Abstract
Aim: The aim of the study was to assess patient selection for embolization of varicoceles based on ultrasonography. An additional objective of the work was to evaluate the results of endovascular treatment. Material and methods: From January 2015 till August 2017, 53 patients with varicoceles diagnosed in an ultrasound examination underwent endovascular treatment in the Department of Interventional Radiology and Neuroradiology in Lublin, Poland. Each ultrasound examination was performed using the Logiq 7 GE Medical System with a linear probe at 6–12 MHz using the B-mode and Doppler functions. The study was performed in both the supine and standing position of the patient. The morphological structures of the scrotum and the width of the pampiniform venous plexus were assessed. Based on clinical signs and symptoms as well as ultrasound findings, the patients were selected for endovascular treatment. This procedure involved the implantation of coils in the distal and proximal parts of the testicular vein and administration of a sclerosing agent between the coils. Results: Varicoceles were confirmed in all patients during a color Doppler scan. Diagnostic venography confirmed venous stasis or retrograde flow in the testicular vein and widened vessels of the pampiniform venous plexus over 2 mm in diameter in all patients undergoing endovascular treatment. The diagnostic efficacy of ultrasound was 100%. The technical success of the procedure was 89%. One patient had a recurrence of varicose veins (2.2%). There were no complications in any of the patients. Conclusions: Ultrasound is the preferred method in the diagnosis of varicoceles and selection for their treatment. Testicular vein embolization is a minimally invasive procedure characterized by high efficacy and safety.
Keywords: diagnostic imaging, ultrasonography, interventional radiology, varicocele
Introduction
A varicocele is a common problem and one of the most frequent causes of worse semen parameters(1). It is characterized by the presence of abnormal, dilated and tortuous veins of the pampiniform venous plexus(2). Varicoceles are located mainly on the left side of the scrotum and occur more frequently in younger individuals. The prevalence of varicoceles in otherwise healthy men averages 15–20% and reaches 35–40% in infertile men(3). It is estimated that they are responsible for primary infertility in 35% of men and for secondary infertility in 75–81% of men(4). The mechanism by which they develop has not been fully explained. It is probably multifactorial(5).
A physical examination is the standard method for the diagnosis of a varicocele but identification of an asymptomatic and impalpable disease is challenging. Also, it is of limited value in patients with high-located testes, those with a history of intervention in the scrotal or inguinal region, men with coexistent hydrocele and in patients with suspected varicocele recurrence. Ultrasound assessment may be very helpful or even necessary in these cases(6).
A varicocele used to be diagnosed using venography, scintigraphy and thermography. Scrotal scintigraphy is described as an accurate and non-invasive method, useful for detection and classification of varicoceles. Radioisotopes were used for imaging of the scrotal vascular bed in order to detect and classify subclinical forms of varicoceles in infertile men with no other apparent causes of infertility. It was also deemed helpful and accurate in cases of recurrent varicoceles(7–9). However, these methods have been replaced with non-invasive scrotal ultrasonography, which is easier to perform and much more accurate.
Ultrasonography is currently the most available and widespread technique for scrotal examination. Additionally, color Doppler ultrasonography (CDUS) is the most sensitive non-invasive method enabling the identification of varicoceles.
Typical abnormalities found in patients with varicoceles during an ultrasound examination are anechogenic tortuous and tubular structures located in the superior and lateral parts of the testicle. The Valsalva maneuver performed during Doppler assessment of the scrotum reveals retrograde blood flow (either constant or intermittent)(6).
However, there are no specified ultrasonographic features that must always be present to enable a diagnosis of a varicocele(10–14). Nevertheless, a commonly acknowledged and used sonographic criterion is the presence of veins with the diameter exceeding 2 mm. In general, clinicians agree that clinically relevant varicoceles are identified if the venous diameter exceeds 2.5–3 mm(10). An ultrasound finding of venous reflux, in turn, is one of the basic and highly significant elements in the diagnosis of varicoceles as its duration >1 s increases the likelihood of infertility(15).
Doppler ultrasonography with a color-coded blood flow function is currently commonly used to diagnose and detect varicose veins. This article also underlines the role of testicular vein embolization in the treatment of varicoceles.
Material and methods
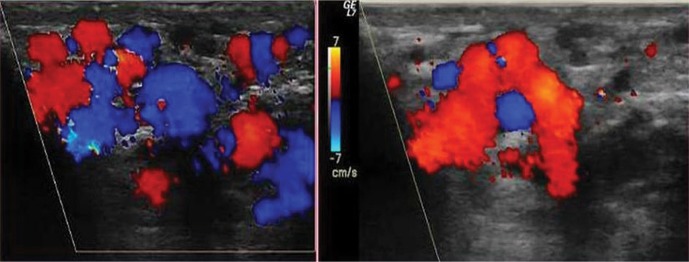
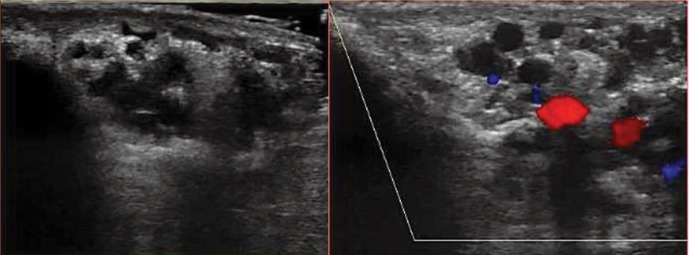
From January 2015 till August 2017, 53 patients with varicoceles, previously diagnosed in an ultrasound examination, underwent endovascular treatment in the Department of Interventional Radiology and Neuroradiology in Lublin, Poland. All patients had varicoceles additionally verified in ultrasonography prior to the procedure. Each ultrasound examination was performed using the Logiq 7 GE Medical System with a linear probe at 6–12 MHz using the B-mode and Doppler functions, i.e. the spectral reading and color-coded blood flow. The study was performed in both the supine (Fig. 1) and standing positions (Fig. 2) of the patient. The first stage involved assessment of the morphological structures of the scrotum and the width of the pampiniform venous plexus. Next, the patient underwent the Valsalva maneuver, in both the supine and standing positions, during which retrograde flow (reflux) was either confirmed or ruled out (Fig. 3). The examination encompassed the inguinal canal, the superior part of the scrotum and the region of the testicle, particularly its superior and lateral aspects, for the presence of a varicocele. Retrograde flow was evaluated with color (Fig. 3) and/or spectral Doppler (Fig. 4) based on alteration in blood flow direction, which was represented by a color change in color Doppler imaging (Fig. 5, Fig. 6) and by flow on the other side of the baseline in spectral Doppler imaging (Fig. 4). Reflux was assessed as positive if it lasted >0.5 s. Based on clinical signs and symptoms as well as ultrasound findings, patients were selected for endovascular treatment. Patients who met two of the following three criteria were selected for treatment: width of the venous plexus over 2 mm (in any position), presence of pathological reflux (in any position) and/or clinical signs and symptoms.
Fig. 1.
Scrotal B-mode ultrasound in the supine position
Fig. 2.
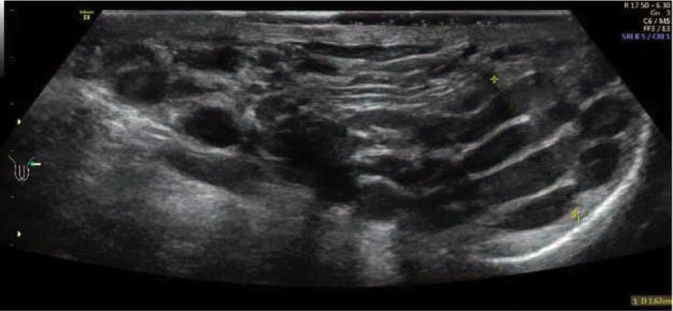
Scrotal B-mode ultrasound in the standing position
Fig. 3.
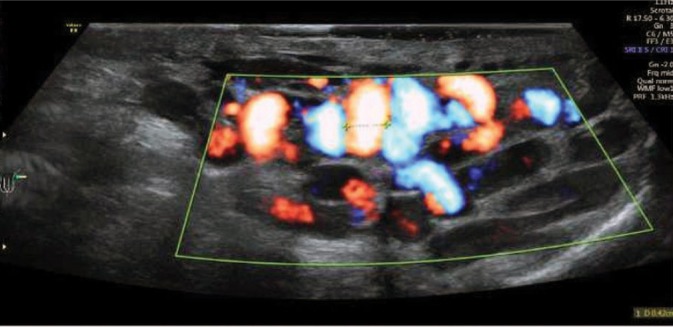
Scrotal ultrasound using color Doppler during the Valsalva maneuver – reflux evaluation
Fig. 4.
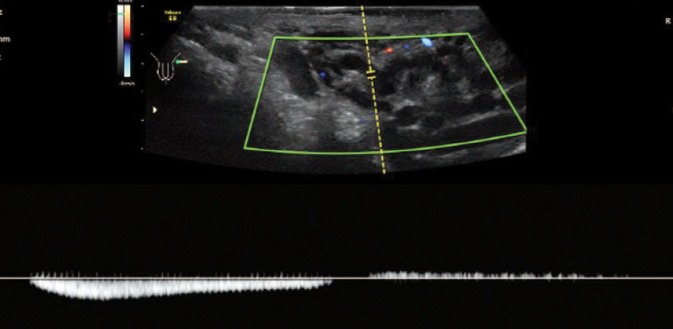
Scrotal ultrasound using spectral Doppler – reflux evaluation. Visible reflux – retrograde blood flow
Fig. 5.
Color Doppler ultrasound during the Valsalva maneuver in the standing position. Color change confirms reflux
Fig. 6.
Standing position – initially B-mode and then color Doppler ultrasound during the Valsalva maneuver
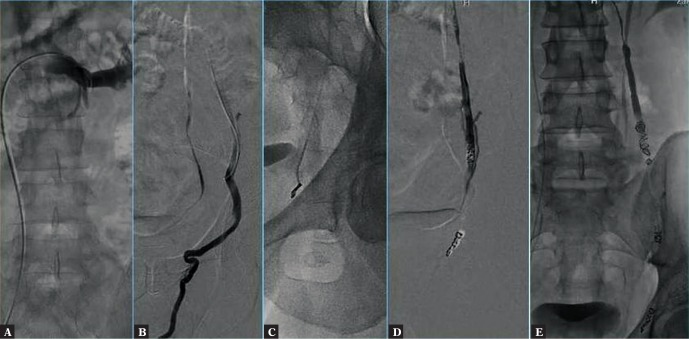
The procedure was performed by puncture of the right femoral vein in local anesthesia (solution of ligocaine 2% in 10 mL) in accordance with the Seldinger technique. After the insertion of a 5 Fr vascular introducer sheath to the right femoral vein, a guidewire was implanted and then a catheter was advanced to the inferior vena cava. Subsequently, Cobra or Levin catheters were used to reach the left renal vein and the left testicular vein, and venography was performed (Fig. 7 A). Venography was conducted during the Valsalva maneuver from the catheter placed in the initial segment of the left testicular vein (Fig. 7 B). This way, venous stasis or reflux and testicular vein dilation were verified and tortuous varicose veins of the pampiniform venous plexus were identified. Diagnostic venography enabled additional accurate visualization of the venous bed, which was helpful for proper planning and conducting of the procedure. In standard cases, the sandwich embolization technique was applied, i.e. coils were implanted in the distal and proximal parts of the testicular vein and a sclerosing agent (aetoxisclerol) was administered between the coils (Fig. 7). In patients with testicular vein tributaries, we additionally performed embolization of the tributary vein (Fig. 7 A–C, Fig. 8). In some cases, a microcatheter was necessary. Final venography revealed effective embolization with no reflux in the testicular vein.
Fig. 7.
A. Venography performed from the catheter placed in the left renal vein. B. Venography performed during the Valsalva maneuver from the catheter placed in the left testicular vein. Visible are venous stasis/reflux and the dilated testicular vein. C. Placing a coil in the distal segment of the testicular vein. D. Coils placed in the testicular vein. E. Final venography from the catheter placed in the initial segment of the testicular vein – no inflow of the contrast medium to the testicular vein
Fig. 8.
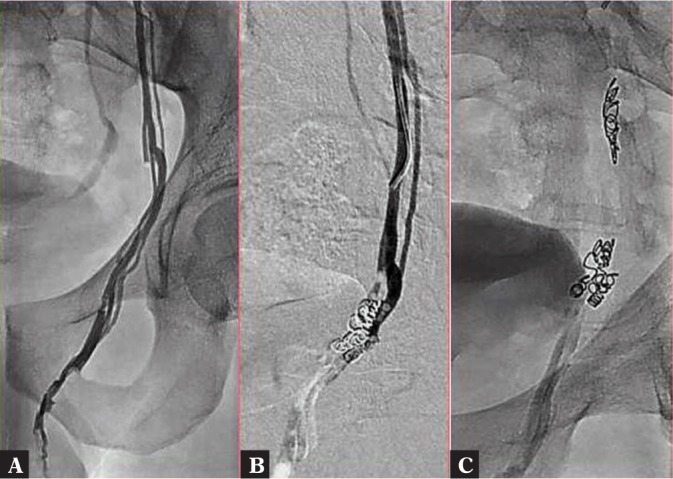
A. Testicular vein with its tributaries. B, C. Coils placed both in the main testicular vein and in its tributary veins
Results
A varicocele, represented by the dilated pampiniform venous plexus and/or the presence of reflux during the Valsalva maneuver, was confirmed in all patients in a color Doppler scan. Of 53 patients selected for testicular vein embolization, 47 underwent the procedure. In 6 patients, selective catheterization of the testicular vein failed. Diagnostic venography confirmed venous stasis or retrograde flow in the testicular vein and widened tortuous vessels of the pampiniform venous plexus over 2 mm in diameter in all patients undergoing endovascular treatment. The efficacy of diagnosing varicocele with ultrasound was 100%. The technical success of the procedure was 89%. One patient had a recurrence of varicose veins (2.2%). There were no complications in any of the patients.
Discussion
A varicocele refers to abnormally dilated veins of the pampiniform venous plexus due to venous insufficiency or the lack of valves in the testicular vein(16,17). Physical examination is the basic method to diagnose clinical varicoceles. However, it is often ambiguous due to its subjective nature and dependence on the examiner’s experience. Also, it is characterized by significant limitations in detecting alterations in blood flow direction. Moreover, this examination is insufficient in the case of minor and subclinical varicose veins, which might also have a significant pathophysiological potential(18). It must also be mentioned that one may encounter diagnostic difficulties in patients with a history of scrotal interventions, with concomitant hydrocele or with suspected recurrence of a varicocele following treatment. That is why, scrotal ultrasound is a useful accessory tool in the diagnosis of varicoceles even though the diagnosis is typically made on the basis of physical examination(6).
Ultrasound techniques have developed considerably in the past years. Owing to this progress, it is currently possible to identify minimally dilated scrotal veins and even slight retrograde venous flow(2,12,13). Ultrasound is the preferred method of all imaging techniques in the diagnosis of varicoceles. Being non-invasive, accessible and inexpensive, ultrasound should be widely used for the detection of this pathology. However, its role is still controversial due to differences in guidelines provided by various urologic societies. This results from insufficient correlations between ultrasonographic classification systems and varicocele clinical severity.
Detection of veins with a diameter over 2–3 mm within the pampiniform venous plexus in an ultrasound examination is considered a diagnostic sign of varicoceles(19). When adding color Doppler imaging, one may assess blood flow and identify retrograde flow. It seems to be the most reliable and practical tool for the detection of subclinical varicoceles(2,12,13). The sensitivity of ultrasonography in varicocele detection is significantly higher than that of physical examination (93% vs 71%)(19). In subclinical varicoceles, its sensitivity and specificity are estimated at 83–95%(7,10,11).
Our results are strictly correlated with those reported by other authors. The sensitivity of ultrasound in our work was 100%. In one study conducted among 63 infertile men, the sensitivity of color Doppler ultrasonography in varicocele detection was 97%(20). Similar results have been reported by Gonda et al. In their work, the sensitivity with which ultrasound detected varicoceles with the diameter of at least 2 mm was 95%(12). It must also be remembered that Doppler imaging is very useful in the diagnosis of subclinical varicoceles and recurrences.
Despite the commonness of ultrasonography, there is still no universal and acknowledged varicocele classification system. There are several methods of their assessment, mainly based on the diameter of veins, resting blood flow direction and Valsalva maneuver. Some classifications additionally include the duration and grade of reflux. Even though the first varicocele classification systems were based on the diameter of scrotal veins, there are still certain discrepancies between methods of their assessment. Apparently, the main reason for this is the lack of clear cutoff values for the diameter of veins in the pampiniform venous plexus(12,14).
Moreover, literature data on the efficacy of techniques and incidence of recurrences and complications depending on the employed treatment method are conflicting. Lurvey et al. performed testicular vein embolization in 101 patients; recurrences were noted in 10% of cases(21). Zampieri et al. reported embolization outcomes in 184 patients. Technical success was achieved in 93.5% of cases, and recurrences were noted in 6.5% of patients(22). In our work, the technical success rate was similar to that of Zampieri et al. (89% vs 93.5%), but the recurrence rate was significantly lower (2.2% vs 6.5 in Zampieri et al. and 10% in Lurvey et al.)(21,22), and there were no complications in any of the patients. The frequency of recurrences and complications in the form of hydrocele after laparoscopy ranges from 1% to 11% and from 2% to even 23%, respectively(23–26). As for open surgery, recurrences are noted in 0–4% of patients, and hydrocele in even 29% of patients(24,27,28). The latest reports state that the best outcomes, as compared with the aforementioned methods, are achieved with the subinguinal microsurgical technique. Numerous authors believe this method to be preferred due to the low recurrence rate (0.8–4%) and no serious complications(29,30). Hydrocele is practically not observed at all (0–1%)(29,31). Nevertheless, recent radiological studies have unequivocally proven that the only case where percutaneous endovascular embolization turned out not to be equivalent to of better than other techniques was bilateral varicoceles(32). Moreover, convalescence is shorter and less painful after embolization as compared with microsurgery(33). It must also be underlined that both treatment methods result in increased semen parameters with no significant differences between them(33). That is why percutaneous embolization is deemed a gold standard in the treatment of varicoceles by numerous authors(32).
Conclusions
This work has shown that ultrasonography can be highly effective in the detection of basic abnormalities indicating possible varicoceles.
It has also reveled high efficacy and safety of endovascular embolization as the treatment of varicoceles. Additionally, due to shorter convalescence and less intense pain after the procedure compared with other techniques, embolization is being more and more frequently considered as the preferred method.
Footnotes
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Franken DR, Acosta AA, Kruger TF, Lombard CJ, Oehninger S, Hodgen GD: The hemizona assay: Its role in identifying male factor infertility in assisted reproduction. Fertil Steril 1993; 59: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 2.Arslan H, Sakarya ME, Atilla MK: Clinical value of power Doppler sonography in the diagnosis of varicocele. J Clin Ultrasound 1998; 26: 229. [DOI] [PubMed] [Google Scholar]
- 3.Masson P, Brannigan RE: The varicocele. Urol Clin North Am 2014; 41: 129–144. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick JI, Goldstein M: Loss of fertility in men with varicocele. Fertil Steril 1993; 59: 613–616. [PubMed] [Google Scholar]
- 5.Practice Committee of the American Society for Reproductive Medicine; Society for Male Reproduction and Urology : Report on varicocele and infertility: A committee opinion. Fertil Steril 2014; 102: 1556–1560. [DOI] [PubMed] [Google Scholar]
- 6.Lorenc T, Krupniewski L, Palczewski P, Gołębiowski M: The value of ultrasonography in the diagnosis of varicocele. J Ultrason 2016; 16: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belay RE, Huang GO, Shen JK, Ko EY: Diagnosis of clinical and subclinical varicocele: how has it evolved? Asian J Androl 2016; 18: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund J, Handelsman DJ, Bautovich GJ, Conway AJ, Morris JG: Detection of varicocele by radionuclide blood-pool scanning. Radiology 1980; 137: 227–230. [DOI] [PubMed] [Google Scholar]
- 9.Paz A, Melloul M: Comparison of radionuclide scrotal blood-pool index versus gonadal venography in the diagnosis of varicocele. J Nucl Med 1998; 39: 1069–1074. [PubMed] [Google Scholar]
- 10.Kim YS, Kim SK, Cho IC, Min SK: Efficacy of scrotal Doppler ultrasonography with the Valsalva maneuver, standing position, and resting-Valsalva ratio for varicocele diagnosis. Korean J Urol 2015; 56: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semiz I, Tokgöz O, Tokgoz H, Voyvoda N, Serifoglu I, Erdem Z: The investigation of correlation between semen analysis parameters and intraparenchymal testicular spectral Doppler indices in patients with clinical varicocele. Ultrasound Q 2014; 30: 33–40. [DOI] [PubMed] [Google Scholar]
- 12.Gonda RL Jr, Karo JJ, Forte RA, O’Donnell KT: Diagnosis of subclinical varicocele in infertility. AJR Am J Roentgenol 1987; 148: 71–75. [DOI] [PubMed] [Google Scholar]
- 13.Aydos K, Baltaci S, Salih M, Anafarta K, Bedük Y, Gülsoy U: Use of color Doppler sonography in the evaluation of varicoceles. Eur Urol 1993; 24: 221–225. [DOI] [PubMed] [Google Scholar]
- 14.Eskew LA, Watson NE, Wolfman N, Bechtold R, Scharling E, Jarow JP: Ultrasonographic diagnosis of varicoceles. Fertil Steril 1993; 60: 693–697. [DOI] [PubMed] [Google Scholar]
- 15.Mihmanli I, Kurugoglu S, Cantasdemir M, Zulfikar Z, Halit Yilmaz M, Numan F: Color Doppler ultrasound in subclinical varicocele: An attempt to determine new criteria. Eur J Ultrasound 2000; 12: 43–48. [DOI] [PubMed] [Google Scholar]
- 16.Kim SW: Varicocele and male infertility. J Korean Med Assoc 2012; 55: 37–46. [Google Scholar]
- 17.Beddy P, Geoghegan T, Browne RF, Torreggiani WC: Testicular varicoceles. Clin Radiol 2005; 60: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 18.Gat Y, Bachar GN, Zukerman Z, Belenky A, Gorenish M: Physical examination may miss the diagnosis of bilateral varicocele: A comparative study of 4 diagnostic modalities. J Urol 2004; 172: 1414–1417. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg S: Diagnostic Ultrasound. Lippincott-Raven, Philadelphia: 1998. [Google Scholar]
- 20.Trum JW, Gubler FM, Laan R, van der Veen F: The value of palpation, varicoscreen contact thermography and colour Doppler ultrasound in the diagnosis of varicocele. Hum Reprod 1996; 11: 1232–1235. [DOI] [PubMed] [Google Scholar]
- 21.Lurvey R, Durbin-Johnson B, Kurzrock EA: Adolescent varicocele: A large multicenter analysis of complications and recurrence in academic programs. J Pediatr Urol 2015; 11: 186.e1–186.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zampieri N, Chironi C, Sulpasso M: Treatment of varicocele with transfemoral retrograde sclero-embolization in pediatric patients under local anesthesia. Minerva Pediatr 2015; 67: 227–229. [PubMed] [Google Scholar]
- 23.Diamond D: Adolescent versus adult varicoceles – how do evaluation and management differ? J Urol 2009; 181: 2418–2419. [DOI] [PubMed] [Google Scholar]
- 24.Riccabona M, Oswald J, Koen M, Lusuardi L, Radmayr C, Bartsch G: Optimizing the operative treatment of boys with varicocele: Sequential comparison of 4 techniques. J Urol 2003; 169: 666–668. [DOI] [PubMed] [Google Scholar]
- 25.Kocvara R, Dvorácek J, Sedlácek J, Díte Z, Novák K: Lymphatic sparing laparoscopic varicocelectomy: A microsurgical repair. J Urol 2005; 173: 1751–1754. [DOI] [PubMed] [Google Scholar]
- 26.Hassan JM, Adams MC, Pope JC 4th, Demarco RT, Brock JW 3rd: Hydrocele formation following laparoscopic varicocelectomy. J Urol 2006; 175: 1076–1079. [DOI] [PubMed] [Google Scholar]
- 27.Misseri R, Gershbein AB, Horowitz M, Glassberg KI: The adolescent varicocele. II: The incidence of hydrocele and delayed recurrent varicocele after varicocelectomy in a long-term follow-up. BJU Int 2001; 87: 494–498. [DOI] [PubMed] [Google Scholar]
- 28.Feber KM, Kass EJ: Varicocelectomy in adolescent boys: Long-term experience with the Palomo procedure. J Urol 2008; 180: 1657–1660. [DOI] [PubMed] [Google Scholar]
- 29.Schiff J, Kelly C, Goldstein M, Schlegel P, Schelgel P, Poppas D: Managing varicoceles in children: Results with microsurgical varicocelectomy. BJU Int 2005; 95: 399–402. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Xia SJ, Liu ZH, Tao L, Ge JF, Xu CM. et al. : Inguinal and subinguinal micro-varicocelectomy, the optimal surgical management of varicocele: A meta-analysis. Asian J Androl 2015; 17: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderBrink BA, Palmer LS, Gitlin J, Levitt SB, Franco I: Lymphatic-sparing laparoscopic varicocelectomy versus microscopic varicocelectomy: Is there a difference? Urology 2007; 70: 1207–1210. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy D, Jarvi K, Grober E, Lo K: Varicocele surgery or embolization: Which is better? Can Urol Assoc J 2012; 6: 266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bou Nasr E, Binhazzaa M, Almont T, Rischmann P, Soulie M, Huyghe E: Subinguinal microsurgical varicocelectomy vs. percutaneous embolization in infertile men: Prospective comparison of reproductive and functional outcomes. Basic Clin Androl 2017; 27: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]