes, cancer, and allergic reactions (Valentová et al., 2014; Vrba et al., 2013). Many studies (Cermak, Landgraf & Wolffram, 2003; Terao, 2009; Terao, Murota & Kawai, 2011) have proposed that QG—the glucuronide form of Qr—functions not only as a detoxified metabolite also as a hydrophobic aglycone precursor and hydrophilic bioactive agent for various ROS-generating systems of quercetin aglycone. Compared with the long history of application and widespread research on Qr, the research on IQ and QG is much less extensive.
Pharmacokinetic studies of drugs are necessary for understanding their in vivo behavior and pharmacological mechanisms. Quercetin flavonols are usually administered orally and can undergo biotransformation by intestinal microorganisms and metabolism in the liver (Day et al., 2003; Murota et al., 2000; Petri et al., 2003; Spencer et al., 1999; Walle, 2004). As a result, the main metabolites in plasma may be the bioactive components. Compared with the parent compounds under different conditions, metabolites may produce similar, stronger or even weaker effects (Crespy, 1998; Day et al., 2000a; Sesink, O’Leary & Hollman, 2001). In addition, since most research on the pharmacokinetic characteristics of Qr, IQ, and QG uses crude herbal extracts, other components of the extracts may affect their pharmacokinetic characteristics, and even contribute contrasting results (Paganga & Rice-Evans, 1997; Sesink, O’Leary & Hollman, 2001). It has been reported that a mutual transformation between Qr and QG may exist in vivo (Guo et al., 2014; Menendez et al., 2011; Yang et al., 2016), and a small number of studies have compared the metabolism of IQ and Qr (Paulke et al., 2012). However, after the separate oral administration of Qr, IQ, and QG in rats, their pharmacokinetics and biotransformation the compounds remain unclear. To address this, in this study we established a sensitive and reliable HPLC-MS method for simultaneous determination of Qr, IQ, and QG in rat plasma and subsequently used the method to systematically compare the pharmacokinetic characteristics of Qr, IQ, and QG in rats. It was expected that the results of this study would help to explain the metabolic relationships between the compounds and could be applied in pharmacological studies for better understanding of the pharmacological effects of each compound.
Materials and Methods
Chemicals and materials
Quercetin (purity ≥ 98%, CAS: 117-39-5) and isoquercitrin (purity ≥ 98%, CAS: 21637-25-2) were provided by the Aladdin Reagent Co. Ltd. (Shanghai, China). Puerarin (purity ≥ 98%, CAS: 3681-99-0) as the internal standard (IS) was purchased from the National Institutes for Food and Drug Control (Beijing, China). Quercetin-3-O-β-D-glucuronide (purity ≥ 96%, CAS: 22688-79-5) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Their structures are shown in Fig. 1. HPLC grade formic acid, acetonitrile, and methanol were provided by Merck (Darmstadt, Germany), and ultrapure water (18 MΩ) was prepared in our laboratory using Milli-Q system (Millipore, Burlington, MA, USA). Other chemicals and reagents were of analytical grade.
Figure 1. Chemical structures of the analytes and IS.
(A) Puerarin (IS), (B) Quercetin (Qr), (C) Isoquercitrin (IQ), (D) Quercetin-3-O-β-D-glucuronide (QG).
Pharmacokinetics study
A total of 15 SPF Sprague-Dawley rats (SD, male, 190–210 g) were provided by Sino-British Sippr/BK Lab Animal Company Ltd. (Shanghai, China). The rats were kept in standard conditions (22–26 °C; 40–60% relative humidity; 12 h light/dark cycle). Throughout the rearing period, the animals had access to food and water ad libitum. Animal experiments were conducted in accordance with the standard ethical guidelines of the Laboratory Animal Management Committee of Jiangsu Province (SYXK (SU) 2016-0011).
Following 1 week of adaptation, the rats were fasted for about 12 h before dosing, with free access to water. A total of 15 rats were randomly divided into Qr, IQ, and QG groups (n = 5), and were dosed accordingly by oral gavage (50 mg/kg/day). After administration of the first dose, blood samples were taken from the retro-orbital vein at 0, 5, 15, 30, 60, 90, 120, 180, 240, 360, 480, and 1,440 min and collected in heparinized tubes. The samples were centrifuged at 3,500 rpm for 10 min at 4 °C and the plasma was stored at −80 °C until analysis.
Instrumentation and HPLC-MS method
An Agilent 1,200 series HPLC system (Agilent Technologies, Waldbronn, Germany) equipped with a quaternary pump on-line degasser, auto-sampler and column oven, and an SL G1946D quadruple mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) source, were used for separation and detection. Data were acquired and analyzed using Agilent ChemStation Software Version A.01.00 (Agilent Technologies, Santa Clara, CA, USA).
The separation was carried out with an Amethyst C18-H column (150 × 4.6 mm, 5 μm, Sepax Technologies, Newark, DE, USA) with a guard column SB-C18 (10 × 4.6 mm, 5 μm, Phenomenex Technologies, Torrance, USA) at a constant temperature of 25 °C. The mobile phase was composed of aqueous solution (0.4% formic acid, A) and acetonitrile (B) and the program of the mobile phase was as follows: 0 min, 5% B; 5 min, 12% B; 10 min, 33% B; 15 min, 50% B; 18 min, 60% B, at a flow rate of 0.8 mL/min with 5 μL sample injection volume.
The mass spectra were acquired using ESI interfaced to a single quadrupole mass spectrometer. The ionization conditions were as follows: drying gas (N2) flow rate of 11 L/min, drying gas temperature of 350 °C, nebulizing gas (N2) pressure of 35 psi, capillary voltage of 3,500 V, quad temperature of 100 °C, and fragment voltage of 110 V. A series of m/z were monitored using a timed program according to the retention times and characteristic ions for the corresponding HPLC-MS peak obtained by scanning from m/z 100–1,000 in the standard sample. The analytes were monitored using negative selected ion monitoring (SIM) at m/z 301.1 for Qr [M-H]−, m/z 463.1 for IQ [M-H]−, m/z 477.1 for QG [M-H]−, and m/z 415.2 for IS [M-H]−.
Preparation of standards and quality control samples
The stock solutions of Qr, IQ, and QG were separately prepared by dissolving the reference standards in methanol at a final concentration of 0.210, 0.192, and 0.200 mg/mL, respectively. The standard working solutions were prepared by diluting with methanol to the desired concentrations. The calibration curves of Qr, IQ, and QG were in the range of 32.8–10,500.0, 24.4–976.0, and 50.0–12,500.0 ng/mL, respectively. The QC samples at low, medium and high concentrations were prepared in the same way and the concentrations at three levels were as follows: 65.6, 525.0, 8,400.0 ng/mL for Qr, 48.8, 244.0, 976.0 ng/mL for IQ, and 100.0, 1,000.0, 10,000.0 ng/mL for QG, respectively. An IS stock solution (0.192 mg/mL) was prepared by dissolving the puerarin standard in methanol and stored at 4 °C.
Sample preparation
Briefly, an aliquot of plasma sample (100 μL) was spiked with 5% formic acid (10 μL) in centrifuge tube. Subsequently, hydrochloric acid (2 mol/L, 10 μL) and IS solution (960.00 ng/mL, 20 μL) were added. Then ethyl acetate/n-butyl alcohol (1:1 v/v; 1,000 μL) was used to extract the components. The samples were then homogenized by vortex mixing and centrifuged for 10 min at 14,000 rpm. After centrifugation, 800 μL of supernatant was transferred to another centrifuge tube and the sample was evaporated to dryness. The residues were dissolved in 100 μL of methanol and centrifuged for 10 min at 12,000 rpm. 50 μL of the supernatant was transferred to the vial and an aliquot of 5 μL was injected into the HPLC-MS system for analysis.
Method validation
The HPLC-MS method used in this study was verified for specificity, linearity, precision, accuracy, extraction recovery, matrix effect, and stability (Food and Drug Administration of the United States, 2013).
Specificity
The specificity was assessed by comparing six independent individual blanks, spiked and drug-administered plasma samples to check for the presence of any potential interference in the peak region of the analytes and IS using the proposed extraction procedure and analytical conditions.
Linearity and lower limits of quantification
A best-fit calibration curve was produced by plotting the area ratios (y-axis) of the analytes to IS against the concentrations of Qr, IQ, and QG (x-axis) in the form of y = bx + a, which was then evaluated using least-squares linear regression analysis weighted (1/x2). The Linearity and lower limits of quantification (LLOQs) was defined as the lowest concentrations of the calibration curve with accuracy (relative error, RE) and precision (relative standard deviation, RSD) not exceeding ± 20%.
Precision and accuracy
The intra- and inter-day precision and accuracy of the method were assessed by replicate analysis (n = 6) of QC samples at low, medium and high concentrations on the same day (intra-day) and three consecutive days (inter-day). Accuracy and precision at each QC concentration were expressed as RE% and RSD%, respectively.
Extraction recovery and matrix effect
To evaluate extraction recoveries, the first set of QC samples (n = 6) were prepared by spiking three flavonoids into blank plasma at three different concentration levels. Then the samples were processed using the procedure described above. The second set of blank plasma samples were extracted and spiked the QC samples. Extraction recovery values for analytes were determined by calculating the ratios of the raw peak areas of the QC samples with those of spike-after-extraction samples. The matrix effect was evaluated by comparing the responses of the analytes at three different levels in the post-extraction spiked blank plasma with those of the corresponding standard solutions.
Stability
The stabilities of Qr, IQ, and QG in plasma were assessed by analyzing QC samples at different storage conditions. The stabilities of three flavonoids in plasma were studied by analyzing three concentration levels of QC samples (n = 5) at room temperature (25 °C) for 12 h, at long-term storage (−80 °C for 15 days), after three freeze (−80 °C) and thaw (room temperature) cycles, and at auto sampler rack storage (4 °C for 24 h).
Data analysis
DAS 2.0 (Chinese Pharmacological Association, Shanghai, China) was used to calculate the pharmacokinetic parameters using the non-compartment model, including the area under curve (AUC), mean residence time (MRT), half-life ( t1/2z), clearance rate (CL), maximum plasma concentration (Cmax), and time (Tmax). All results are expressed as mean ± standard deviation (SD). One way analysis of variance followed by Kruskal–Wallis test was used for comparison of different groups using Graph pad prism 5 software. A p-value of <0.05 was considered statistically significant.
Results
Optimization of HPLC-MS conditions
Under the optimized ESI conditions, a comparison of the responses to Qr, IQ, QG, and IS in positive and negative ion HPLC-MS modes revealed higher responses and lower noise in negative mode. An Amethyst C18-H column was used to separate Qr, IQ, QG, and IS with high efficiency. To achieve good chromatographic behavior, formic acid, acetic acid, and ammonium acetate aqueous solution were investigated. We found that 0.4% formic acid aqueous presented better method sensitivity and could significantly enhance the signal response of all analytes.
Method validation
Specificity
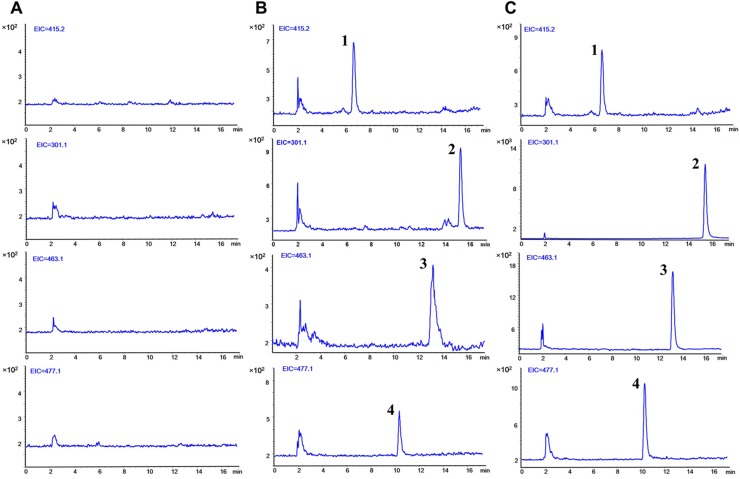
The typical SIM chromatograms of the blank plasma, blank plasma spiked with three analytes at LLOQ level and IS, and plasma obtained 5 min after separate oral administration of Qr, IQ, and QG are presented in Fig. 2. No interference was observed at the respective times of the analytes and IS. The retention time was 15.56 min for Qr, 13.29 min for IQ, 10.53 min for QG, and 6.76 min for IS.
Figure 2. Typical selected ion monitoring (SIM) chromatograms of (1) IS, (2) quercetin, (3) isoquercitrin, and (4) quercetin 3-O-β-D-glucuronide in rat plasma.
(A) blank plasma. (B) blank plasma spiked with the three analytes at lower limit of quantification (LLOQ) and IS. (C) after 5 min oral administration of quercetin (50 mg/kg), isoquercitrin (50 mg/kg), quercetin-3-O-β-D-glucuronide (50 mg/kg) seperately to rats and IS. (1, puerarin, 6.76 min; 2, quercetin, 15.56 min; 3, isoquercitrin, 13.29 min; 4, quercetin-3-O-β-D-glucuronide, 10.53 min).
Calibration curves and LLOQs
The calibration curves and the linear range for the analytes are shown in Table 1. The correlation coefficients (r) for every calibration curves exceeded 0.99, showing good linearity over the concentration range. The accuracy and precision of LLOQs were within ± 20%, which would be sufficient for pharmacokinetic study of three flavonoids.
Table 1. The regression equations, linear range and lower limit of quantification (LLOQ) of the analytes.
| Biosamples | Compounds | Regression equation | Correlation coefficient (r) | Linear range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|---|
| Plasma | Quercetin (Qr) | y = 0.002550x−0.031 | 0.995 | 32.8–10,500.0 | 32.8 |
| Isoquercitrin (IQ) | y = 0.001785x + 0.132 | 0.998 | 24.4–976.0 | 24.4 | |
| Quercetin-3-O-β-D-glucuronide (QG) | y = 0.001876x + 0.208 | 0.996 | 50.0–12,500.0 | 50.0 |
Precision and accuracy
The precision and accuracy were evaluated by determining three levels of QC samples, and the results are summarized in Table S1. The precision ranged from 5.6% to 14.6%, and the accuracy values ranged from −13.8% to 6.4%. These results indicated that the present HPLC-MS method was reliable, accurate and reproducible.
Matrix effect and recovery
The mean extraction recoveries of Qr, IQ, and QG at all QC concentration levels in plasma ranged from 68.9% to 79.2% (n = 6, Table S2). The extraction recoveries of analytes in plasma were stable. The matrix effects for the analytes in plasma ranged from 96.8% to 106.5% at each QC level indicative of a lack of apparent endogenous matrix (Table S2).
Stability
The stabilities of Qr, IQ, and QG in plasma were assessed by analyzing three concentration levels of QC samples at different storage conditions (Table S3). The results showed that these analytes in plasma sample were all stable at room temperature for 12 h, at −80 °C for 15 days, and after three freeze-thaw cycles. Post-preparation stability of the analytes also showed no significant degradation when the extracted samples were kept in the auto sampler rack at 4 °C for 24 h.
Pharmacokinetics study
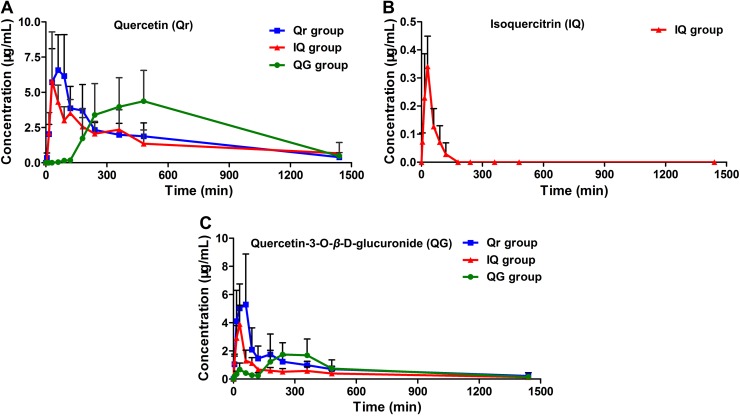
The developed HPLC-MS method was applied to study the pharmacokinetics of Qr, IQ, and QG in rats. The mean plasma concentration-time profiles for Qr, IQ, and QG after oral administration in rats are presented in Fig. 3. The pharmacokinetic parameters are summarized in Table 2. After Qr (50 mg/kg) was orally administrated to rats, Qr could be detected in rat plasma and reached the Cmax (7.47 ± 2.63 ug/mL) at Tmax (54.0 ± 25.1 min) with the AUC0−t (2,590.5 ± 987.9 mg/L*min) (Table 2). After IQ (50 mg/kg) was orally administrated to rats, IQ could be detected in rat plasma and reached the Cmax (0.35 ± 0.11 ug/mL) at Tmax (27.0 ± 6.7 min) with the AUC0−t (17.2 ± 7.3 mg/L*min) (Table 2). Moreover, after QG (50 mg/kg) was orally administrated to rats, QG could be detected in rat plasma and reached the Cmax (2.04 ± 0.85 ug/mL) at Tmax (222.0 ± 119.2 min) with the AUC0−t (962.7 ± 602.3 mg/L*min) (Table 2). The AUC of IQ could only be accessed until 90–120 min, unlike the AUC of others groups. Greater absorption was observed for Qr and QG than for IQ (Table 2). Tmax (222.0 ± 119.2 min) of QG was delayed compared with Tmax (54.0 ± 25.1 min) of Qr (Table 2). In addition, a clear double peak phenomenon was observed in QG mean plasma concentration-time profile after oral administration of QG (Fig. 3C).
Figure 3. Mean plasma concentration-time profiles of (A) quercetin (Qr), (B) isoquercitrin (IQ), and (C) quercetin-3-O-β-D-glucuronide (QG) after oral administration of 50 mg/kg Qr, IQ, and QG separately in rats.
(Mean ± SD, n = 5).
Table 2. Pharmacokinetic parameters of quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG) after oral administration of 50 mg/kg Qr, IQ, and QG separately in rats.
(Mean ± SD, n = 5).
| Pharmacokinetic parameters | Qr group | IQ group | QG group | ||||
|---|---|---|---|---|---|---|---|
| Qr | QG | IQ | Qr | QG | QG | Qr | |
| AUC0−t (mg/L*min) | 2,590.5 ± 987.9 | 1,150.0 ± 454.2 | 17.2 ± 7.3 | 2,212.7 ± 914.1 | 669.3 ± 188.3 | 962.7 ± 602.3 | 3,505.7 ± 1,565.0 |
| MRT0−t (min) | 383.3 ± 24.3 | 315.9 ± 134.7 | 40.6 ± 7.2 | 422.6 ± 148.6 | 355.9 ± 94.6 | 437.8 ± 36.3 | 491.1 ± 22.6 |
| t1/2z (min) | 437.3 ± 54.3 | 545.8 ± 436.6 | 42.6 ± 27.9 | 378.4 ± 338.8 | 523.5 ± 190.4 | 376.4 ± 95.2 | 365.4 ± 152.1 |
| Tmax (min) | 54.0 ± 25.1 | 42.0 ± 16.4 | 27.0 ± 6.7 | 42.0 ± 16.4 | 30.0 ± 0.0 | 222.0 ± 119.2 | 360 ± 120 |
| CL (L/min/kg) | 0.02 ± 0.008 | 0.043 ± 0.029 | 2.91 ± 1.29 | 0.024 ± 0.011 | 0.07 ± 0.02 | 0.071 ± 0.057 | 0.014 ± 0.014 |
| Cmax (ug/mL) | 7.47 ± 2.63 | 6.46 ± 2.94 | 0.35 ± 0.11 | 6.60 ± 2.67 | 3.91 ± 1.34 | 2.04 ± 0.85 | 5.17 ± 1.85 |
Note:
AUC, the area under concentration-time curve; MRT, mean residence time; t1/2z, half-life; Cmax, maximum plasma concentration; and Tmax, time; CL, clearance rate.
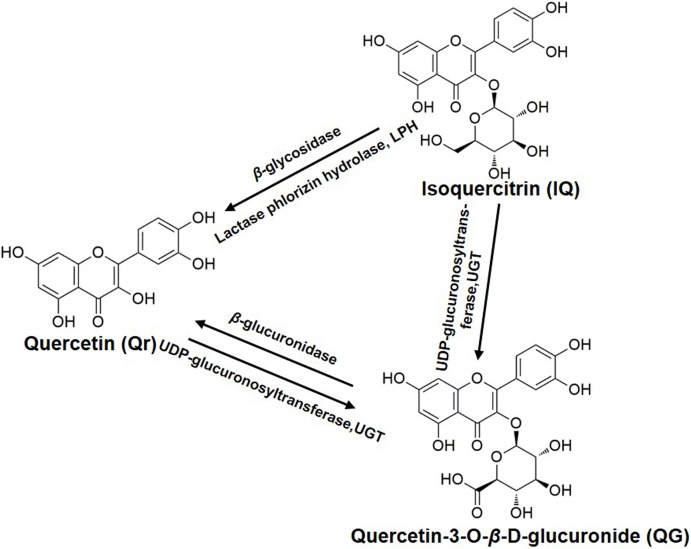
Furthermore, QG was detected in the Qr group (Fig. 3C); Qr and QG were measured in the IQ group (Figs. 3A and 3C); And Qr was found in rat plasma after 1.5 h of QG administration (Fig. 3A). The potential biotransformation relationship is summarized in Fig. 4. In addition, the AUC0−t of Qr in the Qr group (2,590.5 ± 987.9 mg/L*min), IQ group (2,212.7 ± 914.1 mg/L*min), and QG group (3,505.7 ± 1,565.0 mg/L*min) was larger than the AUC0−t of QG in the Qr group (1,150.0 ± 454.2 mg/L*min), IQ group (669.3 ± 188.3 mg/L*min), and QG group (962.7 ± 602.3 mg/L*min) (Table 2). The MRT0−t of Qr in all groups was also larger than that of QG in the corresponding group, and the MRT0−t of IQ was the lowest among all groups. The Tmax of Qr in the QG group was significantly delayed compared with that of Qr in the Qr (p = 0.02) and IQ groups (p = 0.006). The Tmax of QG in the QG group also came after a longer period than that of QG in the Qr and IQ (p = 0.021) groups. And the Tmax, t1/2z, and Cmax of IQ were the shortest and lowest compared with Qr and QG. The CL of IQ was the fastest compared with Qr and QG (Table 2).
Figure 4. The biotransformation among quercetin, isoquercitrin, and quercetin-3-O-β-D-glucuronide.
Discussion
Our results indicated that Qr, IQ, and QG can all be absorbed into plasma; There is a biotransformation between Qr and QG; And IQ can be metabolized into Qr and QG. Under the same intragastric dosage (50 mg/kg) conditions, the AUC values for Qr in the Qr, IQ, and QG dose groups were larger than those for QG in the corresponding groups. And the AUC of IQ was the lowest. In addition, each pharmacokinetic profile (Fig. 3) came from different individual rats, there existed transformations between Qr, IQ, and QG in vivo, and the metabolism and transformation between these three compounds would be affect by many factors, such as gastric emptying rate and intestinal flora (Rowland et al., 2018; Yuan et al., 2016). The above reasons might leaded to the large data variability of Qr, IQ, or QG used for calculating the mean pharmacokinetic profiles and the phenomenon of large data variability also could be found in previous study (Yang et al., 2016).
In previous studies, Sesink, O’Leary & Hollman (2001) and Murota et al. (2010) revealed that after administration of IQ in human subjects, in contrast to QG, Qr and IQ were not detected in plasma. In contrast to these reports, some recent studies have found that after administration of IQ-enriched herbal extracts, intact IQ can be detected in rat plasma (He et al., 2013; Li et al., 2010; Xue et al., 2011; Zhou et al., 2011). These reports all used effective plasma sample extraction and sensitive HPLC or LC/MS analysis methods, however, the metabolism of IQ could be affected by the complex mixtures of herbal extracts (Zuo et al., 2006). Studies by Paulke et al. (2012) and Lu et al. (2013) found that after oral administration of IQ, Qr could be detected in plasma, which agrees with our findings (Fig. 3A). Chang et al. (2005) found that after a single oral dose of IQ (4.5 mg/kg) in rats, quercetin glucuronide and a trace amount of IQ could be found in plasma, which is consistent with our results (Figs. 3C and 3B). Valentová et al. (2014) proposed that although small amounts of intact IQ can be found in plasma after oral administration, IQ still shows many chemical protective effects both in vitro and in vivo.
Quercetin is weakly acidic and can be absorbed in the stomach (Crespy et al., 2002). Unlike Qr, IQ cannot be absorbed (Crespy et al., 2002) and is stable in the stomach (Chang et al., 2005; Zuo et al., 2006). However, IQ can undergo deglycosylation by lactase phlorizin hydrolase present on the outer surface of the intestinal brush border, or by microbial enzymes (Schoefer et al., 2003) to give Qr (Day et al., 2000b). If absorbed intact, IQ can be hydrolyzed to Qr in intestinal cells by cytosolic β-glycosidase (Lesser, Cermak & Wolffram, 2004). These are potential reasons why Qr can be detected in plasma after oral administration of IQ (Fig. 4).
The biotransformation of Qr and IQ can also take place in the liver. LC-MS results showed that the predominant biotransformation processes for Qr and its glycosides were methylation and glucuronidation, produced by isolated human or rat hepatocytes (Vacek et al., 2012); which could account for QG being detected in the blood after IQ or Qr were administrated (Fig. 3C). Studies suggest that Qr can be glucuronidated by UDP-glucuronyltransferases, which are present in human and rat intestine (Cermak, Landgraf & Wolffram, 2003; Lesser, Cermak & Wolffram, 2004) (Fig. 4). Yang et al. (2016) found that QG circulates in the bloodstream after oral administration of Qr. As the hydrophobic glucuronide form of Qr cannot easily cross cell membranes by diffusion, its absorption and bioavailability are hampered. This may be one of the reasons for the delayed Tmax of QG compared with that of Qr (Table 2).
Generally, flavonoid conjugates are hydrolyzed to aglycone before passing through the small intestine, while flavonoid aglycone can be directly absorbed into epithelial cells in the intestine (Kroon et al., 2004; Manach et al., 1997). Spencer et al. (1999) found that the hydrolysate of IQ and glucuronide in isolated rat intestine could release Qr. We also found that after IQ or QG were administrated, Qr could be detected in the blood (Fig. 3A). Yang et al. (2016) demonstrated that double peaks are observed after oral administration of QG in rats, which is consistent with our findings (Fig. 3C). Previous work indicates that delayed gastric emptying, enterohepatic recirculation, and variability of absorption may cause double-peaked pharmacokinetics (Huh, 2007; Lee & Mitchell, 2012; Xing, Chen & Zhong, 2005).
Furthermore, QG was found to be much less toxic compared with its aglycone (Yoshino et al., 2011). The lower toxicity of QG appears to be derived from its extremely high hydrophilicity. In addition, the lack of a free OH group at the three-position contributes to the less toxic effect of QG (Awad et al., 2000; Boots, Haenen & Bast, 2008). QG has been shown to be the more effective anti-oxidative metabolite in rat plasma after oral administration of Qr (Moon et al., 2001; Shirai et al., 2002), and it (10 mM) can inhibit lipid peroxidation (Shirai et al., 2001). It is likely that QG can release Qr by enhanced β-glucuronidase activity during inflammation (Kawai et al., 2008; Shimoi et al., 2001) (Fig. 4).
The results obtained in this study corroborate with the literature and show with pharmacokinetic profiles that there exist biotransformation between Qr and QG after administration of the compounds separately. Also, these results confirm that Qr and QG are metabolites of IQ. The pharmacokinetic profiles obtained after administration of analytical standards help to explain the relation between the compounds. These results can be applied to pharmacokinetic/pharmacodynamic studies for better understanding of the pharmacological effects of each compound.
Conclusions
A sensitive HPLC-MS method was successfully developed, validated, and used to determine the pharmacokinetic profiles of Qr, IQ, and QG. Using liquid–liquid extraction with a mixture of n-butyl alcohol and ethyl acetate to simultaneously analyze complex mixtures of Qr, IQ, and QG with a range of low polarity to high polarity compounds, was more effective than protein precipitation (methanol and acetonitrile). Furthermore, we compared the pharmacokinetics of Qr, IQ, and QG and found that there is biotransformation between Qr and QG, and IQ can be metabolized into Qr and QG. The pharmacokinetic profiles obtained after administration of analytical standards help to explain the relation between the compounds and is helpful for understanding their pharmacodynamics mechanism. In addition, the results provide a guidance for their clinical use.
Supplemental Information
Given data show quality control samples at low, medium and high concentrations on the same day (intra-day) and three consecutive days (inter-day). Accuracy and precision at each quality control concentration were expressed as RE% and RSD%, respectively. RE = relative error; RSD = relative standard deviation.
Given data show the extract recoveries and matrix effects of quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG) in rat plasma. RSD = relative standard deviation.
Given data show that the stabilities of quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG) in plasma were studied by analyzing three concentration levels of quality control samples (n = 5) at room temperature (25 °C) for 12 h, at long-term storage (−80 °C for 15 days), after three freeze (−80 °C) and thaw (room temperature) cycles, and at auto sampler rack storage (4 °C for 24 h). RSD = relative standard deviation.
Raw data exported from the Agilent ChemStation Software applied for preparation for Fig. 2 for the selected ion monitoring chromatograms of puerarin (IS), quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG).
Acknowledgments
We thank the State Key Laboratory of Natural Medicines laboratory at China Pharmaceutical University for the access to their blood sample detection equipment. We thank Sarah Dodds, Ph.D., from Liwen Bianji, Edanz Editing China for editing the English text of a draft of this manuscript.
Funding Statement
This work was supported by the One Hundred Person Project of The Chinese Academy of Sciences, Science and Technology Plan Projects in Xinjiang (No. 2014AB043), the Prospective Joint Research Project of Jiangsu Province (BY2016-078-02) and Xinjiang Science and Technology Assistance Program (2016E02067). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Hongli Yin conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Ji Ma performed the experiments, analyzed the data.
Jichun Han performed the experiments, analyzed the data, authored or reviewed drafts of the paper.
Maoru Li performed the experiments, analyzed the data.
Jing Shang conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Laboratory Animal Management Committee of Jiangsu Province provided full approval for this research (SYXK (SU) 2016-0011).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Aguirre et al. (2014).Aguirre L, Portillo MP, Hijona E, Bujanda L. Effects of resveratrol and other polyphenols in hepatic steatosis. World Journal of Gastroenterology. 2014;20(23):7366–7380. doi: 10.3748/wjg.v20.i23.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad et al. (2000).Awad HM, Boersma MG, Vervoort J, Rietjens IM. Peroxidase-catalyzed formation of quercetin quinone methide–glutathione adducts. Archives of Biochemistry & Biophysics. 2000;378(2):224–233. doi: 10.1006/abbi.2000.1832. [DOI] [PubMed] [Google Scholar]
- Boots, Haenen & Bast (2008).Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. European Journal of Pharmacology. 2008;585(2–3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Cermak, Landgraf & Wolffram (2003).Cermak R, Landgraf S, Wolffram S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. Journal of Nutrition. 2003;133(9):2802–2807. doi: 10.1093/jn/133.9.2802. [DOI] [PubMed] [Google Scholar]
- Chang et al. (2005).Chang Q, Zuo Z, Chow MS, Ho WK. Difference in absorption of the two structurally similar flavonoid glycosides, hyperoside and isoquercitrin, in rats. European Journal of Pharmaceutics and Biopharmaceutics. 2005;59(3):549–555. doi: 10.1016/j.ejpb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Crespy (1998).Crespy V. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Letters. 1998;426(3):331–336. doi: 10.1016/S0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- Crespy et al. (2002).Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C. Quercetin, but not its glycosides, is absorbed from the rat stomach. Journal of Agricultural and Food Chemistry. 2002;50(3):618–621. doi: 10.1021/jf010919h. [DOI] [PubMed] [Google Scholar]
- Day et al. (2000a).Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radical Biology and Medicine. 2000a;29(12):1234–1243. doi: 10.1016/S0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- Day et al. (2000b).Day AJ, Cañada FJ, Dã-Az JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Letters. 2000b;468(2–3):166–170. doi: 10.1016/S0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- Day et al. (2003).Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochemical Pharmacology. 2003;65(7):1199–1206. doi: 10.1016/S0006-2952(03)00039-X. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2014).Ding L, Liu JL, Hassan W, Wang LL, Yan FR, Shang J. Lipid modulatory activities of Cichorium glandulosum Boiss et Huet are mediated by multiple components within hepatocytes. Scientific Reports. 2014;4(1):4715. doi: 10.1038/srep04715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration of the United States (2013).Food and Drug Administration of the United States (FDA) Guidance for industry bioanalytical method validation. 2013. https://www.regulations.gov/document?D=FDA-2013-D-1020-0002. [29 May 2015]. https://www.regulations.gov/document?D=FDA-2013-D-1020-0002
- Fraga, Celep & Galleano (2009).Fraga CG, Celep GS, Galleano M. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. Vol. 3. Hoboken: John Wiley & Sons, Inc.; 2009. Biochemical Actions of Plant Phenolics Compounds: Thermodynamic and Kinetic Aspects; pp. 91–106. [DOI] [Google Scholar]
- Guo et al. (2014).Guo XD, Zhang DY, Gao XJ, Parry J, Liu K, Liu BL, Wang M. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species - associated inflammation. Molecular Nutrition & Food Research. 2014;57(6):1037–1045. doi: 10.1002/mnfr.201200569. [DOI] [PubMed] [Google Scholar]
- He et al. (2013).He J, Feng Y, Ouyang HZ, Yu B, Chang YX, Pan GX, Dong GY, Wang T, Gao XM. A sensitive LC–MS/MS method for simultaneous determination of six flavonoids in rat plasma: application to a pharmacokinetic study of total flavonoids from mulberry leaves. Journal of Pharmaceutical and Biomedical Analysis. 2013;84:189–195. doi: 10.1016/j.jpba.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Huh (2007).Huh JS. Comparison of oral bioavailability of genistein and genistin in rats. International Journal of Pharmaceutics. 2007;337(1–2):148–154. doi: 10.1016/j.ijpharm.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Kashiwada et al. (2005).Kashiwada Y, Aoshima A, Ikeshiro Y, Chen YP, Furukawa H, Itoigawa M, Fujioka T, Mihashi K, Cosentino LM, Morris-Natschke SL, Lee KH. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorganic & Medicinal Chemistry. 2005;13(2):443–448. doi: 10.1016/j.bmc.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Kawai et al. (2008).Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. Journal of Biological Chemistry. 2008;283(14):9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- Kitanov (1988).Kitanov GM. Miquelianin and other polyphenols from Hypericum hirsutum. Chemistry of Natural Compounds. 1988;24(1):119–120. doi: 10.1007/BF00597593. [DOI] [Google Scholar]
- Kroon et al. (2004).Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G. How should we assess the effects of exposure to dietary polyphenols in vitro? American Journal of Clinical Nutrition. 2004;80(1):15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- Lee & Mitchell (2012).Lee J, Mitchell AE. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. Journal of Agricultural & Food Chemistry. 2012;60(15):3874–3881. doi: 10.1021/jf3001857. [DOI] [PubMed] [Google Scholar]
- Lesser, Cermak & Wolffram (2004).Lesser S, Cermak R, Wolffram S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. Journal of Nutrition. 2004;134(6):1508–1511. doi: 10.1093/jn/134.6.1508. [DOI] [PubMed] [Google Scholar]
- Li et al. (2010).Li J, Wang ZW, Zhang L, Liu X, Chen XH, Bi KS. HPLC analysis and pharmacokinetic study of quercitrin and isoquercitrin in rat plasma after administration of Hypericum japonicum thunb. extract. Biomedical Chromatography. 2010;22(4):374–378. doi: 10.1002/bmc.942. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2013).Lu L, Qian D, Yang J, Jiang S, Guo J, Shang EX, Duan JA. Identification of isoquercitrin metabolites produced by human intestinal bacteria using UPLC-Q-TOF/MS. Biomedical Chromatography. 2013;27(4):509–514. doi: 10.1002/bmc.2820. [DOI] [PubMed] [Google Scholar]
- Manach et al. (1997).Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C. Bioavailability of rutin and quercetin in rats. FEBS Letters. 1997;409(1):12–16. doi: 10.1016/s0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- Menendez et al. (2011).Menendez C, Dueñas M, Galindo P, González-Manzano S, Jimenez R, Moreno L, Zarzuelo MJ, Rodríguez-Gómez I, Duarte J, Santos-Buelga C. Vascular deconjugation of quercetin glucuronide: the flavonoid paradox revealed? Molecular Nutrition & Food Research. 2011;55(12):1780–1790. doi: 10.1002/mnfr.201100378. [DOI] [PubMed] [Google Scholar]
- Moon et al. (2001).Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-β-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radical Biology and Medicine. 2001;30(11):1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Murakami, Ashida & Terao (2008).Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Letters. 2008;269(2):315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Murota et al. (2010).Murota K, Matsuda N, Kashino Y, Fujikura Y, Nakamura T, Kato Y, Shimizu R, Okuyama S, Tanaka H, Koda T. α-Oligoglucosylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans. Archives of Biochemistry and Biophysics. 2010;501(1):91–97. doi: 10.1016/j.abb.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Murota et al. (2000).Murota K, Shimizu S, Chujo H, Moon JH, Terao J. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line Caco-2. Archives of Biochemistry and Biophysics. 2000;384(2):391–397. doi: 10.1006/abbi.2000.2123. [DOI] [PubMed] [Google Scholar]
- Paganga & Rice-Evans (1997).Paganga G, Rice-Evans CA. The identification of flavonoids as glycosides in human plasma. FEBS Letters. 1997;401(1):78–82. doi: 10.1016/s0014-5793(96)01442-1. [DOI] [PubMed] [Google Scholar]
- Paulke et al. (2012).Paulke A, Eckert GP, Schubert-Zsilavecz M, Wurglics M. Isoquercitrin provides better bioavailability than quercetin: comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Die Pharmazie. 2012;67(12):991–996. [PubMed] [Google Scholar]
- Petri et al. (2003).Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O’Leary KA, Kroon PA, Knutson L, Forsell P, Eriksson T, Lennernas H, Williamson G. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metabolism and Disposition. 2003;31(6):805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- Rowland et al. (2018).Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition. 2018;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoefer et al. (2003).Schoefer L, Mohan R, Schwiertz A, Braune A, Blaut M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Applied and Environmental Microbiology. 2003;69(10):5849–5854. doi: 10.1128/aem.69.10.5849-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesink, O’Leary & Hollman (2001).Sesink AL, O’Leary KA, Hollman PC. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4′-glucoside. Journal of Nutrition. 2001;131(7):1938–1941. doi: 10.1093/jn/131.7.1938. [DOI] [PubMed] [Google Scholar]
- Shimoi et al. (2001).Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metabolism and Disposition: the biological fate of chemicals. 2001;29(12):1521–1524. [PubMed] [Google Scholar]
- Shirai et al. (2001).Shirai M, Moon JH, Tsushida T, Terao J. Inhibitory effect of a quercetin metabolite, quercetin 3-O-β-D-glucuronide, on lipid peroxidation in liposomal membranes. Journal of Agricultural and Food Chemistry. 2001;49(11):5602–5608. doi: 10.1021/jf010713g. [DOI] [PubMed] [Google Scholar]
- Shirai et al. (2002).Shirai M, Yamanishi R, Moon JH, Murota K, Terao J. Effect of quercetin and its conjugated metabolite on the hydrogen peroxide-induced intracellular production of reactive oxygen species in mouse fibroblasts. Bioscience, Biotechnology, and Biochemistry. 2002;66(5):1015–1021. doi: 10.1271/bbb.66.1015. [DOI] [PubMed] [Google Scholar]
- Spencer et al. (1999).Spencer JP, Chowrimootoo G, Choudhury R, Debnam ES, Srai SK, Rice-Evans C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Letters. 1999;458(2):224–230. doi: 10.1016/S0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- Sun, Simonyi & Sun (2002).Sun AY, Simonyi A, Sun GY. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radical Biology and Medicine. 2002;32(4):314–318. doi: 10.1016/S0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- Terao (2009).Terao J. Dietary flavonoids as antioxidants. Forum of Nutrition. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- Terao, Kawai & Murota (2008).Terao J, Kawai Y, Murota K. Vegetable flavonoids and cardiovascular disease. Asia Pacific Journal of Clinical Nutrition. 2008;17(Suppl 1):291–293. [PubMed] [Google Scholar]
- Terao, Murota & Kawai (2011).Terao J, Murota K, Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food & Function. 2011;2(1):11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- Vacek et al. (2012).Vacek J, Papoušková B, Kosina P, Vrba J, Křen V, Ulrichová J. Biotransformation of flavonols and taxifolin in hepatocyte in vitro systems as determined by liquid chromatography with various stationary phases and electrospray ionization-quadrupole time-of-flight mass spectrometry. Journal of Chromatography B. 2012;899:109–115. doi: 10.1016/j.jchromb.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Valentová et al. (2014).Valentová K, Vrba J, Bancířová M, Ulrichová J, Křen V. Isoquercitrin: pharmacology, toxicology, and metabolism. Food & Chemical Toxicology. 2014;68:267–282. doi: 10.1016/j.fct.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Vrba et al. (2013).Vrba J, Gažák R, Kuzma M, Papoušková B, Vacek J, Weiszenstein M, Křen V, Ulrichová J. A novel semisynthetic flavonoid 7-O-galloyltaxifolin upregulates heme oxygenase-1 in RAW264.7 cells via MAPK/Nrf2 pathway. Journal of Medicinal Chemistry. 2013;56(3):856–866. doi: 10.1021/jm3013344. [DOI] [PubMed] [Google Scholar]
- Walle (2004).Walle T. Absorption and metabolism of flavonoids. Free Radical Biology and Medicine. 2004;36(7):829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Xing, Chen & Zhong (2005).Xing J, Chen X, Zhong D. Absorption and enterohepatic circulation of baicalin in rats. Life Sciences. 2005;78(2):140–146. doi: 10.1016/j.lfs.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Xue et al. (2011).Xue C, Guo J, Qian D, Duan JA, Shang E, Shu Y, Lu Y. Identification of the potential active components of Abelmoschus manihot in rat blood and kidney tissue by microdialysis combined with ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Journal of Chromatography B. 2011;879(5–6):317–325. doi: 10.1016/j.jchromb.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2016).Yang LL, Xiao N, Li XW, Fan Y, Alolga RN, Sun XY, Wang SL, Li P, Qi LW. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Scientific Reports. 2016;6(1):35460. doi: 10.1038/srep35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino et al. (2011).Yoshino S, Hara A, Sakakibara H, Kawabata K, Tokumura A, Ishisaka A, Kawai Y, Terao J. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition. 2011;27(7–8):847–852. doi: 10.1016/j.nut.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Youdim, Shukitt-Hale & Joseph (2004).Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radical Biology and Medicine. 2004;37(11):1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2016).Yuan M, Shi DZ, Wang TY, Zheng SQ, Liu LJ, Sun ZX, Wang RF, Ding Y. Transformation of trollioside and isoquercetin by human intestinal flora in vitro. Chinese Journal of Natural Medicines. 2016;14(3):220–226. doi: 10.1016/s1875-5364(16)30019-x. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2011).Zhou C, Liu Y, Su D, Gao G, Zhou X, Sun L, Ba X, Chen X, Bi K. A sensitive LC–MS–MS method for simultaneous quantification of two structural isomers, hyperoside and isoquercitrin: application to pharmacokinetic studies. Chromatographia. 2011;73(3–4):353–359. doi: 10.1007/s10337-010-1879-0. [DOI] [Google Scholar]
- Zuo et al. (2006).Zuo Z, Zhang L, Zhou L, Chang Q, Chow M. Intestinal absorption of hawthorn flavonoids—in vitro, in situ and in vivo correlations. Life Sciences. 2006;79(26):2455–2462. doi: 10.1016/j.lfs.2006.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Given data show quality control samples at low, medium and high concentrations on the same day (intra-day) and three consecutive days (inter-day). Accuracy and precision at each quality control concentration were expressed as RE% and RSD%, respectively. RE = relative error; RSD = relative standard deviation.
Given data show the extract recoveries and matrix effects of quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG) in rat plasma. RSD = relative standard deviation.
Given data show that the stabilities of quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG) in plasma were studied by analyzing three concentration levels of quality control samples (n = 5) at room temperature (25 °C) for 12 h, at long-term storage (−80 °C for 15 days), after three freeze (−80 °C) and thaw (room temperature) cycles, and at auto sampler rack storage (4 °C for 24 h). RSD = relative standard deviation.
Raw data exported from the Agilent ChemStation Software applied for preparation for Fig. 2 for the selected ion monitoring chromatograms of puerarin (IS), quercetin (Qr), isoquercitrin (IQ), and quercetin-3-O-β-D-glucuronide (QG).
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.