Abstract
Background
In the last decade, increasing evidence has evolved for early and maximal safe resection of diffuse low-grade gliomas (LGGs) regarding survival. However, changes in clinical practice are known to occur slowly and we do not know if the scientific evidence has yet resulted in changes in neurosurgical patterns of care.
Methods
The Swedish Brain Tumor Registry was used to identify all patients with a first-time histopathological diagnosis of LGG between 2005 and 2015. For analysis of surgical treatment patterns, we subdivided assessed time periods into 2005-2008, 2009-2012, and 2013-2015. Population-based data on patient and disease characteristics, surgical management, and outcomes were extracted.
Results
A total of 548 patients with diffuse World Health Organization grade II gliomas were identified: 142 diagnosed during 2005-2008, 244 during 2009-2012, and 162 during 2013-2015. Resection as opposed to biopsy was performed in 64.3% during 2005-2008, 74.2% during 2009-2012, and 74.1% during 2013-2015 (P = .08). There was no difference among the 3 periods regarding overall survival (P = .11). However, post hoc analysis of data from the 4 (out of 6) centers that covered all 3 time periods demonstrated a resection rate of 64.3% during 2005-2008, 77.4% during 2009-2012, and 75.4% during 2013-2015 (P = .02) and longer survival of patients diagnosed 2009 and onward (P = .04).
Conclusion
In this nationwide, population-based study we observed a shift over time in favor of LGG resection. Further, a positive correlation between the more active surgical strategy and longer survival is shown, although no causality can be claimed because of possible confounding factors.
Keywords: diffuse low-grade glioma, glioma/surgery, neurosurgery, patterns of care, treatment outcome
Adult supratentorial World Health Organization (WHO) grade II diffuse low-grade gliomas (LGGs) are slow-growing primary brain tumors.1,2 LGG is a rare cancer with an incidence of approximately 1/100 000/year, typically affecting younger adults who at the time of diagnosis often present with epileptic seizures.3–5 These circumstances make larger observational surgical series relatively uncommon, while surgical trials using randomization of patients are unlikely to be performed because of questionable clinical equipoise.6,7
In lack of consensus, the surgical management of LGG has previously varied among centers.6,8 Currently, the argument frequently favors a more active surgical approach encouraged by growing evidence for the favorable impact of early and maximal safe resection on clinical outcome.6,9–12
To what extent today’s scientific evidence favoring maximal safe resection has resulted in changes of clinical practice remains unknown, but changes in clinical practice are known to occur slowly.13 To address this issue, a nationwide observational, registry-based study of current practice is attractive because registries are equipped to monitor rare diseases. Further, surgical registry-based studies may capture trends in disease characteristics in addition to surgical effectiveness and risks.
The primary aim of this nationwide registry-based study was to explore trends in surgical treatment of LGG patients in Sweden during the last decade (2005-2015). The secondary aims were (i) to investigate differences in patient and disease characteristics in relation to surgical strategy, and (ii) to investigate clinical outcomes over time following different surgical strategies.
Materials and Methods
The Registry
The Swedish Brain Tumor Registry (SBTR) is a regionally based registry covering data from 1999 and onward of adult patients diagnosed with brain tumors. The Swedish health care system is divided into 6 regions that each provide neurosurgical care to patients with tumors in the central nervous system. All regions report data to the SBTR, but the level of coverage from the different regions has varied somewhat over time. In our study, a minimum registration rate of 80% was required to be included in the analysis at any given year for each region to provide representative population-based data. Registration rate was defined as the percentage of diagnoses in the SBTR that corresponds to diagnoses reported to the compulsory National Cancer Registry. For this reason, in the case of 1 region, only data from 2012-2013 were used, while only data from 2009 and onward were used in the case of another region. For the remaining 4 regions, data inclusion covered the entire time period from 2005 to 2015. There have been revisions of the SBTR during the study period, with the latest revision in 2015, and consequently some variables are missing for certain time periods. For further details of the SBTR, see Asklund et al.14
We analyzed data for adults (≥ 18 years) with a first-time diagnose of supratentorial hemispheric diffuse LGG, defined as WHO grade II astrocytoma, oligoastrocytoma or oligodendroglioma according to the 2007 WHO classification of brain tumors.1 Patients with radiologically suspected LGG only were not included in the present study.
Definition of Variables
In Table 1, the definitions of variables according to the SBTR are provided.
Table 1.
Definitions of Variables
| Variable | Definition |
|---|---|
| Age | Years at time of diagnosis |
| Sex | Male or female |
| Symptoms at Diagnosis | • Asymptomatic (yes/no) • Focal deficit (yes/no) • Seizure (yes/no) • ICP related (yes/no; exemplified with H/A and cognitive deficit) |
| Performance Statusa | 0-4 |
| Date of Imaging Diagnosis | dd.mm.yyyy |
| Main Location According to ICD | C71.1 (frontal), C71.2 (temporal), C71.3 (parietal), C71.4 (occipital), C71.8 (corpus callosum or overlapping sites), C71.9 (not specified) |
| Laterality | Left/right/bilateral |
| Multifocal | Yes/no |
| Largest Diameter of Tumor | < 4 cm |
| 4–6 cm | |
| > 6 cm | |
| MRI Preop | Yes/No |
| Type of Surgery | Biopsy or resection |
| Date of Surgery | dd.mm.yyyy |
| Type of Resection (Surgeon Impression or Image Based) | Partial or radical resection |
| Complication Within 30 Days | Yes/No |
| New Focal Deficit Within 30 Days | Yes/No |
| New Seizure Within 30 Days | Yes/No |
| Any Infection Within 30 Days | Yes/No |
| Any VTE Within 30 Days | Yes/No |
| Any Hematoma Within 30 Days | Yes/No |
| Complication Leading to Reoperation Within 30 Days | Yes/No |
| Date of Discharge from Neurosurgical Department | dd.mm.yyyy |
| Histopathology | SNOMED codes Astrocytoma: 94003, 94203, 94113, 94103 Oligoastrocytoma: 93823b Oligodendroglioma: 94503 |
| Planned oncological treatment | Yes/No |
Abbreviations: H/A, headache; ICD, International Classification of Diseases; ICP, intracranial pressure; MRI, magnetic resonance imaging; preop, preoperatively; SNOMED, Systemized Nomenclature of Medicine-Clinical Terms; VTE, venous thromboembolism; WHO, World Health Organization.
aPerformance status according to WHO.
bFor oligoastrocytoma WHO grade II and oligoastrocytoma grade III, the SNOMED code is similar (92823).
The total number of patients was divided over 3 consecutive time periods (2005-2008, 2009-2012, 2013-2015) with the aim of creating balanced group sizes but also taking into account 2 landmark papers that could influence surgical management during these time periods. In 2008, Smith and colleagues showed that extent of resection of LGGs affected overall survival. In 2012, a comparison between 2 Norwegian centers, 1 favoring early resection and the other favoring biopsy only, showed a large and significant survival benefit for the former.12,15 In addition, we assumed there was a natural delay between the appearance of scientific evidence and subsequent changes in clinical practice, justifying the 3 time periods of 2005-2008, 2009-2012, and 2013-2015. Importantly, these time periods were selected prior to data analysis to avoid data-driven analysis.
Statistics
All analyses were performed with SPSS, version 21.0 (Chicago, IL, USA) or newer. Statistical significance level was set to P < .05. All tests are 2 sided. Central tendencies are presented as means ± standard deviation, or median and interquartile range if skewed. Categorical data were analyzed with the Pearson chi-square test. Comparisons of continuous variables between time periods were analyzed using analysis of variance when normally distributed or Kruskal-Wallis test if skewed. Overall survival is presented as Kaplan-Meier curves and compared using the log-rank test. For all statistical tests, the date of diagnosis was defined as the date of surgery.
Ethics Statement
This study was approved by the regional ethical committee in Västra Götaland region (Dnr: 702–16).
Results
Demographic Data
A total number of 548 patients with LGGs were included, 142 patients (26% of total in entire study period) diagnosed during the time period 2005-2008, 244 patients diagnosed during 2009-2012 (44% of total in entire study period), and 162 patients (30% of total in entire study period) diagnosed during 2013-2015.
Temporal Trends
Crude incidence was calculated for the 3 time periods using population data from Statistics Sweden (Swedish public authority on statistics, www.scb.se, accessed February 20, 2018). The incidence as calculated from our cases was 0.78/100 000 in 2005-2008, 0.94/100 000 in 2009-2012, and 0.79/100 000 in 2013-2015.
Patient, tumor, and treatment characteristics are presented in detail in Table 2.
Table 2.
Baseline Characteristics and Factors Related to Surgical Treatment
| 2005-2008 n = 142 |
2009-2012 n = 244 |
2013-2015 n = 162 |
P-Value | |
|---|---|---|---|---|
| Age, Mean (SD) | 47.0 (15.7) | 47.0 (14.4) | 45.2 (15.2) | .43 |
| Female, n (%) | 73 (51.4) | 108 (44.3) | 64 (39.5) | .12 |
| Tumor Size, n (%) | N = 116 | N = 241 | N = 126 | .73 |
| < 4 cm | 51 (44.0) | 96 (39.8) | 47 (37.3) | |
| 4-6 cm | 46 (39.7) | 95 (39.4) | 50 (39.7) | |
| > 6 cm | 19 (16.4) | 50 (20.7) | 29 (23.0) | |
| MRI Preop, n (%) | 132 (93.6) N = 141 |
236 (96.7) | 157 (96.9) | .25 |
| Main Lobe Involved, n (%) | .19 | |||
| Frontal | 66 (46.5) | 127 (52.0) | 94 (58.0) | |
| Temporal | 40 (28.2) | 56 (23.0) | 28 (17.3) | |
| Parietal | 16 (11.3) | 31 (12.7) | 24 (14.8) | |
| Occipital | 5 (3.5) | 10 (4.1) | 5 (3.1) | |
| Overlapping Sites | 5 (3.5) | 6 (2.4) | 3 (1.9) | |
| Not Specified | 10 (7.0) | 14 (5.7) | 8 (4.9) | |
| Laterality, n (%) | N = 138 | N = 242 | N = 160 | .19 |
| Left | 72 (52.2) | 120 (49.6) | 85 (53.1) | |
| Right | 65 (47.1) | 110 (45.5) | 66 (41.3) | |
| Bilateral | 1 (0.7) | 12 (5.0) | 9 (5.6) | |
| Multifocal, n (%) | 15 (10.6) | 20 (8.2) N = 243 |
23 (14.2) | .16 |
| Asymptomatic, n (%) | 7 (6.1) N = 115 | 20 (8.2) | 10 (6.2) | .66 |
| Focal Deficit, n (%) | 44 (31.7) N = 139 |
92 (38.0) N = 242 |
64 (41.8) N = 153 |
.19 |
| Seizures, n (%) | 79 (68.1) N = 116 |
160 (66.1) N = 242 |
99 (64.7) N = 153 |
.84 |
| ICP Related, n (%) | 26 (22.8) N = 114 |
51 (21.1) N = 242 |
49 (32.0) N = 153 | .04 |
| Performance status, n (%) | N = 140 | N = 238 | N = 157 | .03 |
| 0 | 88 (62.9) | 143 (60.1) | 69 (43.9) | |
| 1 | 28 (20.0) | 62 (26.1) | 48 (30.6) | |
| 2 | 17 (12.1) | 25 (10.5) | 31 (19.7) | |
| 3 | 5 (3.6) | 6 (2.5) | 6 (3.8) | |
| 4 | 2 (1.4) | 2 (0.8) | 3 (1.9) | |
| Weeks from Imaging to Surgery, Median (IQR) | 4 (2-8) | 4 (2-12) | 7 (3-15) | .002 |
| Surgery Performed, n (%) | N = 140 | N = 244 | N = 158 | .08 |
| Biopsy | 50 (35.7) | 63 (25.8) | 41 (25.9) | |
| Resection | 90 (64.3) | 181 (74.2) | 117 (74.1) | |
| Radical Resectiona, n (%) | N = 90 40 (44.4) |
N = 181 97 (53.6) |
N = 117 43 (36.8) |
.02 |
| Histopathology, n (%) | <.001 | |||
| Astrocytoma | 90 (63.4) | 112 (45.9) | 71 (43.8) | |
| Oligoastrocytoma | 35 (24.6) | 19 (7.8) | 22 (13.6) | |
| Oligodendroglioma | 17 (12.0) | 113 (46.3) | 69 (42.6) | |
| Postop Complication, n (%) | 23 (16.2) | 64 (26.2) | 46 (28.4) | .03 |
| Postop New Neurological Deficit, n (%) | 16 (17.8) N = 90 |
38 (16.5) N = 231 |
34 (21.0) N = 162 |
.51 |
| Postop New Seizure, n (%) | 1 (1.1) N = 90 |
5 (2.2) N = 230 |
5 (3.1) N = 162 |
.60 |
| Postop Infection, n (%) | 2 (1.7) N = 115 |
8 (3.5) N = 231 |
4 (2.5) N = 162 |
.63 |
| Postop VTE, n (%) | 2 (1.7) N = 115 |
4 (1.7) N = 231 |
5 (3.1) N = 162 |
.62 |
| Postop Hematoma, n (%) | 6 (5.2) N = 115 |
12 (5.2) N = 231 |
8 (4.9) N = 162 |
.99 |
| Reoperation due to Complication, n (%) | 6 (6.7) N = 90 |
15 (6.5) N = 230 |
7 (4.3) N = 162 |
.61 |
| Neurosurgical Ward, Days (IQR) | 5 (3-7) | 4 (3-6) | 4 (3-6) | .04 |
| Planned Oncological Treatment, n (%) | 51 (86.4) N = 59 |
170 (70.2) N = 242 |
110 (69.6) N = 158 |
.03 |
| 30-Day Mortality, n (%) | 2 (1.4) | 3 (1.2) N = 243 |
2 (1.2) | .99 |
| 1-Year Mortality, n (%) | 23 (16.2) | 25 (10.3) N = 243 |
14 (8.6) | .09 |
Abbreviations: ICP, intracranial pressure; IQR, interquartile range; MRI, magnetic resonance imaging; postop, postoperative; preop, preoperatively; VTE, denotes venous thromboembolism.
aRadical resection according to surgeon or imaging, with imaging being more common in later years.
When data are missing, the actual N is provided in individual cells.
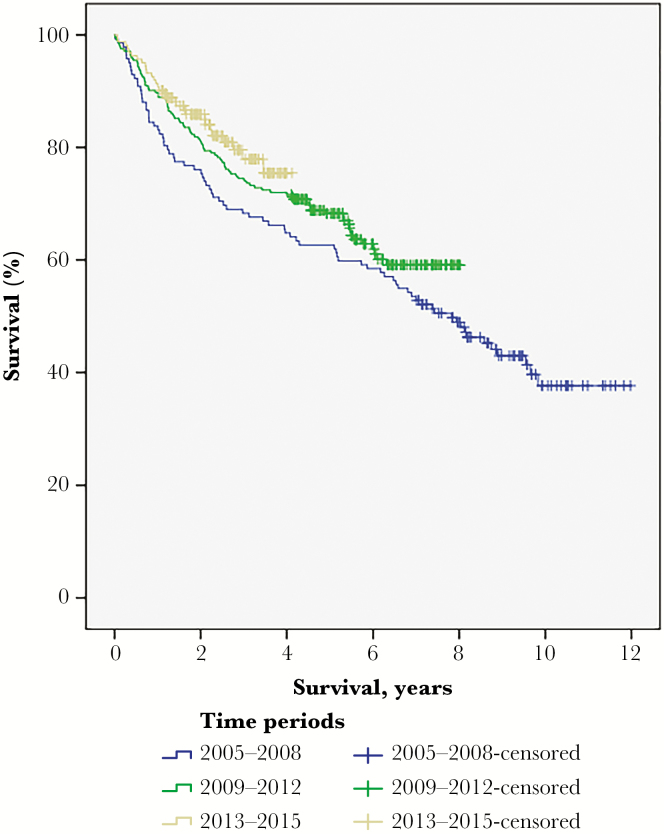
As shown, the more recently treated patients more often had intracranial pressure (ICP)-related preoperative symptoms, lower performance status, and longer time intervals between radiological diagnosis and surgery. In addition, oligodendroglial histology was more frequent. Postoperative complications were more frequent in more recent time periods, but hospital stay in neurosurgical departments was shorter and patients were less often selected for immediate postoperative adjuvant oncological treatment. Tumor resection (as opposed to biopsy) in the different time periods was performed in 64.3% in 2005-2008, 74.2% in 2009-2012, and 74.1% in 2013-2015 (P = .08). There was no survival difference between time periods (Fig. 1).
Fig. 1.
Overall Survival in Different Time Periods. Median survival was 7.8 years (95% confidence interval 6.2-9.4) in the period 2005-2008, but was not reached in the other time periods (log-rank P = .11).
Sensitivity Analysis
To rule out that the results were caused by practice differences among different regions, we performed a sensitivity analysis leaving out the 2 regions that had not provided complete data covering all 3 time periods. As illustrated in Supplementary Table 1, the sensitivity analysis did not significantly alter the main findings presented in Table 2. However, in this analysis we found that resection was preferred over biopsy in 90/140 patients (64.3%) in 2005-2008, 151/191 patients (77.4%) in 2009-2012, and 92/122 patients (75.4%) in 2013-2015 (P = .02).
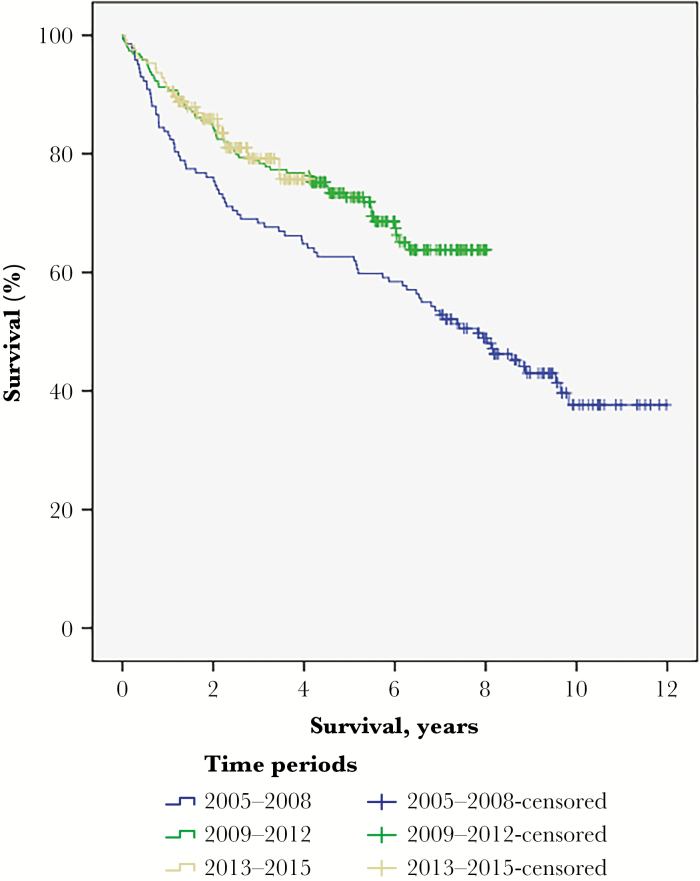
Based on the findings in the sensitivity analysis, we also analyzed survival in the 4 centers contributing data during the entire study period. As shown in Fig. 2, this post-hoc analysis demonstrates that survival improved in the 2 recent time periods. This improvement in survival over time was observed without any increase of patients selected for immediate adjuvant oncological therapy. To exclude the impact of lead-time bias due to upfront surgery, we also analyzed survival from the time point of radiological diagnosis but this did not alter the results (data not shown, log-rank P = .02).
Fig. 2.
Overall Survival in Different Time Periods in the 4 Centers Contributing with Data During the Entire Study Period. Median survival was 7.8 years (95% confidence interval 6.2-9.4) in the period 2005-2008, but was not reached in the other time periods (log-rank P = .04).
Biopsy Only vs Resection
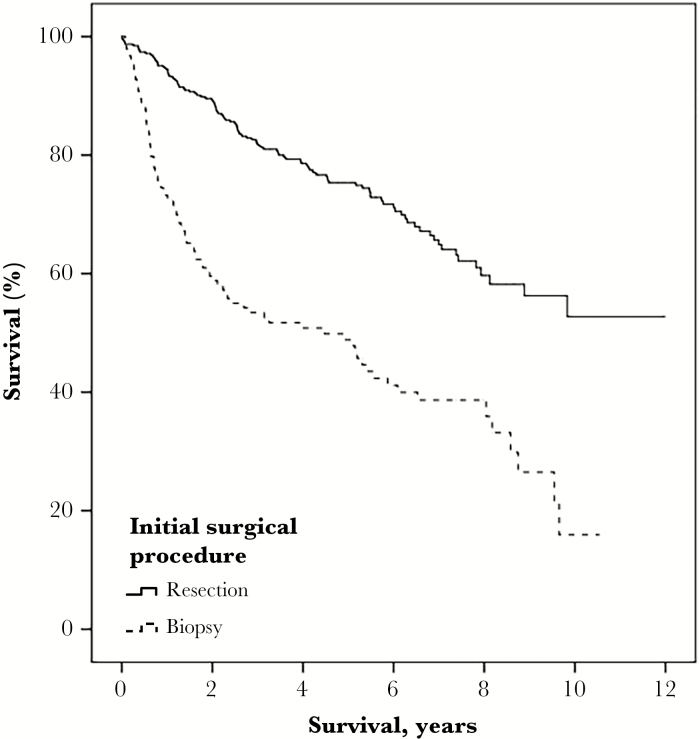
We also explored differences between patients who had undergone a biopsy (n = 154) compared with those who had undergone a resection (n = 388) without stratification into different time periods. The result of this analysis is presented in detail in Table 3. To summarize, patients undergoing biopsy were older, more often had bilateral or multifocal LGGs, and more seldom frontal or temporal tumor location, more focal deficits, and lower performance status. In the biopsy group there were also more astrocytomas, fewer complications, shorter hospital stays in neurosurgical units, and impaired 1-year survival. Survival analysis comparing biopsy and resection showed a median survival of 4.5 years (95% confidence interval 2.2-6.8) following biopsy while median survival was not reached for the group with resection (P < .001) (Fig. 3).
Table 3.
Differences Between Patients Undergoing Initial Biopsy or Resection (n = 542, 6 Cases Missing)
| Biopsy (N = 154) | Resection (N = 388) | P-Value | |
|---|---|---|---|
| Age, Mean (SD) | 53.0 (14.9) | 44.0 (14.2) | <.001 |
| Female, n (%) | 76 (49.4) | 168 (43.3) | .20 |
| Year of Treatment, Median (IQR) | 2010 (2008-2013) | 2011 (2009-2013) | .09 |
| Tumor Size, n (%) | N = 130 | N = 349 | .10 |
| < 4 cm | 59 (45.4) | 134 (38.4) | |
| 4-6 cm | 41 (31.5) | 148 (42.4) | |
| > 6 cm | 30 (23.1) | 67 (19.2) | |
| Main Lobe Involved, n (%) | <.001 | ||
| Frontal | 63 (40.9) | 219 (56.4) | |
| Temporal | 29 (18.8) | 94 (24.2) | |
| Parietal | 24 (15.6) | 47 (12.1) | |
| Occipital | 8 (5.2) | 12 (3.1) | |
| Overlapping Sites | 8 (5.2) | 6 (1.6) | |
| Not Specified | 22 (14.3) | 10 (2.6) | |
| Laterality, n (%) | N = 149 | N = 385 | <.001 |
| Left | 77 (51.7) | 195 (50.6) | |
| Right | 58 (38.9) | 182 (47.3) | |
| Bilateral | 14 (9.4) | 8 (2.1) | |
| Multifocal, n (%) | 34 (22.1) | 23 (5.9) N = 387 |
<.001 |
| Bilateral OR Multifocal, n (%) | 38 (24.7) | 25 (6.5) N = 387 |
<.001 |
| Asymptomatic, n (%) | 7 (5.0) N = 141 |
29 (7.8) N = 374 |
.27 |
| Focal Deficit, n (%) | 76 (50.0) N = 152 |
123 (32.7) N = 376 |
<.001 |
| Seizures, n (%) | 86 (61.0) N = 141 |
246 (67.6) N = 364 |
.16 |
| ICP-Related Symptoms, n (%) | 34 (24.3) N = 140 |
90 (24.8) N = 363 |
.91 |
| Performance Status, n (%) | N = 149 | N = 380 | .001 |
| 0 | 67 (45.0) | 229 (60.3) | |
| 1 | 44 (29.5) | 93 (24.5) | |
| 2 | 24 (16.1) | 48 (12.6) | |
| 3 | 11 (7.4) | 6 (1.6) | |
| 4 | 3 (2.0) | 4 (1.1) | |
| Weeks from Imaging to Surgery, Median (IQR) | 4 (2-8.75) N = 152 |
5 (3-12) N = 384 |
.10 |
| Histopathology, n (%) | <.001 | ||
| Astrocytoma | 120 (77.9) | 152 (39.2) | |
| Oligoastrocytoma | 11 (7.1) | 171 (44.1) | |
| Oligodendroglioma | 23 (14.9) | 65 (16.8) | |
| Postop Complication, n (%) | 14 (9.1) | 117 (30.2) | <.001 |
| Postop New Neurological Deficit, n (%) | 8 (6.2) N = 130 |
78 (22.5) N = 347 |
<.001 |
| Postop New Seizure, n (%) | 1 (0.8) N = 130 |
10 (2.9) N = 346 |
.17 |
| Postop Infection, n (%) | 3 (2.1) N = 141 |
11 (3.0) N = 361 |
.57 |
| Postop VTE, n (%) | 3 (2.1) N = 141 |
7 (1.9) N = 361 |
.89 |
| Postop Hematoma, n (%) | 3 (2.1) N = 141 |
23 (6.4) N = 361 |
.05 |
| Reoperation due to Complication, n (%) | 5 (3.8) N = 130 |
23 (6.6) N = 346 |
.25 |
| Neurosurgical Ward, Days (IQR) | 3 (2-5) | 5 (3-6) | <.001 |
| Planned Adjuvant Oncological Treatment, n (%) | 100 (87.0) | 231 (67.2) | <.001 |
| 30-day Mortality, n (%) | 2 (1.3) N = 153 |
5 (1.3) | .99 |
| 1-Year Mortality, n (%) | 41 (26.8) N = 153 |
21 (5.4) | <.001 |
Abbreviations: ICP, intracranial pressure; IQR, interquartile range; Postop, postoperative; VTE, venous thromboembolism.
When data are missing, the actual N is provided in individual cells.
Fig. 3.
Kaplan-Meier Survival, Biopsy vs Resection. Median survival was not reached for resection, but was 4.5 years (95% confidence interval 2.2-6.8) following biopsy (P < .001).
Discussion
In this nationwide registry-based study spanning from 2005 to 2015, we demonstrated a trend, albeit nonsignificant, toward more resections during the more recent time periods. Interestingly, the analysis that included only centers contributing data for all time periods showed that surgical resection was more common from 2009 and onward.
In addition, we found a higher proportion of patients who had lower performance level and ICP-related symptoms prior to operation during the most recent years. There was also a longer time interval between radiological diagnosis and surgery, a higher frequency of complications, but earlier discharge from neurosurgical units during this period. Our data on the preoperative parameters for patients who had resections compared with those who underwent biopsy demonstrate that patients selected for biopsy were older, had more often unresectable (ie, bilateral or multifocal) tumors, and were in worse clinical condition.
Another interesting observation was that patients selected for surgery had lower performance status in the later period. This may suggest that while “easier” surgical cases were judged amenable for resection in all time periods, the observed increase during the recent periods was an increase in more demanding cases. This may also explain the nonsignificant increase in neurological deficits seen in the later time period, although a change in surgical attitude may be equally important. The longer time interval between detection and surgery seen in the later years could be related to more extensive diagnostic workups, also reflecting more complicated surgical cases. Finally, the observed drop in radical resection over the years may have a similar explanation. Another possible explanation is the more frequent use of postoperative MRI in the recent time periods while during earlier periods the extent of resection was more often based on the surgeon’s subjective impression, which may often be too optimistic.16
Another notable finding was a conspicuous increase over time in the proportion of LGGs with oligodendroglial histology. If this indeed represents a true increase, and not just an artifact in classification, it would naturally influence survival.17 A similar shift in classification in favor of oligodendroglial tumors at the expense of pure astrocytic tumors has, however, been described previously in other studies,18–21 albeit mostly earlier than in our material. Because the histological tumor classification used in the SBTR, as in the abovementioned studies, is based solely on histological appearance without incorporation of the molecular tumor status, the classification is vulnerable to interobserver variability.22 Also, no central review of pathology was possible in this registry-based study. Altogether, we believe it is unlikely that the observed increase in oligodendroglial tumors reflects a true shift in histological subtypes in a population-based cohort like the one presented in this study. A more plausible explanation is that an increased awareness of oligodendroglial tumors, because of their favorable therapeutic response and prognosis, is lying behind this classification drift.20,23
The secondary aim of this study was to explore survival, and we found no statistically significant differences in survival among the 3 time periods. However, post hoc analysis of data from the 4 centers that provided data from the entire study period revealed an improvement in survival during the most recent period. We think that the increased survival during recent years is unlikely to be explained by lead-time bias due to earlier radiological diagnosis (more widespread MRI) since there was no observed increase of asymptomatic patients (eg, incidental LGG) in the later time period. Thus, although no causality can be claimed from an observational study, we believe that the longer survival may reflect the observed change in surgical strategy in the corresponding time periods, consonant with the literature indicating that surgical resection improves survival.6
Apart from survival, functional outcome is another very important factor of risk-benefit of LGG surgery. The observed postoperative neurological deficits in approximately 20% of all patients may seem unacceptable, but it should be noted that the SBTR neither discriminates immediate transient deficits from permanent ones, nor minor from major deficits. In light of this, the results on postoperative outcome presented here may well be comparable to the literature, in which the frequency of “any deficit” at early assessment was reported 30.3%.24 Nevertheless, the occurrence of postoperative neurological deficits is an obvious risk of a more active surgical attitude and this important aspect needs to be studied in greater detail by methods directly evaluating function (eg, neuropsychology, functional tests) and patient-reported outcomes.
Not surprisingly, we demonstrate that the selection of patients for either biopsy or resection is not random, bearing in mind that not all patients are suitable for major surgery. Hence, case series comparing the impact of surgical treatment on outcome are seemingly studies of selection bias rather than of treatment effectiveness.25 Accordingly, we found an accumulation of negative preoperative prognostic factors in the group of patients selected for biopsy. In the biopsy group, there were also 78% astrocytomas compared to 39% in the resection group, although this difference may be somewhat influenced by the more common resections in recent time periods during which LGGs were more frequently classified as oligodendrogliomas. While the 30-day mortality was similar in the 2 groups, a marked difference was observed in 1-year mortality: 26.8% following biopsy and 5.4% following resection. Thus, 1-year mortality was almost 5 times higher in the biopsy group but, as argued previously, this probably related to the multiple negative preoperative prognostic factors in this group.
There have been limited data on patterns of surgical care of LGGs during the recent decade; however, some data from earlier time periods can be used for comparison. In a large North American registry-based study of astrocytomas (1999-2010), no consistent change over time in surgical strategy could be seen when measured as the likelihood for undergoing gross total resection (GTR).26 In a similar study based on the same registry (The Surveillance, Epidemiology, and End Results program), the survival and surgical care for patients with oligodendrogliomas was analyzed over time (1999-2012).27 The survival benefit over time observed in WHO grade III oligodendrogliomas was not seen for WHO grade II oligodendrogliomas. The pattern of surgical practice for WHO grade II oligodendrogliomas was largely unaltered during 1999-2008, but thereafter the proportion of GTRs decreased. The discordance between these studies and our study might be due to their somewhat earlier time setting. There is also a difference in categorization of surgical strategy whereby biopsies only are not separated from subtotal resections, when in fact the latter may often be synonymous with what the surgeon perceives as maximal safe resection.
Limitations of this Study
Our study has several limitations related to the observational design. In the SBTR, only tumors verified by histology are included. Thus, the true incidence of LGGs in the different regions is unknown. Radiological suspicion of an LGG does not automatically warrant surgical intervention, as this decision may depend on severe comorbidity, old age, critically located tumors or patients’ specific wishes. Another limitation is the lack of molecular data on the tumor subtypes, as a result of the older WHO classification that was used during the inclusion period. To reduce interobserver variability, a central review of pathology would be optimal, although this was not feasible in this registry-based study.
At the time of the study, the SBTR still lacked more detailed information on the type and the date of postoperative adjuvant treatment. As a result, we considered only whether patients were “high risk” and thus selected for immediate oncological treatment (a decision typically made at the first multidisciplinary team conference when histopathological diagnosis was available), but we cannot account for the type of treatment in terms of radiotherapy, chemotherapy or combined radiochemotherapy. However, the national guidelines concerning adjuvant treatment were not changed until after the inclusion period as a result of the study by Buckner et al from 2016.28 In sum, this argues against a significant impact of adjuvant therapy on the survival benefit observed in the present study.
Further shortcomings of this study include the variability of registration coverage over time and across regions, which we addressed in the post-hoc analysis. Strengths include the population-based data acquired through standardized, consecutive, and prospective reporting.
Conclusion
The approach to surgical management of LGGs in Sweden during the past decade seems to have drifted in favor of more active strategy, even for patients with lower performance status and presumably more complex tumors. This more active surgical strategy may be related to the observed improved survival during recent years.
Funding
This work was supported in part by grants from the Göteborg Medical Society (Göteborgs Läkaresällskap) and the Agreement Concerning Research and Education of Doctors (Avtal om läkarutbildning och forskning) [ALFGBG-695611] and the Swedish Research Council (Vetenskapsrådet) [2017-00944].
Acknowledgments
This project was made possible by the continuous work of the Swedish Brain Tumor Registry (SBTR), Roger Henriksson (chair), Thomas Asklund, Annika Malmström, Lena Damer, Lena Rosenlund, Rickard Sjöberg, Sofia Hylin, Peter Milos, Thomas Blystad, Sara Kinhult, Göran Hesselager, Petra Witt Nyström, Katja Werlenius, Asgeir S. Jakola, Gregor Tomasevic, Magnus Olivecrona, Margret Jensdottir, Michael Bergqvist, Marie Sjögren, Eskil Degsell, Linnea Nilsson, Kerstin Rehn, Kristina Lundqvist, and Lisa Tykosson.
Conflict of interest statement
None declared.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. [DOI] [PubMed] [Google Scholar]
- 3. Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. [DOI] [PubMed] [Google Scholar]
- 4. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. [DOI] [PubMed] [Google Scholar]
- 5. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jakola AS, Skjulsvik AJ, Myrmel KS, et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weller M. Surgery for patients with ‘lower grade’ glioma: putting assumptions, beliefs and convictions into perspective. Ann Oncol. 2018;29(4):1077. [DOI] [PubMed] [Google Scholar]
- 8. Seiz M, Freyschlag CF, Schenkel S, et al. Management of patients with low-grade gliomas—a survey among German neurosurgical departments. Cent Eur Neurosurg. 2011;72(4):186–191. [DOI] [PubMed] [Google Scholar]
- 9. Capelle L, Fontaine D, Mandonnet E, et al. ; French Réseau d’Étude des Gliomes Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 10. Hayhurst C. Contemporary management of low-grade glioma: a paradigm shift in neuro-oncology. Pract Neurol. 2017;17(3):183–190. [DOI] [PubMed] [Google Scholar]
- 11. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764; discussion 264–266. [DOI] [PubMed] [Google Scholar]
- 12. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 13. Johnston SC. Leaving tiny, unruptured intracranial aneurysms untreated: why is it so hard?JAMA Neurol. 2018;75(1):13–14. [DOI] [PubMed] [Google Scholar]
- 14. Asklund T, Malmström A, Bergqvist M, Björ O, Henriksson R. Brain tumors in Sweden: data from a population-based registry 1999-2012. Acta Oncol. 2015;54(3):377–384. [DOI] [PubMed] [Google Scholar]
- 15. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 16. Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109(5):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 18. Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006;106(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 19. Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015;38(1):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCarthy BJ, Propp JM, Davis FG, Burger PC. Time trends in oligodendroglial and astrocytic tumor incidence. Neuroepidemiology. 2008;30(1):34–44. [DOI] [PubMed] [Google Scholar]
- 21. Youland RS, Schomas DA, Brown PD, et al. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15(8):1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79(7):1381–1393. [DOI] [PubMed] [Google Scholar]
- 23. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 24. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. [DOI] [PubMed] [Google Scholar]
- 25. Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011;115(5):948–965. [DOI] [PubMed] [Google Scholar]
- 26. Dong X, Noorbakhsh A, Hirshman BR, et al. Survival trends of grade I, II, and III astrocytoma patients and associated clinical practice patterns between 1999 and 2010: a SEER-based analysis. Neurooncol Pract. 2016;3(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brandel MG, Alattar AA, Hirshman BR, et al. Survival trends of oligodendroglial tumor patients and associated clinical practice patterns: a SEER-based analysis. J Neurooncol. 2017;133(1):173–181. [DOI] [PubMed] [Google Scholar]
- 28. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]