Abstract
Introduction: Ascites is observed in cancer patients as well as in other non-neoplastic processes. In some patients, it may cause severe symptoms that can become directly life-threatening. The assessment of the degree of ascites seems useful in the determination of treatment effects as well as in the monitoring of fluid accumulation and early planning of decompression procedures. Aim: Determination of the clinical usefulness of a quantitative method of determining the degree of ascites, so-called Ascites Index. Material and methods: The Ascites Index is an ultrasonographic way of assessing the grade of ascites. The examination result is an index which is analogous to the amniotic fluid index determined in pregnant patients. The Ascites Index was determined in patients with ascites in the course of stage III–IV ovarian carcinoma (7 patients) and ovarian hyperstimulation syndrome (12 patients). Results: The patients with ovarian hyperstimulation syndrome required decompressive paracentesis at the median Ascites Index above 290 mm (range: 216–386 mm). In the patients with ovarian carcinoma, the median value of the Ascites Index at which paracentesis was required was 310 mm (range: 273–389 mm). To avoid complications associated with excessive protein loss, 2000 mL of fluid was evacuated at a single occasion. Following the procedure, the median value of the Ascites Index was 129 mm (range: 121–145 mm) in the patients with ovarian hyperstimulation syndrome and 146 cm (119–220 mm) in cancer patients. Conclusions: The proposed index is simple and rapid to determine. It makes evaluation of the degree of ascites considerably easier. Moreover, it only minimally burdens patients and enables assessment of the effect of decompression or treatment. It seems that this method might be useful also in the assessment of ascites caused by other factors, but this requires further clinical studies.
Keywords: ascites, ascites volume, Ascites Index, ovarian hyperstimulation syndrome, ovarian neoplasms
Introduction
Ascites is the buildup of exudate or transudate in the peritoneal cavity. It is observed in 15–50% of cancer patients. Cancers most commonly accompanied by ascites include ovarian carcinoma, endometrial cancer, breast cancer, colorectal cancer, stomach cancer and pancreatic cancer. In these patients, ascites is observed in 80% of cases and frequently portends the terminal phase of the disease. Median survival ranges from 1 to 4 months. Other cancers, more rarely accompanied by ascites include: non-Hodgkin lymphoma, prostate carcinoma, mesothelioma, multiple myeloma and malignant melanoma(1–3).
Ascites can also be caused by non-neoplastic processes. They include liver diseases with portal hypertension (liver cirrhosis, Budd–Chiari syndrome), portal vein thrombosis, congestive heart failure, nephrotic syndrome, pancreatitis, tuberculosis as well as bowel perforation and ovarian hyperstimulation syndrome(2–4).
The above-mentioned neoplastic causes of ascites, i.e. ovarian carcinoma, endometrial cancer, breast cancer, colorectal cancer and pancreatic cancer, are one of the commonest causes of death in women younger than 65 years of age.
The incidence of malignant cancer and its related mortality in women has been increasing for many years. In 2003, breast cancer accounted for 20% of all cases and 13% of deaths due to malignant cancers in Poland. In 2005, the incidence ratio was 67.9 per 100 thousand individuals (http://www.csioz.gov.pl). The risk increases with age. The highest incidence is observed in women older than 50 years of age(5,6).
Endometrial cancer is the most frequent malignancy of female genital organs. In 2005, the incidence ratio in Poland was 21.3 per 100 thousand individuals (http://www.csioz.gov.pl). In 2003, this cancer accounted for 7% of all cases and 2% of deaths due to malignant cancers among Polish women. This disease rarely develops before the age of 45 years. The incidence increases with age in the course of the child-bearing period and is still present after menopause, but to a lower degree(6,7).
Poland belongs to countries with moderate incidence of ovarian carcinoma. In 2003, this disease accounted for 6% of cases and 6% of deaths due to malignant cancers, whereas the incidence ratio in 2005 was 17.7 per 100 thousand individuals (http://www.csioz.gov.pl). As many as 80–90% of cases concern women older than 40 years of age. In Poland, the disease mostly affects women at the age between 45 and 54 years. Ovarian carcinoma is the most frequent cancer complicated by ascites, which is observed in 30–54% of cases(6,8,9).
In Poland, colorectal carcinoma constituted 10% of cases and caused 10% of deaths in 2003, while pancreatic cancer was characterized by the prevalence of 3% and mortality of 5% in women with malignant cancers(6).
In normal conditions, fluid production in the peritoneal cavity is continuous. The produced fluid lubricates the serosa, thus enabling the visceral slide. The fluid is then absorbed. In physiological conditions, approximately 2/3 of peritoneal fluid is absorbed through open diaphragmatic, omental and peritoneal lymphatic channels, and thanks to an important lymph movement factor, i.e. negative thoracic pressure, it is transported to the thoracic duct and the left venous angle through the mediastinal trunks. The amount of fluid depends on portal pressure, plasma oncotic pressure, water and sodium retention, amount of lymph in the body and capillary permeability. It is estimated at approximately 50 cm3. When fluid production in the peritoneal cavity is greater than its absorption, ascites develops(1,9).
The etiopathogenesis of malignant ascites is complex. The mechanisms include the sole presence of a tumor and increased vascular permeability within the lesion. Patients with ovarian carcinoma are also characterized by enhanced angiogenesis within the parietal peritoneum, which is proportional to the size of ascites. Vascular factors, such as vascular endothelial growth factor (VEGF) and vascular permeability factor (VPF), affect the clinical malignancy of cancers and metastasis production. VEGF triggers angiogenesis in tumors and is associated with vascular permeability(1,9).
There are four types of malignant ascites:
the central form, which is observed in 15% of cases and is caused by cancer cell infiltration in the hepatic parenchyma and by compression on the portal vein and lymphatic vessels;
the peripheral form, which is observed in 50% of cases and is associated with cancer cell infiltration on the surface of the peritoneum;
the mixed form, which is found in 15% of cases;
the chylous from, which occurs in the remaining cases and develops as cancer cells infiltrate the retroperitoneal space and prevent flow through lymph nodes, thereby leading to lymph extravasation(10).
The most significant causes of central ascites are: hindered blood outflow from the portal circulation and hypoalbuminemia. As circulating blood volume decreases, glomerular filtration in the kidneys declines and aldosterone secretion increases. In consequence, this induces sodium and water retention in the body. The central form of ascites is mostly caused by non-neoplastic processes.
Peripheral ascites is caused by increased vascular permeability and obstruction of small lymphatic vessels or the thoracic duct (the main path of fluid resorption to the lymphatic system) due to cancerous infiltration(10).
Ovarian hyperstimulation syndrome (OHSS) is a serious complication of ovulation induction. Its most severe manifestation is massive ovarian enlargement and fluid buildup in the third space, which results in blood volume condensation and reduction. The full-blown (critical) form of OHSS may be complicated by ascites, pericardial effusion, tachycardia with tachypnea, hemorrhage from a ruptured ovary, renal failure, oliguria, hypovolemia, thromboembolic events, ischemic stroke, shock, adult acute respiratory distress syndrome, and death. The etiopathogenesis of OHSS has not been fully explained. It seems that the critical role is played by increased capillary permeability induced by substances produced by hyperstimulated ovaries though the action of a triggering factor, i.e. human chorionic gonadotropin (hCG), which leads to ascites. In severe and critical forms of OHSS, fluid accumulates also in other body cavities. There are several vasoactive substances, for example histamine, serotonin, prostaglandins and prolactin. It is currently believed that interleukins, including vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α) and endothelin-1, are the most significant factors in the development of ascites(4).
Ascites deteriorates the quality of life. The main symptoms are: increased abdominal circumference, weight gain, distension and abdominal pain. Symptoms that follow include appetite loss, difficulty sitting and walking, nausea, vomiting, flatulence, recurring heartburn as well as edema of the lower limbs and reproductive organs. In some patients, ascites induces severe symptoms and becomes a directly life-threatening condition (breathing difficulty, dyspnea, tachypnea)(1,2,9,10). Effective treatment of symptoms caused by pressure of ascitic fluid is difficult. The assessment of the degree of ascites is necessary in disease monitoring and selection of appropriate treatment methods.
Ascites can be diagnosed in physical examination with the volume of approximately 1000 cm3. A typical sign is lateral protrusion of the abdomen in the supine position (so-called “frog’s belly”). Moreover, one may observe flattening or protrusion of the umbilicus or, more rarely, umbilical hernia. Also, one may note a positive fluid thrill test and dull notes on percussion at the site where fluid appears, especially upon shifting the position. Imaging comes in handy in the diagnostic process of ascites. It enables the diagnosis of this condition with the fluid volume of 100 cm3(1,2,9,10).
Physical examination and imaging do not allow the differentiation of malignant ascites from ascites that has developed for another cause. That is why, the differential diagnosis includes cancer markers, biochemical tests as well as cytological and microbiological examinations of fluid acquired by paracentesis, biopsy, diagnostic laparoscopy or exploratory laparotomy(1,9,10).
Specific cancer markers in malignant ascites include: sialic acid, human telomerase and chorionic gonadotropin, while non-specific markers are: Ca 125 (cancer antigen 125), CEA (carcino-embryonic antigen) and AFP (alpha-fetoprotein). Elevated levels of Ca 125 in post-menopause women with concomitant ascites and a tumor in the adnexa indicate ovarian carcinoma. Cytological examination of fluid acquired from paracentesis is common, but it has a diagnostic value in only 50–60% of cases(1,9,10).
The aim of the treatment of ascites is to relieve symptoms and improve the quality of life or, in some cases, prolong survival.
Paracentesis is still the basic method used in patients with malignant ascites. This method relieves symptoms but does not prevent recurrences, which are observed in 90% of patients. Paracentesis is performed in patients with painful and tense abdominal integuments and dyspnea. The literature reports that approximately 5000 cm3 of fluid should be evacuated to bring relief to the patient, but it is not recommended to perform a single procedure. At a single occasion, approximately 2000 cm3 of fluid should be evacuated with another 4000–5000 cm3 within 12 hours. In the mixed form of ascites, diuretics can be considered after evacuating approximately 2000 cm3 of fluid. Paracentesis is performed in the inguinal region, at a line joining the umbilicus with the anterior superior iliac spine. The procedure can be conducted on an outpatient basis. Hypovolemic shock may be a complication of paracentesis, but it is rarely observed in cancer patients. Other complications include: fluid outflow from the puncture site, bleeding, perforation of the visceral organs and, more rarely, peritonitis or peritoneal-venous shunt. Le Veen catheters are rarely used for fluid evacuation; Denver shunts are valves placed in peritoneal-venous shunts and can be used in patients with fast-accumulating fluid and good overall condition. The efficacy of this method is 75–85%. Due to high risk of peritonitis, the least common method is a shunt in the peritoneal cavity with a Tenckhoff catheter. When fluid builds up in the peritoneal cavity, repeated paracenteses depend on the occurrence of clinical signs and symptoms(1,2,9–12).
Diuretics are useful in the central and mixed forms of ascites in patients with water and sodium retention. In the mixed form, this method helps reduce the frequency of repeated paracenteses. Loop and potassium-sparing diuretics are mainly used in treatment. Moreover, patients are recommended to reduce sodium intake to 100 mmol/dL and to take fluids. This manner of treatment is not justified in peripheral and chylous ascites(1,2,9–12).
Intraperitoneal chemotherapy is used to achieve high drug concentrations and action directly on cancer cells. This helps avoid various adverse events typical of intravenous chemotherapy. Intraperitoneal chemotherapy is characterized by weaker penetration to the tumor (1 mm) and uneven drug distribution. This therapeutic method is effective in patients with metastases from breast and ovarian cancers after good response to systemic chemotherapy and when neoplastic spread is microscopic. Peritoneal fibrosis occurring during this form of treatment (destruction of the tumor surface triggers this process) causes closure of the peritoneal cavity, thus preventing fluid production(1,10–12).
Hyperthermic chemotherapy may increase cytotoxicity of certain cytostatics. Increasing the temperature of a cytostatic solution to 40.5–43°C improves tissue penetration and decreases resistance to the active substance. After administration of a solution of cisplatin with etoposide at a temperature of 41.5–42°C, ascites subsided in 4 of 5 patients with gastric or colorectal carcinoma(1).
Intraperitoneal radioactive isotopes were first used in local treatment of ascites in 1945. Currently, colloidal phosphorus (32P) and aurum (198Au) isotopes are used. Advantages of this therapeutic method include deep tissue penetration (up to 8 mm) and long half-life (14 days for 32P). The presence of adhesions in the peritoneal cavity is a contraindication to this method. Treatment efficacy ranges from 30% to 80%. This method is currently rarely used in oncology(1,9,10).
Octreotide (sandostatin) lowers blood flow in the abdominal vessels, thus leading to reduced exudate production. Treatment costs are in this case high(13).
Intraperitoneal steroid drugs are an inexpensive and effective treatment method. Intraperitoneal administration of a slowly metabolized corticosteroid (triamcinolone hexacetonide) after prior fluid evacuation effectively relieves symptoms and results in less frequent paracenteses(10,12).
Aim
The aim of the study was to determine the clinical usefulness of a quantitative system to evaluate the degree of ascites, so-called Ascites Index (AsI), in patients with OHSS and stage III–IV ovarian carcinoma. It was also attempted to standardize the method, compare the presented index in patients with these two conditions and determine referential values.
Material and methods
The AsI in the course of OHSS was calculated in 12 patients aged 26–32 years(14). The AsI in the course of stage III–IV ovarian carcinoma was estimated in 7 women aged 56–71 years. The patients were hospitalized in 2011–2015 for severe abdominal pain, dyspnea and increasing ascites. Weight, height and abdominal circumference were measured in all the patients. Body mass index (BMI) was calculated for each of them. Typical treatment was started. The degree of ascites was monitored until symptoms regressed using body weight, abdominal circumference and AsI measurements.
The data were presented as median (Me; range) due to non-normal distribution. Body mass, BMI, abdominal circumference and AsI values from before and after paracentesis were compared using the Wilcoxon signed-rank test. Relationships between the investigated parameters were assessed using the non-parametric Spearman’s rank correlation coefficient (Spearman’s rho). The statistical calculations were done in STATISTICA version 10 (StatSoft, Tulsa, OK, USA).
Ascites Index – examination technique and results
In the Third Department of Gynecology of the Medical University of Lublin, the degree of ascites is estimated in a different way from the ones described elsewhere. It is an ultrasonographic measurement of the degree of ascites in patients with stage III–IV ovarian carcinoma and OHSS with determination of an index, referred to as the Ascites Index (AsI) by the authors of this publication(14–17).
The presented method is based on the physical law formulated by Blaise Pascal in the 17th century, known as the Pascal’s law.
It is assumed that the peritoneal cavity is a closed spherical reservoir, and the fluid inside it exerts external pressure. This makes pressure equal inside the reservoir and equal to the external pressure. The law results from the fact that fluid particles can move in any direction as the pressure from one side changes the movement of particles in all directions(18). In consequence, free fluid in the peritoneal cavity during pressure equalization moves under the diaphragmatic domes and into both iliac fossae, displacing the liver, spleen and intestinal loops medially and forming fluid pockets.
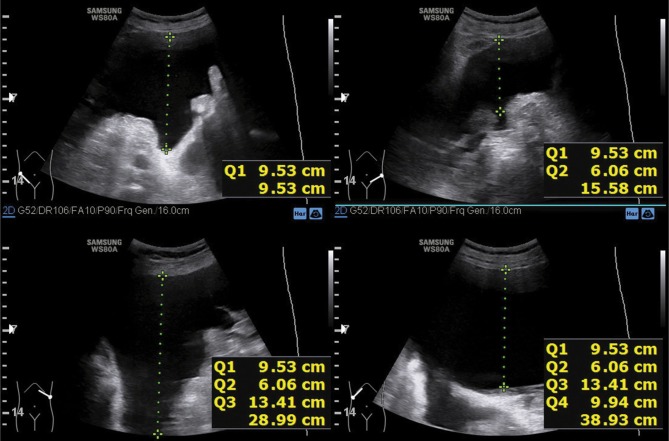
An ultrasound scan conducted to determine the AsI is performed with the convex probe in the supine position on the external abdominal quadrants: in both inguinal regions and in the region of the liver and spleen (Fig. 1, Fig. 2, Fig. 3, Fig. 4). Subsequently, one should measure the depth of the largest free fluid pockets in each quadrant (in mm or cm). The depth is measured in the horizontal plane, perpendicularly to the abdominal circumference tangent. The resultant values are summed up in an analogous manner to the amniotic fluid index determined in pregnant patients (Fig. 5). After a shift in the patient position by 45 degrees along the body axis (due to symptoms of dyspnea and abdominal pain), peritoneal fluid was redistributed. This did not affect AsI values.
Fig. 1.
Ultrasound evaluation of a free fluid pocket in the peritoneal cavity in the right inguinal region
Fig. 2.
Ultrasound evaluation of a free fluid pocket in the peritoneal cavity in the left inguinal region
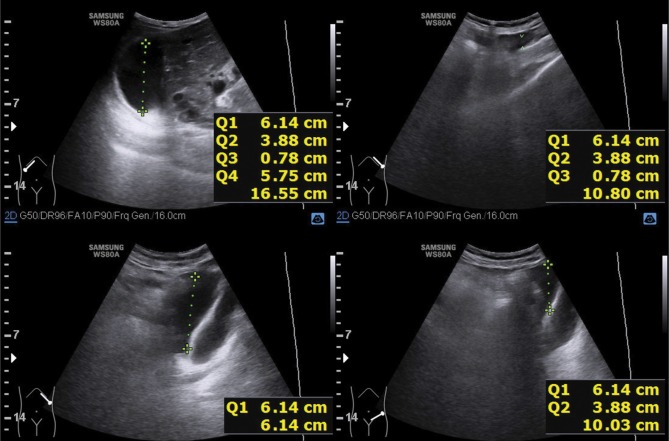
Fig. 3.
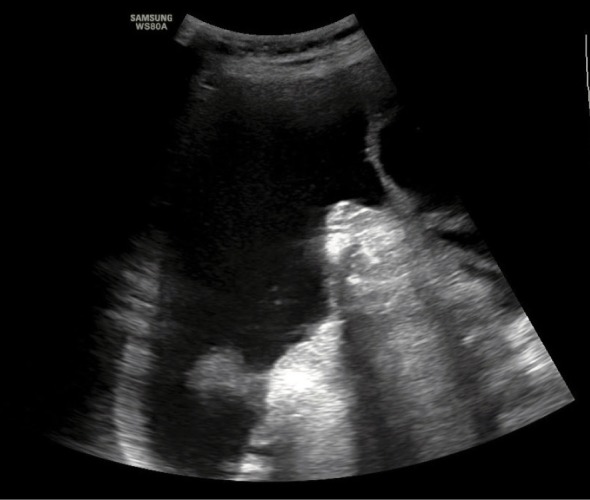
Ultrasound evaluation of a free fluid pocket in the peritoneal cavity in the region of the left diaphragmatic dome (spleen)
Fig. 4.
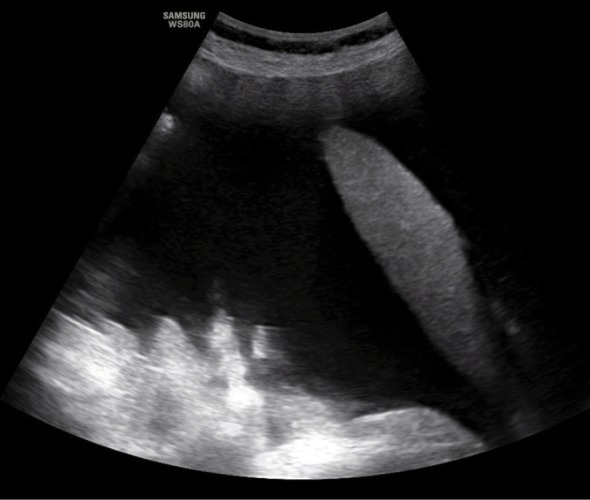
Ultrasound evaluation of a free fluid pocket in the peritoneal cavity in the region of the right diaphragmatic dome (liver)
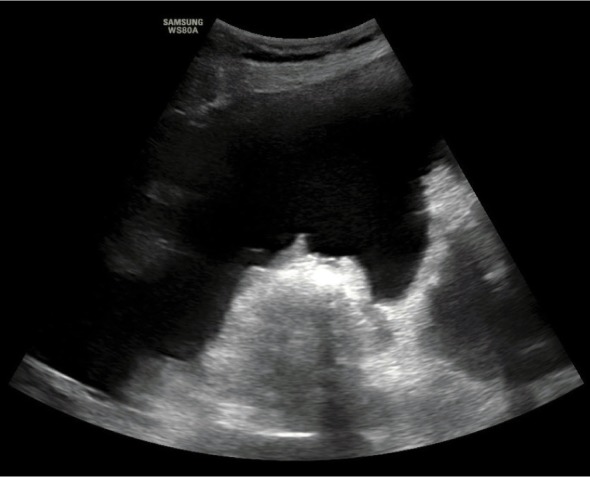
Fig. 5.
Measurement technique of the Ascites Index
The patients with ovarian hyperstimulation syndrome required decompressive paracentesis due to dyspnea with accompanying tachypnea at the (median, Me) Ascites Index above 290 mm (range: 216–386 mm). In the patients with ovarian carcinoma, the value of the Ascites Index (Me) at which paracentesis was required was 310 mm (range: 273–389 mm). To avoid complications associated with excessive protein loss, 2000 mL of fluid was evacuated at a single occasion.
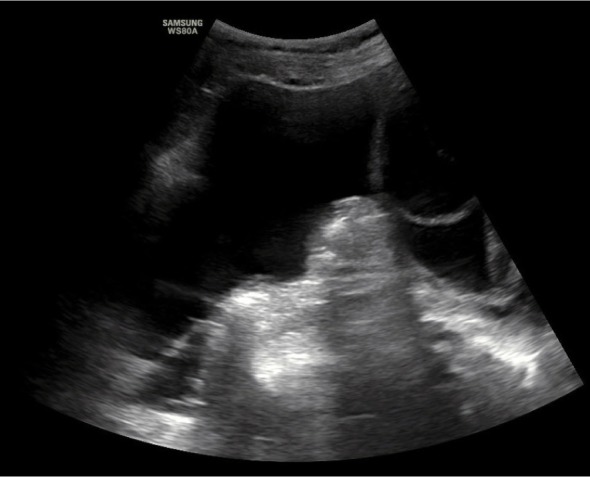
Following the procedure (day 1 after paracentesis), median AsI was 129 mm (range: 121–145 mm) in the patients with OHSS (Fig. 6) and 146 cm (119–220 mm) in cancer patients.
Fig. 6.
Ascites Index measured after paracentesis
Differences between AsI, body mass, BMI and abdominal circumference before and after paracentesis were statistically significant (p < 0.05), thus making the Ascites Index as effective as other methods for ascites monitoring. There were no statistically significant correlations between the AsI and the compared anthropometric methods for ascites monitoring before and after paracentesis.
Discussion
As long as the diagnosis of ascites based on available diagnostic methods is not problematic, quantitative assessment of its degree is challenging. The peritoneal cavity approximates a sphere, but organs inside it make it an irregular geometrical form. That is why it is difficult to estimate the degree of ascites. The assessment of ascites seems justified in the determination of treatment effects as well as in fluid accumulation monitoring and early planning of decompression procedures.
Physical examination is often inaccurate. Noninvasive diagnostic procedures, such as computed tomography or ultrasonography, are sensitive and specific in diagnosing ascites, but they are also expensive and currently incapable of assessing ascites volume. The indicator-dilution technique (IDT) may be used for calculating the ascites volume, but it carries a risk of infection, bowel perforation or hemorrhage(19).
The methods of ascites monitoring used to date include: serial measurements of body mass, abdominal circumference or protrusion index (an index of ascites intensity), which is a subjective scoring system assessing compression-induced deflection of the abdominal integuments measured in a line between the xiphoid process and pubic symphysis. Weight changes can indirectly indicate decreasing or increasing ascites. Of note is also the fact that changes in body weight and structure can be affected by the course of the underlying disease and the way of its treatment. Moreover, abdominal circumference measurements and the protrusion index assume that both the amount of fatty tissue and muscle mass do not change(20,21). The lack of correlations between the AsI and the above-mentioned anthropometric methods indirectly indicates that the method proposed by the authors herein is independent of the course of the underlying disease.
Currently, new methods of measuring ascites are proposed. They are based on ultrasonography and computed tomography (CT)(19,22).
Ultrasound assessment of ascites requires the patient to be placed horizontally with the abdomen down and supported on the hands and knees for 10 minutes. In this position, the ascitic fluid collects in the lowest point between the intestinal surface and the posterior surface of the anterior abdominal wall. The examination is conducted with a linear probe on the anterior abdominal wall upwards. The largest fluid pocket should be identified and measured. Next, the abdominal circumference is measured. It is assumed that the abdominal cavity is modelled as a sphere, and the volume is calculated according to the following formula: fluid volume = 1/3[ϖd2 (3r–d)], where „d” is the width of the largest fluid pocket and „r” is the radius of the abdominal cavity, calculated from the formula r = abdominal circumference/2ϖ(19).
Moreover, a 5-grade evaluation of ascites volume has been proposed on the basis of a computed tomography scan. After performing a series of transverse sections of the abdominal cavity, the width of fluid pockets is measured in 5 sites: bilaterally in the subdiaphragmatic spaces (points A and B) and in the paracolonic spaces (points C and D) as well as in the space between the pubic symphysis and the urinary bladder (point E). The subdiaphragmatic and paracolonic measurements are performed in the transverse section, while the space anterior to the urinary bladder is evaluated in the sagittal section. The volume of ascites is calculated according to the following formula: fluid volume = ([A + B + C + D + E] × 200 [mL])(22).
Computed tomography enables the use a 3D rendering technique to calculate the ascites volume. It provides the most reliable data as compared to the above-described methods(22).
From the clinical point of view, it is unnecessary to know the volume of fluid in the peritoneal cavity to evaluate the extent of ascites, its complications and treatment outcomes. This knowledge is irrelevant even in selecting patients for paracentesis as the peritoneal cavity is not emptied completely during the procedure, and the amount of evacuated fluid is limited due to simultaneous albumin loss. This is relevant not only in OHSS or ovarian carcinoma, but also in other diseases with ascites.
Conclusions
The proposed index is simple and rapid to determine. It makes the evaluation of the extent of ascites considerably easier. Moreover, it only minimally burdens patients and enables the assessment of the effect of decompression or treatment as well as helps predict the occurrence of subsequent severe symptoms. The AsI may be determined with simple (including portable) ultrasound equipment, also in hospices and home settings.
It seems that this method might be useful also in the assessment of ascites caused by other factors than those investigated herein, but this requires further clinical studies.
Footnotes
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Adam RA, Adam YG: Malignant ascites: past, present, and future. J Am Coll Surg 2004; 198: 999–1011. [DOI] [PubMed] [Google Scholar]
- 2.Becker G, Galandi D, Blum HE: Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 2006; 42: 589–597. [DOI] [PubMed] [Google Scholar]
- 3.Garcia N, Sanyal AJ: Ascites. Curr Treat Options Gastroenterol 2001; 4: 527–537. [DOI] [PubMed] [Google Scholar]
- 4.Zivi E, Simon A, Laufer N: Ovarian hyperstimulation syndrome: definition, incidence, and classification. Semin Reprod Med 2010; 28: 441–447. [DOI] [PubMed] [Google Scholar]
- 5.Matkowski R: Epidemiologia raka piersi In: Markowska J. (ed.): Ginekologia onkologiczna. Urban & Partner, Wrocław: 2006: 1013–1016. [Google Scholar]
- 6.Zwierko M, Wronkowski Z: Epidemiologia nowotworów narządu rodnego In: Markowska J. (ed.): Ginekologia onkologiczna. Urban & Partner, Wrocław: 2006: 3–22. [Google Scholar]
- 7.Gabryś M: Epidemiologia i etiopatogeneza raka błony śluzowej trzonu macicy In: Markowska J. (ed.): Ginekologia onkologiczna. Urban & Partner, Wrocław: 2006: 683–694. [Google Scholar]
- 8.Markowska J, Markowska A: Epidemiologia i etiopatogeneza raka jajnika In: Markowska J. (ed.): Ginekologia onkologiczna. Urban & Partner, Wrocław: 2006: 805–812. [Google Scholar]
- 9.Parsons SL, Watson SA, Steele RJ: Malignant ascites. Br J Surg 1996; 83: 6–14. [DOI] [PubMed] [Google Scholar]
- 10.Terlikiewicz J, Marciniak L: Wodobrzusze. Pol Med Paliatywna 2003; 2: 105–109. [Google Scholar]
- 11.Choudhury J, Sanyal AJ: Treatment of ascites. Curr Treat Options Gastroenterol 2003; 6: 481–491. [DOI] [PubMed] [Google Scholar]
- 12.Velamati PG, Herlong HF: Treatment of refractory ascites. Curr Treat Options Gastroenterol 2006; 9: 530–537. [DOI] [PubMed] [Google Scholar]
- 13.Cairns W, Malone R: Octreotide as an agent for the relief of malignant ascites in palliative care patients. Palliat Med 1999; 13: 429–430. [DOI] [PubMed] [Google Scholar]
- 14.Szkodziak PR, Czuczwar P, Wrona W, Paszkowski T, Szkodziak F, Woźniak S: Ascites Index – a novel technique to evaluate ascites in ovarian hyperstimulation syndrome: a concept-proof study. Ginekol Pol 2018; 89: 183–189. [DOI] [PubMed] [Google Scholar]
- 15.Brezinka C: Tipps und Tricks im Gyn-Ultraschall: Der Abdominalschallkopf beim OHSS. J für Gynäkologische Endokrinol 2013; 7: 33–34. [Google Scholar]
- 16.Szkodziak PR, Wozniak S, Czuczwar P, Kludka-Sternik M, Paszkowski M, Paszkowski T: P31.01: Ascites index – a new method of ultrasound evaluation of ascites volume in patients with ovarian cancer. Ultrasound Obstet Gynecol 2010; 36: 289. [Google Scholar]
- 17.Szkodziak PR, Wozniak S, Czuczwar P, Paszkowski T: P11.03: Ascites Index: a new method of ultrasound evaluation of ascites volume in patients with ovarian hyperstimulation syndrome. Ultrasound Obstet Gynecol 2012; 40: 214. [Google Scholar]
- 18.Fairman JG: Pascal’s Principle and Hydraulics. NASA Official; 1996. Available from: https://www.grc.nasa.gov/www/k-12/WindTunnel/Activities/Pascals_principle.html. [Google Scholar]
- 19.Inadomi J, Cello JP, Koch J: Ultrasonographic determination of ascitic volume. Hepatology 1996; 24: 549–551. [DOI] [PubMed] [Google Scholar]
- 20.Stanley MM, Ochi S, Lee KK, Nemchausky BA, Greenlee HB, Allen JI. et al. : Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with Ascites. N Engl J Med 1989; 321: 1632–1638. [DOI] [PubMed] [Google Scholar]
- 21.Wapnick S, Grosberg SJ, Evans MI: Randomized prospective matched pair study comparing peritoneovenous shunt and conventional therapy in massive ascites. Br J Surg 1979; 66: 667–670. [DOI] [PubMed] [Google Scholar]
- 22.Oriuchi N, Nakajima T, Mochiki E, Takeyoshi I, Kanuma T, Endo K. et al. : A new, accurate and conventional five-point method for quantitative evaluation of ascites using plain computed tomography in cancer patients. Jpn J Clin Oncol 2005; 35: 386–390. [DOI] [PubMed] [Google Scholar]