ABSTRACT
Long non-coding RNAs (lncRNAs) have been found to participate in the regulation of human spermatogenic cell development. However, little is known about the abnormal expression of lncRNAs associated with spermatogenic failure and their molecular mechanisms. Using lncRNA microarray of testicular tissue for male infertility and bioinformatics methods, we identified the relatively conserved lncRNA Gm2044 which may play important roles in non-obstructive azoospermia. The UCSC Genome Browser showed that lncRNA Gm2044 is the miR-202 host gene. This study revealed that lncRNA Gm2044 and miR-202 were significantly increased in non-obstructive azoospermia of spermatogonial arrest. The mRNA and protein levels of Rbfox2, a known direct target gene of miR-202, were regulated by lncRNA Gm2044. Furthermore, the miR-202-Rbfox2 signalling pathway was shown to mediate the suppressive effects of lncRNA Gm2044 on the proliferation of human testicular embryonic carcinoma cells. Understanding of the molecular signalling pathways for lncRNA-regulated spermatogenesis will provide new clues into the pathogenesis and treatment of patients with male infertility.
KEYWORDS: LncRNA, Gm2044, non-obstructive azoospermia, miR-202
Introduction
It has been reported that reproductive infertility occurs in 10–15% of couples in childbearing age, about half of which is caused by male factors including reproductive tract obstruction, inflammation, and sexual dysfunction (Matzuk and Lamb 2002; Pan et al. 2018). However, 60–75% of male infertility patients, known as idiopathic male infertility, are often accompanied by azoospermia or oligospermia, showing non-obstructive azoospermia (NOA). NOA is a complex disease caused by multiple factors with high genetic heterogeneity and phenotypic heterogeneity. Common genetic causes include chromosome abnormality, gene mutations, and epigenetic modifications (Shinjo et al. 2013; Vij et al. 2018). According to differences in spermatogenic disorders, NOA is mainly divided into maturation arrest, Sertoli cell only syndrome, and hypospermatogenesis. Spermatogenic maturation arrest, including early stage arrest (spermatogonial and spermatocyte stasis) and late stage arrest (sperm stasis), is one of the most complicated causes and most difficult to treat in male infertility. At present, the aetiology and mechanism of maturation arrest remain unknown in many spermatogenic arrest patients, which leads to great obstacles in the diagnosis and treatment of reproductive diseases (Miyamoto et al. 2012; Miyamoto et al. 2017). In-depth studies of spermatogenic maturation arrest regarding the related genetic factors, pathogenesis, and formation mechanism are needed.
LncRNAs are a class of endogenous non-coding RNAs with a length of more than 200 nucleotides and have been shown to participate in the regulation of human spermatogenic cell development (Kopp and Mendell 2018; Maeda et al. 2018). Research into the function and molecular mechanism of lncRNAs in male infertility is in the ascendant. LncRNA AK015322 is highly expressed in spermatogonial stem cells and regulates the proliferation of spermatogonial stem cells by acting as a miR-19b-3p sponge (Hu et al. 2017). LncRNA H19 can regulate the proliferation and apoptosis of male germline stem cells via the IGF-1 signalling pathway (Lei et al. 2018). The lncRNA Mrhl inhibits the Wnt signalling pathway by interacting with the p68 protein and regulates Sox8 expression through binding to the Sox8 promoter in mouse spermatogonial cells, thus ensuring normal spermatogenesis (Arun et al. 2012; Kataruka et al. 2017). LncRNA NLC1-C is down-regulated in the testicular tissue of male infertility patients with spermatocyte maturation arrest and is involved in the regulation of spermatogenesis as competitive endogenous RNA of miR-302a and miR-383 (Lu et al. 2015). Male mice without lncRNA Tslrn1 show a significant decrease in spermatozoa production (Wichman et al. 2017).
miRNAs are a class of endogenous non-coding RNAs with a length of about 22 nucleotides and have been demonstrated to be involved in the regulation of germ cell development (Ambros 2004; Bartel 2004; Wu et al. 2012; Kotaja 2014; Wang and Xu 2015; Hilz et al. 2016). The testis-determining factor SOX9 binds to the pri-miR-202 promoter and then transcriptionally activates miR-202 expression in testis differentiation which suggest the crucial roles of SOX9-miR-202 signalling pathway during testis development (Wainwright et al. 2013). Furthermore, miR-202 mediates the GDNF and RA (retinoic acid) regulatory network and affects the self-renewal and differentiation of spermatogonial stem cells by directly targeting Rbfox2 (RNA binding fox-1 homolog 2) (Chen et al. 2017). In zebrafish, miR-202 is mainly expressed in oocytes during follicular development and exhibits a typical primordial germ cell-specific expression marker throughout embryogenesis (Zhang et al. 2017). Deletion of miR-202 in female medaka led to no egg production or dramatically decreased the number of eggs that could not be fertilised, ultimately resulting in no successful pregnancy (Gay et al. 2018). In addition, upregulated miR-202 enhances the progression of endometriosis by directly targeting SOX6 and its downstream pathways including p21, cyclin D1, and pRb (Zhang et al. 2015). miR-202 has been confirmed to function as a tumour suppressor in the progression of endometrial adenocarcinoma by targeting the oncogene FOXR2 (Deng et al. 2017). The lncRNA NORAD promotes colorectal cancer cell proliferation, migration, and invasion and is associated with a poor prognosis by acting as an endogenous competitor of RNA of miR-202 (Zhang et al. 2018b). Taken together, miR-202 play critical roles in the activity, health, and disease of human beings.
In this study, the testicular tissues of male infertility patients were used to reveal abnormal expression status using lncRNA microarray. The results suggest that lncRNA may be involved in the process of spermatogenic differentiation and meiosis. We identified the abnormal expression of the conservative lncRNA Gm2044, miR-202, and Rbfox2 (a known direct target gene of miR-202) in non-obstructive azoospermia in spermatogonial arrest, and found that the miR-202-Rbfox2 signalling molecular pathway mediates the suppressive effects of lncRNA Gm2044 on the proliferation of the human testicular embryonic carcinoma cell line NCCIT.
Materials and methods
Cell culture and transfection
NCCIT cells were grown in RPMI-1640 medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) foetal bovine serum (Life Technologies) and 1% penicillin–Streptomycin (100 U/ml penicillin and 100 μg/ml streptomycin) (Life Technologies) at 37°C in 5% carbon dioxide incubator. Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was selected to transfect the NCCIT cells following the manufacturer’s instructions.
RNA extract and RT-qPCR
Total RNA was extracted using Trizol (Invitrogen) from testicular tissues and NCCIT cells. The relative expression levels of lncRNA Gm2044, Rbfox2 mRNA, and miR-202 were analysed by RT-qPCR according to our previous studies (Hu and Liang 2017; Hu et al. 2018).
Western blotting
The protein of testicular tissues and NCCIT cells was isolated using radio immunoprecipitation assay lysis buffer (Millipore, Bedford, MA, USA) with a 1% protease inhibitor cocktail (Roche, Indianapolis, IN, USA), separated on SDS-PAGE, transferred onto nitrocellulose membrane filters (Amersham Biosciences, Freiburg, Germany), and then immunoblotted with antibody. Anti-RBFOX2 and anti-β-actin antibodies were obtained from Thermo Fisher Scientific (Rockford, IL, USA).
Cell proliferation analysis
CCK-8 reagent (Dojindo Laboratories, Kumamoto, Japan) was used to determine the proliferation of transfected NCCIT cells according to the manufacturer’s protocol. The absorbance at 450 nm in transfected NCCIT cells was detected using a microplate reader.
Statistics
Each experiment was performed at least three times. The data were analysed using Student’s t-test or ANOVA, and are presented as mean ± SEM. Differences were considered to be statistically significant when P < 0.05.
Results
LncRNA Gm2044 and miR-202 are highly expressed in non-obstructive azoospermia with spermatogonial arrest
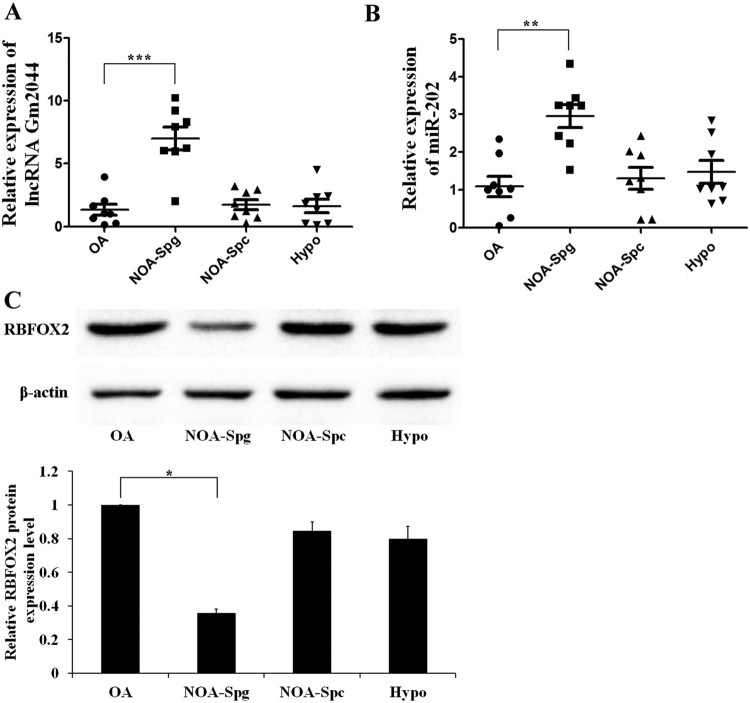
Using an lncRNA microarray on testicular tissue obtained from male infertility patients and bioinformatics methods, we identified the relatively conserved lncRNA Gm2044, which may play important roles in non-obstructive azoospermia (NOA). The relative expression of lncRNA Gm2044 in obstructive azoospermia (OA), NOA with spermatogonial arrest (NOA-Spg), NOA with spermatocyte arrest (NOA-Spc), and hypospermatogenesis (Hypo) was measured by RT-qPCR. The results show that lncRNA Gm2044 was significantly increased in NOA-Spg (Figure 1(A)). Additionally, lncRNA Gm2044 was weakly expressed in spermatogonia and highly expressed in healthy spermatocytes (Hu et al. 2018). The abnormal expression of lncRNA Gm2044 in spermatogonia cells in NOA-spg male infertility testicular tissue may play a critical role in blocking spermatogonia cell development.
Figure 1.
The relative expression of lncRNA Gm2044 and miR-202. A and B, LncRNA Gm2044 (A) and miR-202 (B) were significantly increased in NOA-Spg. Total RNA was isolated from OA, NOA-Spg, NOA-Spc, and Hypo, and then subjected to RT-qPCR analysis for lncRNA Gm2044 (A) and miR-202 (B). C, RBFOX2 protein was significantly decreased in NOA-Spg. Protein was extracted from OA, NOA-Spg, NOA-Spc, and Hypo, and then subjected to western blotting analysis for RBFOX2 and β-actin. The top panel shows the band of western blotting and the bottom panel gives the statistical summary of the above band. In A and B, β-actin mRNA and U6 snRNA were used as the reference genes, respectively. OA, obstructive azoospermia; NOA-Spg, non-obstructive azoospermia of spermatogonial arrest; NOA-Spc, non-obstructive azoospermia of spermatocyte arrest; Hypo, hypospermatogenesis; **, P ≤ 0.01; ***, P ≤ 0.001.
The UCSC Genome Browser showed that lncRNA Gm2044 is the miR-202 host gene. RT-qPCR demonstrated that miR-202 was also highly expressed in NOA-spg male infertility testicular tissue (Figure 1(B)). Previous research found that miR-202 can directly target Rbfox2 and regulate spermatogenesis (Chen et al. 2017). This study revealed that the expression level of RBFOX2 protein was significantly lower in NOA-spg male infertility testicular tissue by western blotting analysis (Figure 1(C)).
LncRNA Gm2044 inhibits Rbfox2 expression
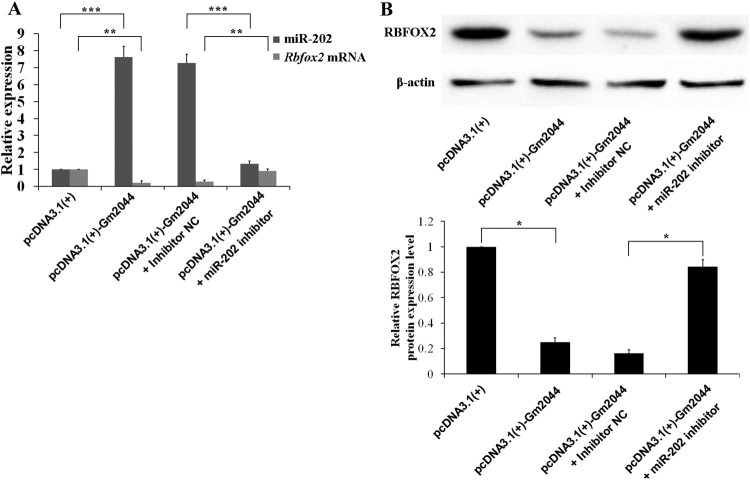
To determine whether lncRNA Gm2044 regulates Rbfox2 expression through miR-202, overexpression of Gm2044 was carried out in human testicular embryonic carcinoma cells (NCCIT) by transfection with pcDNA3.1(+)-Gm2044 plasmid. The result demonstrated that overexpression of Gm2044 led to a high level of miR-202, which may due to the transformation of Gm2044 (Figure 2(A)). Overexpression of lncRNA Gm2044 significantly decreased the Rbfox2 mRNA and protein level (Figure 2(A and B)). However, knockdown of miR-202 rescued the suppressive effects of lncRNA Gm2044 on Rbfox2 mRNA and protein expression (Figure 2(A and B)). These results suggest that miR-202 can mediate the effect of upregulated lncRNA Gm2044 in NOA-Spg on regulating Rbfox2 expression.
Figure 2.
LncRNA Gm2044 suppresses the Rbfox2 expression. A, The level of Rbfox2 mRNA was inhibited by overexpression of lncRNA Gm2044 and rescued by knockdown of miR-202. NCCIT cells transfected with pcDNA3.1(+)/pcDNA3.1(+)-Gm2044/inhibitor NC/miR-202 inhibitor were used to isolate RNA, and then subjected to RT-qPCR detection for lncRNA Gm2044 and miR-202. B, The level of RBFOX2 protein was inhibited by overexpression of lncRNA Gm2044 and rescued by knockdown of miR-202. NCCIT cells transfected with pcDNA3.1(+)/pcDNA3.1(+)-Gm2044/inhibitor NC/miR-202 inhibitor were used to extract protein, and then subjected to western blotting for RBFOX2 and β-actin. The top panel shows the band of western blotting and the bottom panel gives the statistical summary of the above band. NC, negative control; **, P ≤ 0.01; ***, P ≤ 0.001.
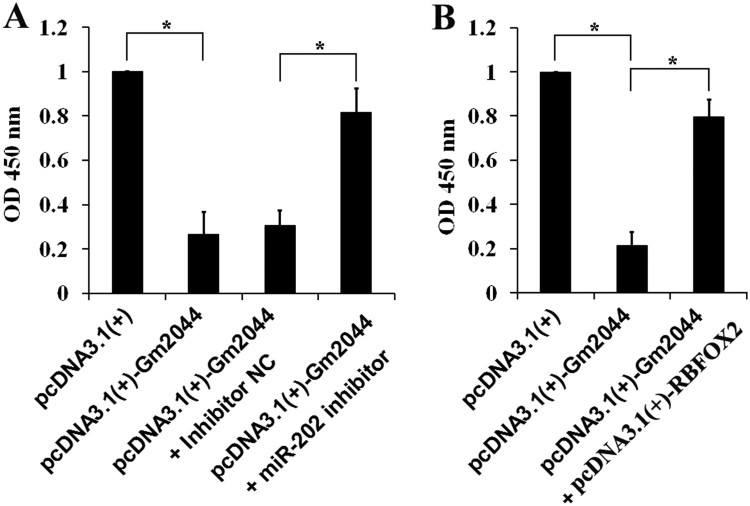
The miR-202-Rbfox2 signalling mediates the inhibitory effects of lncRNA Gm2044 on NCCIT cell proliferation
Human testicular embryonic carcinoma cells (NCCIT, a pluripotent extragonadal germ cell tumour cell line) was used to study the effect of lncRNA Gm2044 in vitro. Overexpression of lncRNA Gm2044 in NCCIT cells significantly inhibited cell proliferation (Figure 3(A and B)). Knockdown of miR-202 (Figure 3(A)) or overexpression of Rbfox2 (Figure 3(B)) attenuated the inhibitory effects of lncRNA Gm2044 on NCCIT proliferation. Taken together, the miR-202-Rbfox2 molecular signalling pathway appears to mediate the inhibitory effects of lncRNA Gm2044 on the proliferation of human testicular embryonic carcinoma cells.
Figure 3.
LncRNA Gm2044 inhibits NCCIT cell proliferation through the miR-202-Rbfox2 signalling pathway. A, The cellular proliferation of NCCIT was inhibited by the overexpression of lncRNA Gm2044 and rescued by knockdown of miR-202. NCCIT cells transfected with pcDNA3.1(+)/pcDNA3.1(+)-Gm2044/inhibitor NC/miR-202 inhibitor for 48 h were used to analyse proliferation. B, The proliferation of NCCIT cells was inhibited by overexpression of lncRNA Gm2044 and rescued by overexpression of RBFOX2. NCCIT cells transfected with pcDNA3.1(+)/pcDNA3.1(+)-Gm2044/pcDNA3.1(+)-RBFOX2 for 48 h were analysed for proliferation using the CCK-8 reagent. NC, negative control; *, P ≤ 0.05.
Discussion
In recent years, we have focused on studies of lncRNAs in spermatogenesis (Liang et al. 2014; Hu et al. 2017; Hu et al. 2018). The expression of lncRNAs and mRNAs in male germ cells at different critical stages was analysed by microarray. The results show that lncRNAs and mRNAs show coordinated expression (Liang et al. 2014). High levels of the lncRNA AK015322 enhance spermatogonial stem cell (C18-4) proliferation by regulating the miR-19b-3p-ETV5 signalling pathway (Hu et al. 2017). In addition, we also found that lncRNA Gm2044 was enriched in spermatocytes and inhibited translation of the adjacent reproductive gene Utf1 by interacting with Utf1 mRNA (Hu et al. 2018). In this study, we mainly explored the roles of lncRNA in male infertility. Upregulated lncRNA Gm2044 was found to be a potential cause of impaired spermatogonial development.
The transition of spermatogonia to spermatocytes is a regulatory processes that involves germ cell differentiation from spermatogonial stem cells (As, a single spermatogonia) (Raverdeau et al. 2012). The spermatogonial stem cell divides into two paired A (Ap) spermatogonia and then to 4–32 aligned (Aal) spermatogonia (de Rooij and Russell 2000; Nakagawa et al. 2010). Subsequently, Aal spermatogonia in turn give rise to A1, A2, A3, A4, In, and B spermatogonia, which then generate premeiotic spermatocytes. LncRNA033862 regulates the self-renewal and survival of spermatogonial stem cells by interation with Gfrα1 chromatin (Li et al. 2016). LncRNA Mrhl can mediate the function of Wnt signalling pathway in mouse spermatogonia (Akhade et al. 2016). LncRNA HSVIII plays crucial roles in spermatocyte meiosis by affecting Prss42 and Tessp2 expression (Yoneda et al. 2016). However, little is known about the regulation of lncRNAs in the transition of spermatogonia to spermatocytes. This study revealed that lncRNA Gm2044 was significantly increased in NOA-Spg and led to abnormal expression of the miR-202-Rbfox2 molecular pathway, indicating that lncRNA Gm2044 and its downstream signalling have the ability to regulate male infertility.
Studies on the mechanism of the mutual regulation for lncRNAs and miRNAs have drawn considerable attention in cellular development (Janakiraman et al. 2018; Xiao et al. 2018). lncRNAs/miRNAs interact with RNA binding proteins to mediate the TGF-β molecular signalling pathway during post-transcriptional genetic regulation (Janakiraman et al. 2018). Because the abnormal expression and dysregulation of lncRNAs and miRNAs occurs in cancer, lncRNAs-miRNAs have been identified as biomarkers in certain cancers (Zhang et al. 2018a). LncRNA MEG3 is down-regulated in gastric cancer and plays critical roles in gastric cancer by suppressing miR-21 expression (Dan et al. 2018). Hypermethylation of CpG islands on the promoter lead to the loss of miR-31 and its host gene lncRNA LOC554202 in triple-negative breast cancer, which then promotes breast cancer growth and metastasis (Augoff et al. 2012). LncRNA MIR100HG, an miRNA-host gene lncRNA, interacts with HuR/ELAVL1 to modify RNA-binding protein ability in human cells (Sun et al. 2018). LncRNA Gm2044 is the miR-202 host gene, and we demonstrated that lncRNA Gm2044 and miR-202 are significantly upregulated in NOA-Spg male infertility testicular tissue. miR-202 can mediate the function of upregulated lncRNA Gm2044 in NOA-Spg by regulating Rbfox2 expression and modulating NCCIT cell proliferation.
In summary, lncRNA Gm2044 is significantly elevated in NOA-Spg and suppresses Rbfox2 expression by acting as miR-202 host gene. Furthermore, the miR-202-Rbfox2 molecular signalling pathway mediates the inhibitory effects of lncRNA Gm2044 on the proliferation of the human testicular embryonic carcinoma cell NCCIT. Understanding the signalling pathway for lncRNAs in male reproduction will provide new clues to the pathogenesis and treatment of male infertility.
Funding Statement
This study was funded by the National Natural Science Foundation of China (81801516), the Natural Science Foundation of Anhui Province (China) (1608085QH199), and the Key Project of Natural Science Foundation of Anhui Provincial Department of Education (China) (KJ2018A0992).
Acknowledgements
We thanks Professor Fei Sun (Shanghai Jiaotong University, China) for providing the NCCIT cell line.
Compliance with ethical standards
This study received ethical approval from the institutional review board of Bengbu Medical College.
Author contributions
Meng Liang and Yaping Liao conceived of the study; Meng Liang performed the research and wrote the manuscript; Ke Hu analysed the results; Chaofan He and Jinzhao Zhou reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Akhade VS, Dighe SN, Kataruka S, Rao MR.. 2016. Mechanism of Wnt signaling induced down regulation of mrhl long non-coding RNA in mouse spermatogonial cells. Nucleic Acids Res. 44:387–401. doi: 10.1093/nar/gkv1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V.2004 Sep 16. The functions of animal microRNAs. Nature. 431:350–355. Epub 2004/09/17. doi: 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- Arun G, Akhade VS, Donakonda S, Rao MR.. 2012. mrhl RNA, a long noncoding RNA, negatively regulates Wnt signaling through its protein partner Ddx5/p68 in mouse spermatogonial cells. Mol Cell Biol. 32:3140–3152. doi: 10.1128/MCB.00006-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augoff K, McCue B, Plow EF, Sossey-Alaoui K.. 2012. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer. 11:5. doi: 10.1186/1476-4598-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP.2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Chen J, Cai T, Zheng C, Lin X, Wang G, Liao S, Wang X, Gan H, Zhang D, Hu X, et al. 2017. MicroRNA-202 maintains spermatogonial stem cells by inhibiting cell cycle regulators and RNA binding proteins. Nucleic Acids Res. 45:4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J, Wang J, Wang Y, Zhu M, Yang X, Peng Z, Jiang H, Chen L.. 2018. LncRNA-MEG3 inhibits proliferation and metastasis by regulating miRNA-21 in gastric cancer. Biomed Pharmacother. 99:931–938. doi: 10.1016/j.biopha.2018.01.164 [DOI] [PubMed] [Google Scholar]
- Deng X, Hou C, Liang Z, Wang H, Zhu L, Xu H.. 2017. miR-202 suppresses cell proliferation by targeting FOXR2 in endometrial adenocarcinoma. Dis Markers. 2017:2827435. doi: 10.1155/2017/2827435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD.. 2000. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 21:776–798. [PubMed] [Google Scholar]
- Gay S, Bugeon J, Bouchareb A, Henry L, Delahaye C, Legeai F, Montfort J, Le Cam A, Siegel A, Bobe J, et al. 2018. MiR-202 controls female fecundity by regulating medaka oogenesis. PLoS Genet. 14:e1007593. doi: 10.1371/journal.pgen.1007593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz S, Modzelewski AJ, Cohen PE, Grimson A.. 2016. The roles of microRNAs and siRNAs in mammalian spermatogenesis. Development. 143:3061–3073. doi: 10.1242/dev.136721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Li L, Liao Y, Liang M.. 2018. LncRNA Gm2044 highly expresses in spermatocyte and inhibits Utf1 translation by interacting with Utf1 mRNA. Genes Genomics. 40:781–787. doi: 10.1007/s13258-018-0690-4 [DOI] [PubMed] [Google Scholar]
- Hu K, Liang M.. 2017. Upregulated microRNA-224 promotes ovarian cancer cell proliferation by targeting KLLN. In Vitro Cell Dev Biol Anim. 53:149–156. doi: 10.1007/s11626-016-0093-2 [DOI] [PubMed] [Google Scholar]
- Hu K, Zhang J, Liang M.. 2017. LncRNA AK015322 promotes proliferation of spermatogonial stem cell C18-4 by acting as a decoy for microRNA-19b-3p. In Vitro Cell Dev Biol Anim. 53:277–284. doi: 10.1007/s11626-016-0102-5 [DOI] [PubMed] [Google Scholar]
- Janakiraman H, House RP, Gangaraju VK, Diehl JA, Howe PH, Palanisamy V.. 2018. The long (lncRNA) and short (miRNA) of It: TGFbeta-mediated control of RNA-binding proteins and noncoding RNAs. Mol Cancer Res: MCR. 16:567–579. doi: 10.1158/1541-7786.MCR-17-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataruka S, Akhade VS, Kayyar B, Rao MRS.. 2017. Mrhl long noncoding RNA mediates meiotic commitment of mouse spermatogonial cells by regulating Sox8 expression. Mol Cell Biol. 15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp F, Mendell JT.. 2018. Functional classification and experimental dissection of long noncoding RNAs. Cell. 172:393–407. doi: 10.1016/j.cell.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja N.2014. MicroRNAs and spermatogenesis. Fertil Steril. 101:1552–1562. doi: 10.1016/j.fertnstert.2014.04.025 [DOI] [PubMed] [Google Scholar]
- Lei Q, Pan Q, Li N, Zhou Z, Zhang J, He X, Peng S, Li G, Sidhu K, Chen S, et al. 2018. H19 regulates the proliferation of bovine male germline stem cells via IGF-1 signaling pathway. J Cell Physiol. 234(1):915–926. Aug 1. doi: 10.1002/jcp.26920 [DOI] [PubMed] [Google Scholar]
- Li L, Wang M, Wu X, Geng L, Xue Y, Wei X, Jia Y.. 2016. A long non-coding RNA interacts with Gfra1 and maintains survival of mouse spermatogonial stem cells. Cell Death Dis. 7:e2140. doi: 10.1038/cddis.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Li W, Tian H, Hu T, Wang L, Lin Y, Li Y, Huang H, Sun F.. 2014. Sequential expression of long noncoding RNA as mRNA gene expression in specific stages of mouse spermatogenesis. Sci Rep. 4:5966. doi: 10.1038/srep05966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Tian H, Cao YX, He X, Chen L, Song X, Ping P, Huang H, Sun F.. 2015. Downregulation of miR-320a/383-sponge-like long non-coding RNA NLC1-C (narcolepsy candidate-region 1 genes) is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation. Cell Death Dis. 6:e1960. doi: 10.1038/cddis.2015.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda RK, Sitnik JL, Frei Y, Prince E, Gligorov D, Wolfner MF, Karch F.. 2018. The lncRNA male-specific abdominal plays a critical role in Drosophila accessory gland development and male fertility. PLoS Genet. 14:e1007519. doi: 10.1371/journal.pgen.1007519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ.. 2002. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 4:s41–s49. doi: 10.1038/ncb-nm-fertilityS41 [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Minase G, Shin T, Ueda H, Okada H, Sengoku K.. 2017. Human male infertility and its genetic causes. Reprod Med Biol. 16:81–88. doi: 10.1002/rmb2.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Tsujimura A, Miyagawa Y, Koh E, Namiki M, Sengoku K.. 2012. Male infertility and its causes in human. Adv Urol. 2012:384520. doi: 10.1155/2012/384520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S.. 2010. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 328:62–67. doi: 10.1126/science.1182868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MM, Hockenberry MS, Kirby EW, Lipshultz LI.. 2018. Male infertility diagnosis and treatment in the era of in vitro fertilization and intracytoplasmic sperm injection. Med Clin North Am. 102:337–347. doi: 10.1016/j.mcna.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB.. 2012. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 109:16582–16587. doi: 10.1073/pnas.1214936109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjo E, Shiraishi K, Matsuyama H.. 2013. The effect of human chorionic gonadotropin-based hormonal therapy on intratesticular testosterone levels and spermatogonial DNA synthesis in men with non-obstructive azoospermia. Andrology. 1:929–935. doi: 10.1111/j.2047-2927.2013.00141.x [DOI] [PubMed] [Google Scholar]
- Sun Q, Tripathi V, Yoon JH, Singh DK, Hao Q, Min KW, Davila S, Zealy RW, Li XL, Polycarpou-Schwarz M, et al. 2018. MIR100 host gene-encoded lncRNAs regulate cell cycle by modulating the interaction between HuR and its target mRNAs. Nucleic Acids Res. 46:10405–10416. doi: 10.1093/nar/gky696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij SC, Sabanegh Jr. E, Agarwal A.. 2018. Biological therapy for non-obstructive azoospermia. Expert Opin Biol Ther. 18:19–23. doi: 10.1080/14712598.2018.1380622 [DOI] [PubMed] [Google Scholar]
- Wainwright EN, Jorgensen JS, Kim Y, Truong V, Bagheri-Fam S, Davidson T, Svingen T, Fernandez-Valverde SL, McClelland KS, Taft RJ, et al. 2013. SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biol Reprod. 89:34. doi: 10.1095/biolreprod.113.110155 [DOI] [PubMed] [Google Scholar]
- Wang L, Xu C.. 2015. Role of microRNAs in mammalian spermatogenesis and testicular germ cell tumors. Reproduction. 149:R127–R137. doi: 10.1530/REP-14-0239 [DOI] [PubMed] [Google Scholar]
- Wichman L, Somasundaram S, Breindel C, Valerio DM, McCarrey JR, Hodges CA, Khalil AM.. 2017. Dynamic expression of long noncoding RNAs reveals their potential roles in spermatogenesis and fertility. Biol Reprod. 97:313–323. doi: 10.1093/biolre/iox084 [DOI] [PubMed] [Google Scholar]
- Wu W, Hu Z, Qin Y, Dong J, Dai J, Lu C, Zhang W, Shen H, Xia Y, Wang X.. 2012. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol Hum Reprod. 18:489–497. doi: 10.1093/molehr/gas022 [DOI] [PubMed] [Google Scholar]
- Xiao B, Zhang W, Chen L, Hang J, Wang L, Zhang R, Liao Y, Chen J, Ma Q, Sun Z, et al. 2018. Analysis of the miRNA-mRNA-lncRNA network in human estrogen receptor-positive and estrogen receptor-negative breast cancer based on TCGA data. Gene. 658:28–35. doi: 10.1016/j.gene.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Yoneda R, Satoh Y, Yoshida I, Kawamura S, Kotani T, Kimura AP.. 2016. A genomic region transcribed into a long noncoding RNA interacts with the Prss42/Tessp-2 promoter in spermatocytes during mouse spermatogenesis, and its flanking sequences can function as enhancers. Mol Reprod Dev. 83:541–557. doi: 10.1002/mrd.22650 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li XY, Hu P, Ding YS.. 2018b. LncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li Y, Tian J, Zhang H, Wang S.. 2015. MiR-202 promotes endometriosis by regulating SOX6 expression. Int J Clin Exp Med. 8:17757–17764. [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu W, Jin Y, Jia P, Jia K, Yi M.. 2017. MiR-202-5p is a novel germ plasm-specific microRNA in zebrafish. Sci Rep. 7:7055. doi: 10.1038/s41598-017-07675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Pian C, Chen Z, Zhang J, Xu M, Zhang L, Chen Y.. 2018a. Identification of cancer-related miRNA-lncRNA biomarkers using a basic miRNA-lncRNA network. PloS One. 13:e0196681. doi: 10.1371/journal.pone.0196681 [DOI] [PMC free article] [PubMed] [Google Scholar]