Abstract
Aims
To prospectively investigate the relation between cannabis use expectancies and cannabis use prior to and during a self-initiated cannabis cessation attempt.
Design
Cohort design that followed participants for 4 weeks following a self-initiated cessation attempt.
Setting
United States Department of Veterans Affairs medical center.
Participants
One hundred cannabis dependent military veterans.
Measurements
The Marijuana Effects Expectancy Questionnaire at baseline; the timeline follow-back procedure at baseline and during the cessation attempt.
Findings
Cannabis use at baseline was associated with positive (P = 0.01), but not negative (P = 0.25), expectancies. Cannabis lapse was associated with positive (P = 0.03) and negative expectancies (P = 0.01), and relapse was associated with positive (P = 0.04), but not negative (P = 0.21), expectancies. The trajectory of average cannabis use during the cessation period was associated with positive (P = 0.03), but not negative (P = 0.96), expectancies. Results were similar in effect and statistical significance when adjusting for demographic factors, motivation to quit cannabis, mental disorder diagnoses, and alcohol and tobacco use, and when analyzing complete data sets obtained through multiple imputation.
Conclusions
In the USA, cannabis use expectancies, especially those regarding the positive effects of cannabis use, appear to be strongly and consistently linked to cannabis use and quit failure.
Keywords: Cannabis, cannabis dependence, cessation, expectancies, lapse, marijuana, relapse
INTRODUCTION
Rates of cannabis abuse and dependence among vulnerable populations (e.g. veterans [1,2]) have drastically increased in recent years. Most individuals abusing or dependent on cannabis attempt to quit on their own [3–5], despite the existence of empirically supported psychotherapies (e.g. cognitive-behavioral therapy [6]) and moderate rates of documented treatment-seeking behavior [1]. However, high rates of lapse (slip or violation of the abstinence goal [7]) and relapse (return to previous level of use, abandonment of abstinence goal [7]) occur among individuals who self-initiate a cessation attempt or engage in treatment [8–10]. Research is needed to identify malleable risk factors that are associated with lapse/relapse and that can be targeted prior to a quit attempt so as to enhance achievement of abstinencerelated goals.
Substance use expectancies (i.e. beliefs regarding the anticipated consequences of substance use) are theorized to contribute to substance use and abuse and patterns of lapse and relapse following a cessation attempt [11–14]. Empirical studies have found that expectancies regarding the positive effects of a substance are more strongly linked to substance use than expectancies regarding negative effects [15,16]. However, the majority of this research has been conducted with alcohol use expectancies, which may differ from expectancies regarding other substances owing to differences in substance effects and use consequences [17].
A much smaller amount of research has demonstrated that cannabis use expectancies are associated with cannabis use patterns, both cross-sectionally [18] and prospectively [17], and problematic cannabis use [19]. Similar to alcohol, positive expectancies are a stronger predictor of cannabis use and dependence severity among treatment seekers than negative expectancies [20]. However, studies including less severe college student samples have found that negative expectancies were more highly related to cannabis use and problems than positive ones [18,19]. To date, no studies have investigated cannabis use expectancies among a sample composed entirely of individuals diagnosed with cannabis dependence or as predictors of quit success following a cessation attempt.
The goal of the current study was to prospectively investigate the relation between positive and negative cannabis use expectancies and outcomes among cannabis-dependent military veterans prior to and following a self-initiated cessation attempt. We measured lapse (defined as any use of cannabis)—as lapse is a strong predictor of further lapses, relapse and problematic use following a cessation attempt [9]. We also measured relapse (operationalized as use on 4 out of 7 days [9,21]), and patterns of average cannabis use during the 4-week cessation period. Measuring patterns of use is important, as an immediate lapse is not necessarily problematic if it is followed by an overall reduction in cannabis use [22]. Consistent with prior work that included a sample most similar to our own [20], we hypothesized that positive expectancies would be more strongly linked to cannabis use at baseline, lapse/ relapse, and patterns of use following cessation, than negative expectancies. As a test of the specificity of our effects, we statistically adjusted for demographic factors, motivation to quit cannabis, mental disorder diagnoses, and alcohol and tobacco use in a subset of our analyses.
METHODS
Participants
Participants were 100 cannabis-dependent military veterans who were predominately male and middle-aged (see Table 1). Participants reported their race/ ethnicity as White/Caucasian (38.4%), Black/Non- Hispanic (33.3%), Hispanic (14.1%), ‘Other’ (12.1%), Black/Hispanic (1.0%) and Asian (1.0%). All participants were recruited to take part in a study investigating predictors of relapse to cannabis use following a self-initiated quit attempt. Study inclusion criteria involved (i) being a military veteran; (ii) meeting diagnostic criteria for current cannabis dependence; (iii) reporting a current level of motivation to quit of at least 5 on a 10-point scale (0 = ‘no interest in quitting’, 10 = ‘definite interest in quitting’); and (iv) being interested in making a serious self-initiated quit attempt. Criteria for a cannabis dependence diagnosis were consistent with DSM-IV-TR [23], with the addition of withdrawal, as proposed for DSM-V [24]. Exclusion criteria included (i) limited mental competency and the inability to give informed, voluntary, written consent to participate; (ii) a significant reduction (>25%) in amount of cannabis smoked per day during the previous month; (iii) pregnancy or current breastfeeding; and (iv) current suicidal ideation. Seven additional participants were recruited, but were excluded from data analyses because they did not complete the expectancy measure at baseline (i.e. 38–100% of responses missing).
Table 1.
Descriptive statistics.
| Variable | Mean (SD) or % (n) |
|---|---|
| Covariates | |
| Age | 50.89 (10.00) |
| % Male | 95.0 (95) |
| % Caucasian | 52.5 (52) |
| % Current substance abuse/dependence other | 94.0 (94) |
| than cannabis | |
| % Current anxiety disorder | 44.0 (44) |
| % Current mood disorder | 60.0 (60) |
| Motivation to quit cannabis (0.00–10.00) | 7.14 (1.40) |
| Mean alcohol use at baseline (prior 90 days) | 2.24 (4.90) |
| (0.00−∞a) | |
| Mean alcohol use across 28-day quit period | 0.47 (1.17) |
| (0.00−∞a) | |
| Mean cigarette use at baseline (prior 90 days) | 6.35 (8.10) |
| (0.00−∞a) | |
| Mean cigarette use across 28-day quit period | 4.68 (5.88) |
| (0.00−∞a) | |
| Cannabis use expectancies | |
| Positive cannabis use expectancies | 3.46 (0.63) |
| (1.00–5.00) | |
| Negative cannabis use expectancies | 2.67 (0.80) |
| (1.00–5.00) | |
| Cannabis use | |
| Mean cannabis use at baseline (prior 90 days) | 5.70 (2.07) |
| (0.00–8.00) | |
| Mean cannabis use at week 1 (0.00–8.00) | 1.87 (2.20)b |
| Mean cannabis use at week 2 (0.00–8.00) | 1.70 (2.21)b |
| Mean cannabis use at week 3 (0.00–8.00) | 1.57 (2.32)b |
| Mean cannabis use at week 4 (0.00–8.00) | 1.58 (2.04)b |
| % Lapse in 4-week period | 77.4 (65) |
| % Relapse in 4-week period | 59.0 (49) |
There was no a priori defined upper limit to the number of drinks or cigarettes a participant could consume in a given time-period.
Value is significantly different (all P < 0.001) from cannabis use at baseline as found by repeated measures ANOVA conducted after square-root transforming variables to increase normal approximations of the distributions.
Almost all participants had a current diagnosis of abuse or dependence of a substance other than cannabis, and almost half of the sample met criteria for a current anxiety, and/or mood disorder.
Measures
Mental disorder diagnoses
Prevalence of current Axis-I diagnoses was determined by the Structured Clinical Interview-Non-Patient Version for DSM-IV (SCID-I-N/P; [25]), while the Clinician Administered Post-Traumatic Stress Disorder Scale (CAPS; [26]) was used to identify individuals meeting criteria for PTSD [27]. These audio-recorded interviews were administered by trained research assistants who were supervised by a clinical psychologist. In terms of training, before administering the SCID-I-N/P and CAPS in the context of the study, each trainee was required to (i) view 3–4 videotaped or live administrations by senior interviewers at the National Center for PTSD, with comparison of the trainees’ ratings with those of the senior interviewer; and (ii) administer 6–10 interviews in the presence of a senior interviewer with the requirement that trainees’ diagnoses match those of the senior interviewer on at least 4 of 5 consecutive administrations. Additionally, all interviews were audio-recorded and all diagnoses were confirmed by the last author (M.O. B-M.) following review of recorded interviews. M.O. B-M. was blind to diagnostic status at the time of audio review, and no discrepancies between research assistants and M.O. B-M. were noted.
Substance use and lapse/relapse
The clinician-administered timeline follow-back (TLFB) procedure [28] was used to assess self-reported use of cannabis, alcohol and tobacco at baseline (each day for the prior 90 days), and each day during the 28-day cessation period. Substance use was calculated as the mean (a) quantity of use per day on an 8-point scale for cannabis, (b) number of drinks consumed per day for alcohol, and (c) number of cigarettes (or equivalent) consumed per day for tobacco. The TLFB has been used extensively among cannabis-dependent participants and has been found to be as effective at assessing cannabis use/ abstinence as objective tests [29–31].
Cannabis use expectancies
Cannabis use expectancies were measured using the Marijuana Effects Expectancy Questionnaire (MEEQ) [32]. The MEEQ includes 78 items that comprise six analytically derived factors: ‘Relaxation and Tension Reduction’, ‘Perceptual/Cognitive Enhancement’, ‘Social/Sexual Facilitation’, ‘Craving/Physical Effects’, ‘Cognitive/ Behavioral Impairment’ and ‘Global Negative Effects’ [32]. The first four factors were highly associated [mean r(100) = 0.45 to 0.70, all P < 0.001], as were the latter two factors [r(100) = 0.66, P < 0.001]. Therefore, a positive expectancy scale was calculated as the mean of items comprising the first four factors, and a negative expectancy scale was calculated as the mean of items comprising the latter two factors. The MEEQ is accepted as a good measure of cannabis use expectancies [17–19,32], and positive and negative expectancy scales demonstrated high reliability in the current sample (Cronbach’s α > 0.91). See Table 1 for descriptive statistics.
Motivation to quit
Motivation to quit was measured by a single 10-point Likert-scale item (1 = ‘I enjoy using marijuana and have decided not to quit using marijuana for my lifetime’; 10 = ‘I have quit using marijuana and I will never use again’).
Procedure
Responding to flyers posted throughout a Veterans Administration medical center, individuals who were interested in quitting cannabis were provided with a detailed description of the study via phone. Participants were then initially screened for eligibility, with those eligible scheduled for a baseline appointment 1 day prior to the day that they chose to make a serious cessation attempt. Upon arrival to the laboratory participants provided written consent to participate in the research study, and were administered the SCID I-N/P and CAPS by trained interviewers to assess key exclusionary and inclusionary criteria. Eligible participants then completed the TLFB and a battery of self-report measures (including the MEEQ). At the conclusion of this appointment, participants were compensated with $75 and instructed to make a serious cessation attempt the morning of the following day. Participants returned to the laboratory to complete the TLFB at 1, 2, 3 and 4 weeks post-quit. Participants were compensated with $15 at the conclusion of each follow-up appointment. Study recruitment took place between 2009 and 2012. All procedures were approved by the Stanford University Institutional Review Board.
Data analysis
A priori statistical power calculations were conducted to determine the sample size needed to test with adequate power the effects of anxiety vulnerabilities on cessation outcomes of cannabis-dependent individuals. A sample of 120 participants would be required to detect with adequate power the medium-sized effects found in studies of the most robust anxiety-related predictors of early lapse and relapse [33–35]. The sample for this study included all participants who enrolled and completed the MEEQ.
We first examined correlations between study variables at baseline, with and without adjustment for baseline covariates. We next conducted two sets of analyses to predict cannabis use outcomes following a cessation attempt from standardized positive and negative cannabis expectancy expectancies, entered simultaneously, at baseline. We predicted (i) the percentage of the sample that lapsed and relapsed within the 28 days of the cessation attempt using logistic regressions; and (ii) the trajectory of mean cannabis use across the 28-day cessation attempt (aggregated to four time-points representing mean use during each of the 4 weeks). The latter analysis utilized zero-inflated negative-binomial mixed effects models (ZINBMEM; as implemented by the glmmADMB package in R [36–38]) to account for the sizable portion of participants who did not use cannabis during the cessation period [39,40]. We first conducted analyses unadjusted for covariates, then adjusted for demographic variables (age, gender, race/ethnicity), participant-reported motivation to quit cannabis, cooccurring diagnoses, and standardized cannabis, alcohol and tobacco use at baseline, and in logistic regression and ZINBMEM analyses, alcohol and tobacco use across the cessation period. We note that there is no valid statistical method to statistically ‘control’ for diagnostic status [41]. Regardless, subsidiary analyses included diagnostic status as a covariate.
For ZINBMEM analyses, a trajectory for each participant was modeled yielding estimates of each individual’s score at week 1, which served as the intercept, and the individual’s slope and error (the fit of the linear model to participant’s data). Between-person parameters were estimated for (i) the mean cannabis use score at the intercept for all participants; (ii) the effects of expectancies on mean cannabis use scores at the intercept; (iii) the average slope over time; (iv) the effects of expectancies on the average slope (i.e. a measure of change per unit time associated with expectancies after accounting for baseline scores and the non-independence of observations); and (v) for covariates. We found that a generalized linear model best accounted for the data based on examination of plotted data representing mean cannabis use of participants at weeks 1–4 post-quit (see Table 1). In our analysis, the intercept was allowed to vary between participants, and an unstructured covariance specification was used. Initially, we allowed the slope to vary between participants, but removed this parameter from analyses because the variance for the random slope was close to zero and its removal did not affect any other model parameter estimate in a meaningful way.
Complete cannabis use data were obtained for 91 participants at week 1, 82 participants at week 2, 81 participants at week 3 and 78 participants at week 4. Participants with complete cannabis use data for all time-points (n = 76) did not differ from those with incomplete data in terms of positive or negative expectancies (all P > 0.31), motivation to quit using cannabis (P > 0.73) and demographic variables (all P > 0.25) other than race/ethnicity [χ2(5) = 12.82; P = 0.03]. Because not all participants were included in all analyses owing to missing data, thus potentially biasing results, we examined the reliability of our results by re-conducting analyses on complete data sets obtained through multiple imputation procedures, and pooling results [42]). For correlation and logistic regression analyses 80 complete data sets were imputed with the chained-equations algorithm, MICE 2.9 [43] (with 200 iterations) implemented by R. Plots of imputed parameters versus iteration number indicated that convergence had been achieved [43]. For ZINBMEM analyses, 50 complete data sets were imputed using R. Covariates were imputed using MICE 2.9 (with 50 iterations), and the outcome variable was imputed using the StatMatch package [44], which adequately handles a sizable preponderance of zeros within a data set through implementation of the random hot deck algorithm [45,46]. Trace plots and Gewecke diagnostic statistics [47] obtained through post hoc Markov Chain Monte Carlo estimation indicated that convergence had been achieved.
RESULTS
As shown in Table 1, demonstrating the commitment of participants to quit using cannabis, the mean use of cannabis was significantly reduced from baseline to weeks 1–4 [all P < 0.001, all effect sizes (ηp2) = 0.64]. Although the sample as a whole reduced its cannabis use during the cessation period, a substantial majority of the sample lapsed and relapsed to cannabis use by the end of the 28-day cessation period.
Correlation analyses at baseline
As shown in Table 2, cannabis use at baseline was positively associated with positive expectancies and not highly associated with negative expectancies, with and without adjustment for covariates. Additionally, positive and negative expectancies were significantly associated, with and without adjustment for covariates. Results were replicated when conducting correlation analyses on imputed data sets (Table 3).
Table 2.
Zero-order correlations between cannabis use at baseline, and positive and negative expectancies without (below diagonal) and with (above diagonal) adjustment for covariates.
| Cannabis use |
Positive expectancies |
Negative expectancies |
|
|---|---|---|---|
| Cannabis use | 0.26* | 0.07 | |
| Positive expectancies | 0.26* | 0.32** | |
| Negative expectancies | 0.12 | 0.32** | |
n ranged from 79 to 100 owing to incomplete data
P < 0.05
P < 0.01
Table 3.
Pooled zero-order correlations between cannabis use at baseline, and positive and negative expectancies without (below diagonal) and with (above diagonal) adjustment for covariates following imputation of data.
| Cannabis use |
Positive expectancies |
Negative expectancies |
|
|---|---|---|---|
| Cannabis use | 0.25* | 0.06 | |
| Positive expectancies | 0.26* | 0.29* | |
| Negative expectancies | 0.12 | 0.32** | |
P < 0.05
P < 0.01
Predicting cannabis lapse and relapse
Positive and negative expectancies together significantly predicted cannabis lapse over the 28-day cessation period [omnibus χ2(2) = 9.78; P = 0.008] and predicted relapse over the 28-day cessation period at the level of a trend [omnibus χ2(2) = 4.84; P = 0.09], and these effects were significant when adjusting for covariates [predicting lapse: omnibus χ2(2) = 21.47; P < 0.001; predicting relapse: omnibus χ2(2) = 9.94; P = 0.007].
As shown in Table 4, examination of individual predictors revealed that both positive (odds ratio [OR] = 3.21) and negative expectancies (OR = 0.33) significantly predicted lapse when statistically adjusting for each other, and these effects remained significant when adjusting for covariates (ORs = 15.71, 0.08). Positive (OR = 2.39), but not negative (OR = 0.68), expectancies significantly predicted relapse when statistically controlling for each other, and both positive and negative expectancies significantly predicted relapse when adjusting for covariates (ORs = 4.40, 0.43). Results were replicated when conducting logistic regression analyses on imputed data sets (Table 5).
Table 4.
Parameter estimates for logistic regressions simultaneously predicting lapse and relapse from positive and negative expectancies.
| Predicting lapse |
Predicting relapse |
|||
|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | |
| Unadjusted for covariates | ||||
| Positive expectancies | 1.17 (0.53)* | 0.17–2.28 | 0.87 (0.42)* | 0.08–1.74 |
| Negative expectancies | −1.12 (0.42)** | −2.01−(−)0.35 | −0.39 (0.31) | −1.01–0.21 |
| Adjusted for covariates | ||||
| Positive expectancies | 2.75 (0.96)** | 1.13–5.03 | 1.48 (0.61)* | 0.38–2.79 |
| Negative expectancies | −2.54 (0.78)** | −4.42−(−)1.24 | −0.84 (0.40)* | −1.69−(−)0.09 |
n ranged from 79 to 84 owing to incomplete data
P < 0.05
P < 0.01
Table 5.
Pooled parameter estimates for logistic regressions simultaneously predicting lapse and relapse from positive and negative expectancies following imputation of data.
| Predicting lapse |
Predicting relapse |
|||
|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | |
| Unadjusted for covariates | ||||
| Positive expectancies | 1.29 (0.50)* | 0.29–2.29 | 0.99 (0.41)* | 0.18–1.80 |
| Negative expectancies | −1.23 (0.40)** | −2.04−(−)0.43 | −0.51 (0.30) | −1.10–0.08 |
| Adjusted for covariates | ||||
| Positive expectancies | 2.32 (0.84)** | 0.64–3.99 | 1.52 (0.59)* | 0.35–2.70 |
| Negative expectancies | −1.88 (0.66)** | −3.20−(−)0.56 | −0.76 (0.38)* | −1.52−(−)0.002 |
P < 0.05
P < 0.01
Predicting mean cannabis use trajectories
As shown in Table 6, positive and negative expectancies significantly predicted mean cannabis use at the intercept, and positive, but not negative, expectancies predicted change in cannabis use during the cessation period when statistically accounting for each other (i.e. the Time × Expectancy parameter was significant for positive expectancies). These effects remained significant when adjusting for covariates. To determine effect size, we calculated the proportion reduction of level 1 (i.e. time) and level 2 (i.e. participant) variance when comparing a model including positive and negative expectancies to one without these variables [48,49]. Reduction in level 1 variance was minimal (=–0.04 in models with and without covariates), while reduction in level 2 variance, the primary level of interest, was moderate (model without covariates = 0.25, model with covariates = 0.3 7). Results were replicated when conducting ZINBMEM analyses on imputed data sets (Table 7).
Table 6.
Parameter estimates for generalized linear mixed modeling analyses simultaneously predicting mean cannabis use from positive and negative expectancies.
| Positive expectancies β (SE) |
Negative expectancies β (SE) |
||
|---|---|---|---|
| Unadjusted for covariates | |||
| Intercept | −0.69 (1.65) | ||
| Time (i.e. slope) | 0.51 (0.22)* | ||
| Expectancy | 1.47 (0.48)** | −1.30 (0.39)*** | |
| Time × expectancy | −0.15 (0.07)* | −0.00 (0.06) | |
| Adjusted for covariates | |||
| Intercept | 2.24 (2.84) | ||
| Time (i.e. slope) | 0.51 (0.23)* | ||
| Expectancy | 1.83 (0.38)*** | −1.38 (0.38)*** | |
| Time × expectancy | −0.15 (0.07)* | 0.01 (0.06) | |
ns were 85 and 91 owing to incomplete data
P < 0.05
P < 0.01
P < 0.001
Table 7.
Pooled parameter estimates for generalized linear mixed modeling analyses simultaneously predicting mean cannabis use from positive and negative expectancies following imputation of data.
| Positive expectancies β (SE) |
Negative expectancies β (SE) |
||
|---|---|---|---|
| Unadjusted for covariates | |||
| Intercept | −0.46 (1.55) | ||
| Time (i.e. slope) | 0.49 (0.23)* | ||
| Expectancy | 1.26 (0.47)** | −0.95 (0.42)* | |
| Time × expectancy | −0.15 (0.07)* | 0.00 (0.06) | |
| Adjusted for covariates | |||
| Intercept | 2.56 (2.56) | ||
| Time (i.e. slope) | 0.53 (0.24)* | ||
| Expectancy | 1.34 (0.50)** | −0.96 (0.39)* | |
| Time × expectancy | −0.17 (0.07)* | 0.02 (0.06) | |
P < 0.05
P < 0.01
As shown in Fig. 1, individuals high versus low in positive expectancies, and individuals low versus high in global negative effects expectancies tended to use a larger amount of cannabis in the first week following the cessation attempt. Additionally, higher levels of positive expectancies were associated with greater decreases in cannabis use over time, whereas lower levels of these expectancies were associated with relatively stable patterns of cannabis use over time.
Figure 1.
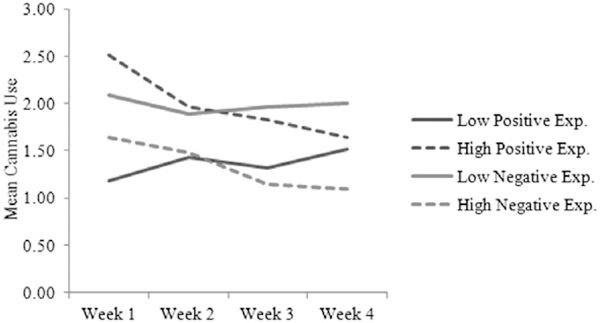
Trajectories of mean cannabis use separately for participants low and high (categorized through a median split of positive and negative expectancies, separately) in positive and negative expectancies
DISCUSSION
In the first study to investigate cannabis use expectancies and quit success among a sample entirely composed of individuals diagnosed with cannabis dependence, we demonstrated that cannabis use expectancies were cross-sectionally and prospectively associated with cannabis use. Consistent with previous research among a sample seeking treatment for cannabis use [20], and with the alcohol expectancy literature more generally [15,16], we found that expectancies regarding the positive effects of cannabis were more strongly and consistently linked to cannabis use than expectancies regarding negative effects. These results were not attributable to demographic factors, participants’ motivation to quit using cannabis, mental disorder diagnoses, or alcohol or tobacco use. Furthermore, the consistency of results obtained from non-imputed and imputed data sets increases our confidence in the reliability of our results [42].
Expanding upon studies of cannabis use expectancies [17–20], we found that positive expectancies were associated with higher levels of cannabis use at baseline, and greater odds of cannabis lapse and relapse. As demonstrated by our ZINBMEM analyses, participants high in positive expectancies used more cannabis in the first week following the cessation attempt than those low on this scale, but eventually reduced their cannabis intake to levels similar to those low in positive expectancies, who had minimal and slightly increasing rates of use over the cessation period. Thus, although they lapsed at greater rates, participants high in positive effects expectancies reduced their overall use.
Individuals, including experienced cannabis users, commonly use cannabis to relax, reduce tension, facilitate social/sexual relationships, enhance perception and cognition, stimulate appetite and facilitate sleep [50–52]. Furthermore, use of cannabis for these purposes has been found to be positively associated with cannabis dependence [50]. Thus, the removal of cannabis during a cessation attempt among those who expect benefit from it for these purposes, and do not have other means to achieve related goals (e.g. relaxation), may explain the observed positive associations between positive expectancies and cannabis lapse/relapse. However, as the duration of the cessation period lengthened, participants may have found other ways to achieve the positive effects they believed were associated with cannabis use (e.g. through exercise [53,54]), or no longer used cannabis for these purposes, thus reducing cannabis intake.
Negative expectancies significantly and inversely predicted lapse (with and without adjusting for covariates) and relapse (adjusting for covariates) during the cessation period. Thus, consistent with previous research [15,20], individuals high in negative expectancies tended to have better lapse/relapse-related outcomes than individuals low in negative expectancies. However, individuals low in negative expectancies tended to remain stable in their use of a moderate amount of cannabis across the cessation period. Thus, our results suggest that a lack of negative expectancies regarding cannabis may be more detrimental in terms of early cannabis cessation than high positive expectancies.
The current research has two primary implications. First, results are consistent with a harm-reduction perspective [22], as even individuals who quickly lapsed to high rates of cannabis use, such as those with high positive expectancies, reduced their cannabis intake over the cessation period. Thus, patterns of cannabis use may become more adaptive, even for those who do not initially achieve cessation goals (e.g. abstinence). This finding highlights the benefits of observing multiple indices of cannabis use following a cessation attempt [55], rather than abstinence alone. Second, results suggest that specific types of expectancies are likely to be useful targets of interventions designed to reduce cannabis use following a cessation attempt. Increasing the balance of negative to positive expectancies prior to a cessation attempt may result in greater achievement of cannabis cessationrelated goals. Indeed, interventions designed to challenge the validity of alcohol expectancies have been shown to be associated with reductions in alcohol intake, with both brief and intensive interventions associated with changes in expectancies and prevention of alcohol use [56,57].
Although this study has important implications, it is not without limitation. First, our statistical analyses may have been underpowered owing to a limited sample size, although results would not be expected to differ with additional participants owing to the lack of trend-level findings. Related to this, results obtained from analyses of non-imputed data may have been biased owing to missing participant data, although this bias is likely to be small given the consistency between results obtained from analyses of non-imputed and imputed data sets. Third, because of a lack of control group we cannot be certain that the associations found are specific to individuals attempting to quit cannabis, or to dependent cannabis users more broadly. Fourth, although we only employed self-report measures, we note that (i) conviction in beliefs is likely to be validly measured via self-report; and (ii) our self-report measure of substance use has been found to be as effective at assessing use as biological measures [29–31]. Furthermore, no explicit incentive was provided for abstinence, thereby reducing potentially inflated reports of abstinence and reduced use. A related limitation concerns our lack of assessment of the desirability of positive and negative expectancies, as these data may have allowed for an investigation of the influence of demand characteristics on associations between expectancies and indices of cannabis use cessation while also increasing our ability to predict such indices [58,59]. Fifth, we were limited in our abilities to measure the particular species (e.g. C. Sativa) or strains (e.g. White Widow) of cannabis used. Different species and strains of cannabis are likely to have different psychological and physical effects [60,61], and therefore may be differently related to cannabis use expectancies and patterns. Additionally, the generalizability of our findings is limited because our sample consisted mostly of males, all of whom were military veterans. Future research would benefit from extending these findings to female and nonmilitary samples, as well as assessing strain of cannabis used at each time-point. Lastly, the extent to which findings generalize to individuals attempting to quit cannabis on their own may be limited by the fact participants in this study were compensated for attending a study session prior to their quit attempt.
Acknowledgments
This work was supported by a VA Clinical Science Research and Development (CSR&D) Career Development Award—2 granted to Dr Bonn-Miller. The expressed views do not necessarily represent those of the Department of Veterans Affairs.
Footnotes
Declaration of interests
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41 HHS Publication No. (SMA) 11–4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. [Google Scholar]
- 2.Bonn-Miller MO, Harris AHS, Trafton JA Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychol Serv 2012; 9: 404–16. [DOI] [PubMed] [Google Scholar]
- 3.Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict 2006; 15: 8–14. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham J Remission from drug dependence: is treatment a prerequisite? Drug Alcohol Depend 2000; 59: 211–13. [DOI] [PubMed] [Google Scholar]
- 5.Weiner M, Sussman S, McCuller W, Lichtman K Factors in marijuana cessation among high-risk youth. J Drug Educ 1999; 29: 337–57. [DOI] [PubMed] [Google Scholar]
- 6.Roffman RA, Stephens RS Cannabis Dependence: Its Nature, Consequences, and Treatment. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 7.Larimer ME, Palmer RS, Marlatt GA Relapse prevention: an overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health 1999; 23: 151–60. [PMC free article] [PubMed] [Google Scholar]
- 8.Budney AJ, Radonovich KJ, Higgins ST, Wong CJ Adults seeking treatment for marijuana dependence: a comparison with cocaine-dependent treatment seekers. Exp Clin Psychopharmacol 1998; 6: 419–26. [DOI] [PubMed] [Google Scholar]
- 9.Moore BA, Budney AJ Relapse in outpatient treatment for marijuana dependence. J Subst Abuse Treat 2003; 25: 85–9. [DOI] [PubMed] [Google Scholar]
- 10.Stephens RS, Roffman RA, Simpson EE Adult marijuana users seeking treatment. J Consult Clin Psychol 1993; 61: 1100–4. [DOI] [PubMed] [Google Scholar]
- 11.Brown S Reinforcement expectancies and alcoholism treatment outcome after a one-year follow-up. J Stud Alcohol 1985; 46: 304–8. [DOI] [PubMed] [Google Scholar]
- 12.Conway KP, Swendsen JD, Merikangas KR Alcohol expectancies, alcohol consumption, and problem drinking: the moderating role of family history. Addict Behav 2003; 28: 823–36. [DOI] [PubMed] [Google Scholar]
- 13.Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol 1992; 101: 139–52. [DOI] [PubMed] [Google Scholar]
- 14.Leventhal AM, Schmitz JM The role of drug use outcome expectancies in substance abuse risk: an interactional-transformational model. Addict Behav 2006; 31: 2038–62. [DOI] [PubMed] [Google Scholar]
- 15.Jones BT, Corbin W, Fromme K A review of expectancy theory and alcohol consumption. Addiction 2001; 96: 57–72. [DOI] [PubMed] [Google Scholar]
- 16.Leigh BC, Stacy AW Alcohol outcome expectancies: scale construction and predictive utility in higher order confirmatory models. Psychol Assess 1993; 5: 216–29. [Google Scholar]
- 17.Aarons GA, Brown SA, Stice E, Coe MT Psychometric evaluation of the marijuana and stimulant effect expectancy questionnaires for adolescents. Addict Behav 2001; 26: 219–36. [DOI] [PubMed] [Google Scholar]
- 18.Simons JS, Arens AM Moderating effects of sensitivity to punishment and sensitivity to reward on associations between marijuana effect expectancies and use. Psychol Addict Behav 2007; 21: 409–14. [DOI] [PubMed] [Google Scholar]
- 19.Buckner JD, Schmidt NB Marijuana effect expectancies: relations to social anxiety and marijuana use problems. Addict Behav 2008; 33: 1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor JP, Gullo MJ, Feeney GF, Young RM Validation of the Cannabis Expectancy Questionnaire (CEQ) in adult cannabis users in treatment. Drug Alcohol Depend 2011; 115: 167–74. [DOI] [PubMed] [Google Scholar]
- 21.Hall SM, Havassy BE, Wasserman DA Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol 1990; 58: 175–81. [DOI] [PubMed] [Google Scholar]
- 22.Marlatt GA Harm reduction: come as you are. Addict Behav 1996; 21 :779–88. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders, 4th edn Text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 24.Budney AJ, Hughes JR, Moore BA, Vandrey R Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 2004; 161: 1967–77. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW Structured Clinical Interview for DSM-IV (SCID-I) (User’s Guide and Interview) Research Version. New York: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- 26.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 27.Weathers FW, Keane TM, Davidson JR Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001; 13: 132–56. [DOI] [PubMed] [Google Scholar]
- 28.Sobell L, Sobell M Timeline Follow-back: a technique for assessing self-reported ethanol consumption In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods, Totowa, NJ: Humana Press; 1992, p. 41–72. [Google Scholar]
- 29.Budney AJ, Hughes JR, Moore BA, Novy PL Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry 2001; 58: 917–24. [DOI] [PubMed] [Google Scholar]
- 30.Hjorthøj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending CapOpus randomized clinical trial. Addiction 2012; 107: 1123–31. [DOI] [PubMed] [Google Scholar]
- 31.Sobell LC, Sobell MB Alcohol abuse and smoking: dual recoveries. Alcohol Health Res World 1996; 20: 124–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer J, Brown SA Marijuana and cocaine effect expectancies and drug use patterns. J Consult Clin Psychol 1991; 59: 558–65. [DOI] [PubMed] [Google Scholar]
- 33.Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE Anxiety sensitivity: relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addict Behav 2001; 26: 887–99. [DOI] [PubMed] [Google Scholar]
- 34.Zvolensky MJ, Bernstein A, Cardenas SJ, Colota VA, Marshall EC, Feldner MT Anxiety sensitivity and early relapse to smoking: a test among Mexican daily, low-level smokers. Nicotine Tob Res 2007; 9: 483–91. [DOI] [PubMed] [Google Scholar]
- 35.Zvolensky MJ, Bernstein A, Marshall EC, Feldner MT Panic attacks, panic disorder, and agoraphobia: associations with substance use, abuse, and dependence. Curr Psychiatry Rep 2006; 8: 2 79–85. [DOI] [PubMed] [Google Scholar]
- 36.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder M et al. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 2012; 27: 233–49. [Google Scholar]
- 37.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 38.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B Generalized linear mixed models using AD model builder. R package. Version 0.7.3; 2012. [Google Scholar]
- 39.Atkins DC, Baldwin SA, Zheng C, Gallop RJ, Neighbors C A tutorial on count regression and zero-altered count models for longitudinal substance use data. Psychol Addict Behav 2013; 27: 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall DB Zero-inflated poisson and binomial regression with random effects: a case study. Biometrics 2000; 56: 1030–9. [DOI] [PubMed] [Google Scholar]
- 41.Miller GA, Chapman JP Misunderstanding analysis of covariance. J Abnorm Psychol 2001; 110: 40–8. [DOI] [PubMed] [Google Scholar]
- 42.Graham JW Missing Data: Analysis and Design. New York: Springer; 2012. [Google Scholar]
- 43.van Buuren S, Groothuis-Oudshoorn K Mice: multivariate imputation by chained equations in R. J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 44.D’Orazio M Package ‘StatMatch’. R package. Version 1.2.0; 2012. [Google Scholar]
- 45.Andridge RR, Little RJA A review of hot deck imputation for survey non-response. Int Stat Rev 2010; 78: 40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuller WA, Kim JK Hot deck imputation for the response model. Surv Methodol 2005; 31: 139–49. [Google Scholar]
- 47.Geweke J Evaluating the accuracy of sampling-based approaches to calculating posterior moments In: Bernardo JM, Berger JO, Dawiv AP, Smith AFM, editors. Bayesian Statistics, vol. 4 Oxford, UK: Clarendon Press; 1992, p. 169–93. [Google Scholar]
- 48.Raudenbush SW, Bryk AS Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd edn Newbury Park, CA: Sage; 2002. [Google Scholar]
- 49.Singer JD, Willett JB Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 50.Johnson K, Mullin JL, Marshall EC, Bonn-Miller MO, Zvolensky M Exploring the meditational role of coping motives for marijuana use in terms of the relation between anxiety sensitivity and marijuana dependence. Am J Addict 2010; 19: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons J, Correia CJ, Carey KB A comparison of motives for marijuana and alcohol use among experienced users. Addict Behav 2002; 25: 153–60. [DOI] [PubMed] [Google Scholar]
- 52.Simons J, Correia CJ, Carey KB, Borsari BE Validating a five-factor marijuana motives measure: relations with use, problems, and alcohol motives. J Couns Psychol 1998; 45: 265–73. [Google Scholar]
- 53.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J Exp Biol 2012; 215: 1331–6. [DOI] [PubMed] [Google Scholar]
- 54.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A Exercise activates the endocannabinoid system. Neuroreport 2003; 14: 2209–11. [DOI] [PubMed] [Google Scholar]
- 55.Hughes JR, Peters EN, Callas PW, Budney AJ, Livingstone AE Attempts to stop or reduce marijuana use in non-treatment seekers. Drug Alcohol Depen 2008; 97: 180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darkes J, Goldman MS Expectancy challenge and drinking reduction: experimental evidence for a mediational process. J Consult Clin Psychol 1993; 61: 344–53. [DOI] [PubMed] [Google Scholar]
- 57.Kivlahan DR, Marlatt GA, Fromme K, Coppel DB, Williams E Secondary prevention with college drinkers: evaluation of an alcohol skills training program. J Consult Clin Psychol 1990; 58: 805–10. [DOI] [PubMed] [Google Scholar]
- 58.Fromme K, Stroot EA, Kaplan D Comprehensive effects of alcohol: development and psychometric assessment of a new expectancy questionnaire. Psychol Assess 1993; 5: 19–26. [Google Scholar]
- 59.Ham LS, Stewart SH, Norton PJ, Hope DA Psychometric assessment of the comprehensive effects of alcohol questionnaire: comparing a brief version to the original full scale. J Psychopathol Behav 2005; 27: 141–58. [Google Scholar]
- 60.Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX et al. Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology 2007; 52: 1079–87. [DOI] [PubMed] [Google Scholar]
- 61.Tambaro S, Bortolato M Cannabinoid-related agents in the treatment of anxiety disorders: current knowledge and future perspectives. Recent Pat CNS Drug Discov 2012; 7: 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


