Abstract
Zinc deficiency and excess can result in adverse health outcomes. There is conflicting evidence regarding whether excess or deficient zinc in the diet can contribute to carcinogenicity. The objective of this study was to characterize zinc carbonate basic for use as a source of dietary zinc in a rodent toxicity and carcinogenicity study investigating the effects of zinc deficiency and excess. Because of the complex chemistries of zinc carbonate basic compounds, inconsistent nomenclature, and literature and reference spectra gaps, it was necessary to employ multiple analytical techniques, including Karl Fischer titration, combustion analysis, inductively coupled plasma–optical emission spectrometry, X-ray diffraction, infrared spectroscopy, X-ray fluorescence spectrometry, and thermogravimetric analysis to characterize the test article. Based on the collective evidence and through the process of elimination, the test article was found to be composed mainly of zinc carbonate basic with zinc oxide as a minor component. The zinc content was determined to be 56.6% (w/w) with heavy metals such as arsenic, cadmium, mercury and lead below the limit of quantitation of less than or equal to 0.01%. The test material was stable at ambient temperature. Based on the work described in this manuscript, the test article was suitable for use as a source of zinc in studies of deficiency and excess in the diet.
Keywords: characterization, zinc carbonate basic, zinc deficiency, zinc fortification, zinc oxide synthesis
INTRODUCTION
Zinc is an essential element in humans and animals that plays a critical role in many diverse biological processes, and is present in more than 300 metalloenzymes involved in catalysis, structure, and ion regulation (Chasapis et al. 2012, Corbo and Lam 2013). Zinc deficiency impacts one-fifth of the world’s population and is a significant factor in the genesis and progression of chronic disease, including diabetes (Khalid et al. 2014, Lindenmayer, Stoltzfus, and Prendergast 2014, Agarwal et al. 2013). The role of zinc in protein and transcription factors involved in DNA damage response and repair have led to reports hypothesizing that deficiency is linked to increased DNA damage (Ho 2004, Yan et al. 2008). No direct link has been established between chronic zinc deficiency, DNA damage, and carcinogenicity. However, some animal studies have shown that dietary zinc deficiency can result in early proliferative changes in the esophageal tissue and there have been isolated reports of esophageal cancer in rodents (Barney, Orgebin-Crist, and Macapinlac 1968, Swenerton and Hurley 1968, Diamond, Swenerton, and Hurley 1971, Newberne, Schrager, and Broitman 1997). More established is the influence that dietary zinc deficiency has on the carcinogenicity of other known carcinogens such as methylbenzylnitrosamine or N-nitrosomethylbenzylamine (Schrager et al. 1986, Barch and Fox 1987, Newberne, Schrager, and Broitman 1997, Lin et al. 1976). Human epidemiological studies have also identified an association between dietary zinc deficiency and the development of gastrointestinal cancers, although many of these studies show inconsistent associations (Li et al. 2014). Zinc deficiency has also been linked with many other adverse health outcomes, including spatial learning and memory loss, depression-like symptoms, suppressed immune function, impaired reproductive capacity, and poor maternal and neonatal health (Bruno et al. 2007, Raqib et al. 2007, Tassabehji et al. 2008, Tahmasebi Boroujeni et al. 2009, Dumrongwongsiri et al. 2015, Wang et al. 2015).
Excess dietary zinc can also result in adverse health outcomes, including copper deficiency and anemia, as well as gastrointestinal and hematopoietic system effects depending on the source of dietary zinc (Broun et al. 1990, CDC 1983, Fischer, Giroux, and L’Abbé 1984, Moore 1978, Porter et al. 1977, Prasad et al. 1978, Salzman, Smith, and Koo 2002, Samman and Roberts 1987, Yadrick, Kenney, and Winterfeldt 1989). The importance of zinc homeostasis has spurred many investigations of the agrochemical factors associated with zinc deficiency, the therapeutic administration for disease treatment and control, and food fortification with various zinc-containing compounds (Alloway 2009, Brewer 2014, Impa et al. 2013, Della Lucia et al. 2014).
In the current literature, there is conflicting evidence regarding whether excess or deficient zinc in the diet can contribute to carcinogenicity. The variability in the literature can be associated with many factors, including differences in bioavailability of the different dietary sources of zinc, the interaction of zinc with other trace elements (e.g., calcium, copper, cadmium, iron) or dietary fiber (i.e., phytate), animal ages in toxicology studies, and the duration and levels of exposure. For example, the LD50 ranges from 186 to 623 mg/kg in rats and mice, depending on which form of zinc was evaluated (i.e., zinc acetate, zinc nitrate, zinc chloride, and zinc sulfate) (Domingo et al. 1998).
The potentially high bioavailability and the palatability of zinc carbonate basic compounds when mixed at relevant doses with rodent diet contributed to their selection as the test article in a zinc-deficiency toxicological investigation (Du et al. 2006). These compounds have a variety of industrial applications and have been used as drilling fluid additives (Cameron 2005), used in respirators (Okutani et al. 1993, Zhang, Fortier, and Dahn 2004), and as synthetic precursors for reactive metal oxide fine particles (Said et al. 1990, Koga and Tanaka 2005) for use in solar cells, cosmetic products, gas sensors, paints, pigments, and a variety of other products (Kanari et al. 2004, Feng et al. 2005, Cao et al. 2009, Du, Liu, and Chen 2009, Zhang et al. 2009, Anžlovar et al. 2015).
Multiple zinc carbonate basic compounds have been described in the literature as hydrozincate (Zn5(CO3)2(OH)6) (Koga and Tanaka 2005), zinc carbonate hydroxide ((Zn5(CO3)2(OH)6) (Yamada, Tsukumo, and Koga 2009), basic zinc carbonate ([ZnCO3]2·[Zn(OH)2]3) (Zhang, Chi, and Li 2002, Sadeek and Refat 2005), basic zinc carbonate (Zn4CO3(OH)6) (Feng et al. 2005), hydrozincite (Zn5(CO3)2(OH)6) (Stoilova, Koleva, and Vassileva 2002a), and as many other compounds. These inorganic compounds are complex and have inconsistent nomenclature, and knowledge about them remains imperfect and fragmentary (Jambor 1963, Bitenc, Marinšek, and Crnjak Orel 2008, Alhawi et al. 2015). Multiple synthetic routes employing an array of reagents and preparation procedures have been reported in the literature (Zhang, Fortier, and Dahn 2004, Sadeek and Refat 2005, Frost and Hales 2007, Wahab et al. 2008, Zhang et al. 2009). In addition, subtle differences in the synthesis of zinc carbonate basic can lead to the presence of different test chemical impurities (Sawada, Murakami, and Nishide 1996, Musić, Dragčević, and Popović 2007). Therefore, complex chemistry and potential impurities require thorough characterization of a test article prior to use in toxicology studies investigating effects of zinc deficient and excess diets in rodent models.
The objective of this investigation was to characterize a zinc carbonate basic test article and assess its stability under appropriate storage conditions to confirm that it was an acceptable source of dietary zinc in rodents for use in studies of the toxicity and carcinogenicity of deficient or excess zinc in the diet. Because of the complexity of the test article, differences in nomenclature, and the general lack of reference spectra, thorough characterization required the use of multiple analytical techniques. A combination of Fourier transform infrared (FTIR) spectrometry and X-ray diffraction spectrometry (XRD) gathered critical structural information through comparison of observed vibration and diffraction patterns against those obtained from known compounds. Karl Fischer titration was used to determine water content and combustion was employed to determine carbon and hydrogen content. X-ray fluorescence (XRF) and inductively coupled plasma–optical emission spectrometry (ICP-OES) were used for qualitative and quantitative elemental analyses, respectively, including the determination of zinc content, and thermogravimetric analysis (TGA) was used to assess chemical changes as a function of increasing temperature. Taken collectively, data from these platforms were critical in characterizing the test article and ensuring that it was a suitable source of dietary zinc for the proposed rodent studies.
MATERIALS AND METHODS
Test Chemicals and Procured Compounds
The zinc carbonate basic test article (58% zinc) was received from Sigma-Aldrich (St. Louis, MO, USA) at RTI International. Representative aliquots were removed upon receipt and stored in a freezer (nominal −20 °C) for use as reference samples to assess stability; the remaining bulk was stored at ambient temperature per vendor recommendation. Zinc oxide (Sigma-Aldrich) and zinc carbonate hydroxide (Strem Chemicals, Newburyport, MA, USA) were also purchased to aid in the identification of a potential impurity and to aid in characterization as a structurally similar reference compound to the test article, respectively.
Analytical Methods for Characterization
X-ray Diffraction
Aliquots of the zinc carbonate basic test article, the zinc carbonate basic frozen reference, and the zinc oxide and zinc carbonate hydroxide samples were analyzed using a Shimadzu XRD-6000 instrument (Kyoto, Japan). Aliquots were transferred to aluminum holders, flattened using a circular motion to compress and avoid preferred orientation of particles, analyzed with a diffraction angle (2-theta) range of between 5 and 65 degrees and a step size of 0.02 degrees. XRD results were searched against a database of nearly 300,000 known standards leased from the International Center for Diffraction Data (ICDD; Newtown Square, PA, USA), Powder Diffraction File (PDF4+).
Fourier Transform Infrared Spectrometry
FTIR analyses of the zinc carbonate basic article, the zinc carbonate basic frozen reference, zinc oxide, and zinc carbonate hydroxide were completed by using a nitrogen-purged Thermo Fisher Nicolet 6700 instrument (Waltham, MA, USA) equipped with OMNIC (version 7.3) software. Before conducting analyses, pellets were prepared from approximately 2 mg of sample and 200 mg of potassium bromide. The pellets were prepared under pressure (approximately 5 to 8 tons) for 1 to 3 minutes under a vacuum.
Thermogravimetric Analysis
A TA Instruments TGA Q50 (New Castle, DE) thermogravimetric analysis system equipped with Advantage (version 2.5.0.255) and Universal Analysis 2000 (version 4.3A) operation and processing software was used to analyze the zinc carbonate basic test article and the zinc carbonate basic frozen reference for weight loss as a function of temperature. Approximately 10 mg of the sample were weighed into an aluminum sample pan, which was then transferred to a platinum TGA pan for analysis. Each sample was analyzed in duplicate manner, with the temperature ramping to 400°C at a rate of 5°C/minute, under nitrogen gas balance and sample flow rates of 40.0 mL/min and 60.0 mL/min, respectively.
X-ray Fluorescence Spectrometry
Aliquots of the zinc carbonate basic test article were placed in 32 mm sample cups, sealed with a Mylar sheet and Teflon ring, and qualitatively analyzed with a Thermo ARL Quant’X (Waltham, MA, USA) energy dispersive X-ray fluorescence instrument equipped with WinTrace (version 4.1 Build 9, Patch 1) software. Samples were scanned to determine the presence of elements with atomic weights ranging from sodium to uranium. If an element was determined to be present after the preliminary scan, it was then re-analyzed with a filter condition optimized for that element (i.e., palladium thin, palladium thick, cellulose, and no filter).
Inductively Coupled Plasma–Optical Emission Spectrometry
Concentrations of zinc and a panel of secondary elements (i.e., antimony, arsenic, cadmium, calcium, cobalt, chromium, copper, iron, lead, manganese, mercury, nickel, palladium, platinum, thallium, and tin) were determined in the zinc carbonate basic test article by using a Thermo Jarrell Ash Atomscan-16 ICP-OES instrument (Franklin, MA, USA). The ICP-OES instrument was calibrated with standards prepared from National Institute of Standards and Technology (NIST)–traceable, single element 1,000 µg/mL standards for each element. NIST–traceable, 1,000 µg/mL of internal standard (yttrium) and gold (to stabilize mercury) solutions were obtained from the same vendor. Zinc carbonate basic aliquots were dissolved in high-purity, Ultrex-grade nitric acid (HNO3) from J.T. Baker (Phillipsburg, NJ, USA), and were quantitatively transferred to and brought to volume in 25 mL volumetric flasks with multiple rinses of deionized water (approximately 18 MΩ quality; Pure Water Solutions; Hillsborough, NC, USA). The concentrations were yttrium (5 µg/mL), gold (25 µg/mL), and HNO3 (5% [v/v]). ICP-OES was also used to compare the zinc emission response from aliquots of the test chemical stored at ambient temperature and stored at 60 °C for 15 days to assess stability.
Elemental Analysis, Ion Analysis, and Water Determination
The zinc carbonate basic test article was submitted to Galbraith Laboratories (Knoxville, TN, USA) for the duplicate determination of carbon, hydrogen, and water percentages and to QTI Laboratories (Whitehouse, NJ, USA) for determination of a suite of anions (fluoride, chloride, nitrite, bromide, phosphate, sulfate, and nitrate) and cations (lithium, sodium, potassium, and ammonium). Briefly, for elemental analysis, aliquots of the test chemical were introduced to an elemental analyzer that burned a small portion of sample in an oxygen atmosphere to create combustion by-products for spectroscopic quantitation of carbon and hydrogen. A purge-trap method (approximately 160 °C) followed by a Karl Fischer titration were used to quantify the percentage of water in the samples. It was necessary to employ this purge-trap procedure because conventional Karl Fischer titration reagents alone could not dissolve the test chemical.
Optical Microscopy
Photomicrographs of representative ambient temperature and frozen reference aliquots of the zinc carbonate basic test article were captured to examine the potential differences resulting from different storage conditions. An Olympus QColor 3.0 megapixel digital camera (Minneapolis, MN) equipped with QCapture 2.8.0 software was used to capture images (magnifications of 50x and 200x).
RESULTS AND DISCUSSION
X-ray Diffraction
Diffraction patterns collected from the zinc carbonate basic test article are presented in Figure 1a. The broad hump within these patterns indicates the presence of a significant amount of amorphous material, and the diffraction angle peak at approximately 8 degrees (2-theta) can be attributed to sample holder interference. Collected patterns were searched against the ICDD PDF4+ database for potential matches. It is important to note that significant inconsistencies exist within the XRD reference database literature with respect to zinc carbonate compounds. This perhaps arises from both inconsistent zinc carbonate compound nomenclature and from reference pattern submitters not knowing the exact chemical composition of their submitted reference. As a result, it is not surprising that collected XRD diffraction patterns did not yield a conclusive match as zinc carbonate basic because a definitive reference spectrum does not exist.
Figure 1.
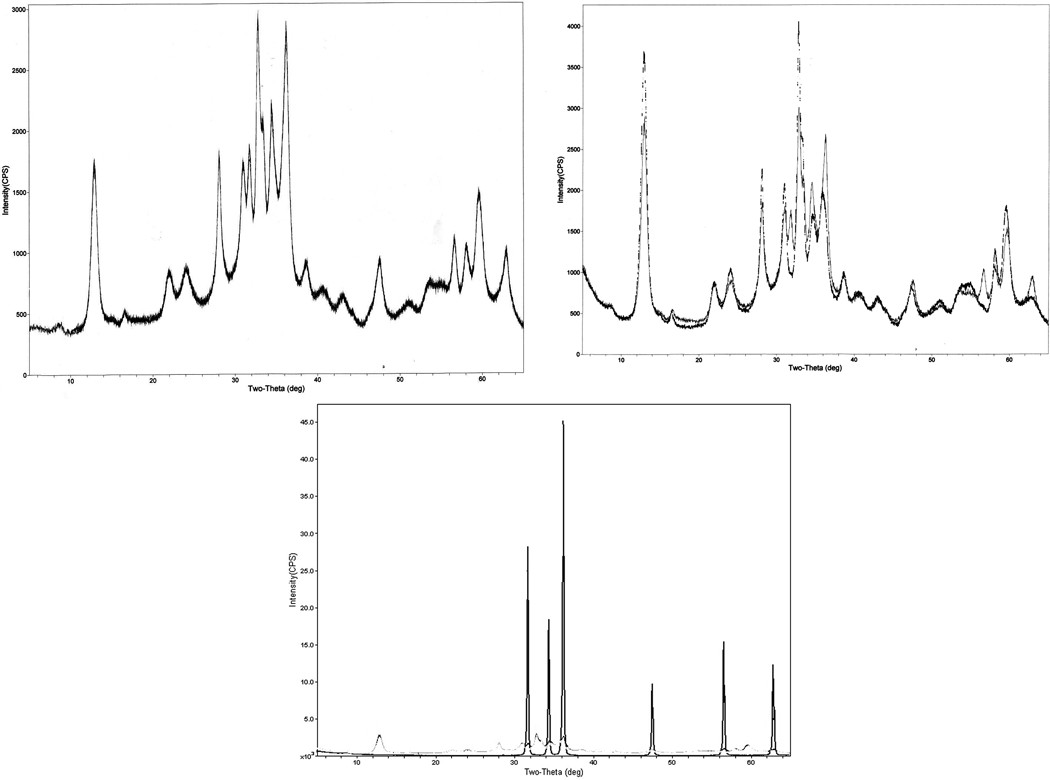
X-ray diffraction pattern from (a) zinc carbonate basic test article, (b) zinc carbonate basic test article (gray) and zinc carbonate hydroxide (black), and (c) zinc carbonate basic test article, zinc carbonate hydroxide, and zinc oxide. Peaks where the test article and zinc carbonate hydroxide differ correspond to zinc oxide peaks.
The database returned 43 possible matches, but only three scored with match figures of merit less than 5.0 (a perfect match has figure of merit equal to 1.0). The database assigns figures of merit as a function of both peak location and relative peak height distribution. These potential matches included zinc oxide (ZnO; PDF4+ reference 03–065-3411; figure of merit = 3.0), zinc hydroxide (Zn(OH)2; PDF4+ reference 00–048-1066; figure of merit = 3.5), and zinc carbonate hydroxide hydrate (Zn4(CO3)2(OH)6·H2O; PDF4+ reference 00–003-0787; figure of merit = 4.0). The ICDD PDF4+ database was also queried to produce the closest possible chemical formula match to zinc carbonate basic and returned reference spectra for both hydrozincite (Zn5(CO3)2(OH)6; PDF4+ reference 00–014-0256) and smithsonite (ZnCO3; PDF4+ reference 00–001-1036). Peak diffraction angles and relative intensities from the zinc carbonate basic lot were closely compared against these five reference compounds (Table 1). Relative peak height distributions for the most abundant zinc hydroxide, zinc carbonate hydroxide hydrate, smithsonite, and hydrozincite reference peaks did not correlate with peaks observed from the zinc carbonate basic test lot. In addition, peaks for each of these reference card pattern did not have any matches with observed peaks collected from the zinc carbonate basic test lot. In contrast, all of the peak diffraction angles for zinc oxide corresponded with matching peak diffraction angles from the test chemical, but relative peak height intensity distributions of the most abundant peaks for zinc oxide did not correlate with the zinc carbonate basic test lot. Taken collectively, these data suggest that zinc oxide is likely present in the sample, but as a possible minor component.
Table 1.
Comparison of zinc carbonate basic test article and reference card X-ray diffraction patterns.
|
Peak # |
Observed ZCBa 2-θ (Height %) |
Zinc Oxide 2-θb (Height %) |
Zinc Hydroxide 2-θb (Height %) |
Zinc Carbonate Hydroxide Hydrate 2-θb (Height %) |
Hydrozincite 2-θb (Height %) |
Smithsonite 2-θb (Height %) |
|---|---|---|---|---|---|---|
| 1 | 12.940 (100.0) | 0.089 (100.0) | ||||
| 2 | 16.560 (5.3) | −0.015 (10.0) | −0.066 (6.0) | |||
| 3 | 22.062 (13.4) | −0.003 (80.0) | 0.144 (13.0) | |||
| 4 | 24.141 (12.2) | −0.154 (80.0) | 0.226 (13.0) | |||
| 5 | 28.099 (48.5) | 0.047 (16.0) | ||||
| 6 | 30.999 (23.3) | 0.048 (20.0) | ||||
| 7 | 31.797 (17.7) | −0.005 (55.2) | 0.045 (59.0) | |||
| 8 | 32.859 (69.4) | −0.061 (100.0) | 0.043 (23.0) | −0.081 (100.0) | ||
| 9 | 33.459 (30.1) | |||||
| 10 | 34.500 (28.7) | −0.060 (39.2) | −0.006 (63.0) | −0.013 (40.0) | ||
| 11 | 36.240 (67.1) | 0.036 (100.0) | 0.086 (100.0) | −0.180 (28.0) | 0.102 (13.0) | |
| 12 | 38.600 (10.8) | 0.030 (16.0) | 0.010 (6.0) | 0.183 (33.0) | ||
| 13 | 40.724 (5.1) | 0.073 (6.0) | ||||
| 14 | 42.996 (6.8) | 0.041 (27.0) | ||||
| 15 | 47.541 (17.1) | 0.019 (20.2) | −0.216 (4.0) | 0.027 (6.0) | ||
| 16 | 50.998 (3.8) | 0.000 (4.0) | ||||
| 17 | 53.540 (6.1) | 0.027 (4.0) | ||||
| 18 | 54.940 (3.0) | |||||
| 19 | 56.600 (15.6) | 0.022 (30.0) | −0.002 (25.0) | |||
| 20 | 58.098 (14.3) | −0.124 (24.0) | ||||
| 21 | 59.620 (33.6) | −0.002 (36.0) | −0.190 (10.0) | |||
| 22 | 62.998 (16.0) | −0.121 (27.2) | −0.108 (26.0) | −0.251 (8.0) | ||
| Unmatched line | 31.802 (59.0) | 29.981 (8.0) | 13.283 (32.0) | 25.136 (66.0) | ||
| Unmatched line | 35.472 (12.0) | 19.936 (6.0) | 46.789 (40.0) | |||
| Unmatched line | 23.267 (3.0) | 51.595 (13.0) | ||||
| Unmatched line | 28.401 (16.0) | 53.887 (80.0) | ||||
| Unmatched line | 29.858 (3.0) | 62.259 (40.0) | ||||
| Unmatched line | 30.591 (100.0) | |||||
| Unmatched line | 39.312 (6.0) | |||||
| Unmatched line | 40.041 (3.0) |
aObserved zinc carbonate basic diffraction (ZCB) angle 2-θ and relative peak intensity (in parentheses) for test lot.
bSignifies relative difference in diffraction angle of reference card for each compound and angle observed from test lot.
Because none of the database reference cards produced an unequivocal match with the observed diffraction pattern from the zinc carbonate basic test lot, zinc carbonate hydroxide with the same nominal formula as the zinc carbonate basic test article (both are [ZnCO3]2·[Zn(OH)2]3) was procured and analyzed by XRD. It was difficult to identify and procure potential reference compounds with the same nominal formula, so only this one additional compound was obtained. Zinc carbonate hydroxide had the same nominal formula as the test article, but had a different CAS number at the time of purchase. However, the zinc carbonate hydroxide vendor now offers this compound under the same CAS number as the zinc carbonate basic. This change in CAS numbers suggests that zinc carbonate hydroxide and the test zinc carbonate basic article may be the same material.
Diffraction patterns from procured zinc carbonate hydroxide are presented in Figure 1b for comparison with those obtained from the test article. Overall, the diffraction patterns generally correspond with each other. In addition, these same diffraction patterns are overlaid with data obtained from procured zinc oxide (Figure 1c). Each of the overlapping peaks for the procured zinc oxide is paralleled with an increase in the peak abundance in the zinc carbonate basic test article spectrum relative to the procured zinc carbonate hydroxide spectrum. Taken collectively, these data suggest that the test article is structurally similar or equivalent to the procured zinc carbonate hydroxide, but with zinc oxide as a minor component.
Fourier Transform Infrared Analysis
The infrared spectrum from the zinc carbonate basic test article is presented in Figure 2, with prominent peaks summarized in Table 2 against a zinc carbonate basic reference spectrum (Nyquist and Kagel 1971). Observed peaks from the zinc carbonate basic test article closely match those obtained from the reference spectrum across a wide range of wavenumbers, including O-H, CO32−, and Zn-O functional groups. For additional comparison, infrared spectra were obtained for aliquots of the zinc carbonate hydroxide and zinc oxide compounds and are also presented in Figure 2. Visual inspection of the spectra from the zinc carbonate basic test article and zinc carbonate hydroxide and comparison of prominent peaks further suggests that these compounds are structurally very similar to each other and to the zinc carbonate basic reference spectrum. A strong Zn-O stretch peak (approximately 450 cm−1) was also observed from the test chemical spectrum and the spectra from the procured zinc carbonate hydroxide and zinc oxide compounds, suggesting that zinc oxide is a plausible minor component of the test article.
Figure 2.
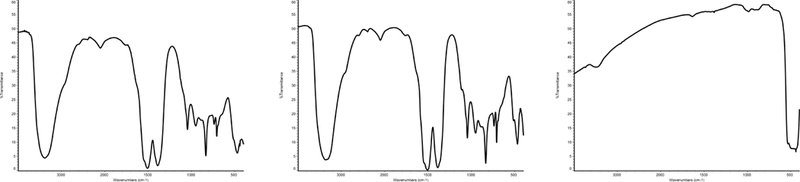
(a) Infrared spectra from the (a) zinc carbonate basic test article, (b) zinc carbonate hydroxide, and (c) zinc oxide.
Table 2.
Infrared spectra of zinc carbonate basic test article and zinc compounds.
| Band # | Observed ZCB Wave Numbera (cm−1) |
Ref. ZCB Wavenumberb (cm−1) |
Observed ZCH Wavenumberc (cm−1) |
Observed ZnO Wavenumberd (cm−1) |
Peak Assignmente |
|---|---|---|---|---|---|
| 1 | 3,383 | ~3,300 | 3,365 | Unmatched | O-H stretch |
| 2 | 1,507 | ~1,510 | 1,507 | Unmatched | ν3
(E’) (νasCO32−) |
| 3 | 1,390 | ~1,390 | 1,388 | Unmatched | ν3
(E’) (νasCO32−) |
| 4 | 1,046 | ~1,045 | 1,046 | Unmatched | OH libration |
| 5 | 951 | ~945 | 952 | Unmatched | OH libration |
| 6 | 835 | ~830 | 835 | Unmatched | ν2 (A2”) (out- of-plane CO32− bending) |
| 7 | 708 | ~710 | 708 | Unmatched | ν4 (E’) (in- plane CO32− bending) |
| 8 | 471 | ~460 | 468 | 441 | Zn-O stretch |
a Observed zinc carbonate basic peaks.
b Identified peaks for zinc carbonate basic reference spectrum (Nyquist and Kagel 1971).
c Observed peaks from procured zinc carbonate hydroxide.
d Observed peaks from procured zinc sample.
e Assignments for Bands 1–7 (Stoilova, Koleva, and Vassileva 2002b) and for Band 8 (Wahab et al. 2007).
Thermogravimetric Analysis
Multiple aliquots (n=6) of the zinc carbonate basic test article were analyzed by TGA, which is based on the measurement of weight loss as a function of temperature. For the zinc carbonate basic test article, weight loss can be attributed to the evaporation of volatile contaminants (i.e., water), volatilization of major sample components, and decarbonation and dehydroxylation. A representative plot of weight percent (relative to the initial sample weight) as a function of temperature was prepared (Figure 3). Weight loss during the analysis was calculated, and the rate of weight loss as a function of temperature was presented as a first derivative curve on the same plot. The single peak for the first derivative curve shown in Figure 3 depicts simultaneous decarbonation and dehydroxylation of the material as represented by (Koga and Tanaka 2005):
| (Eq. 1) |
Figure 3.
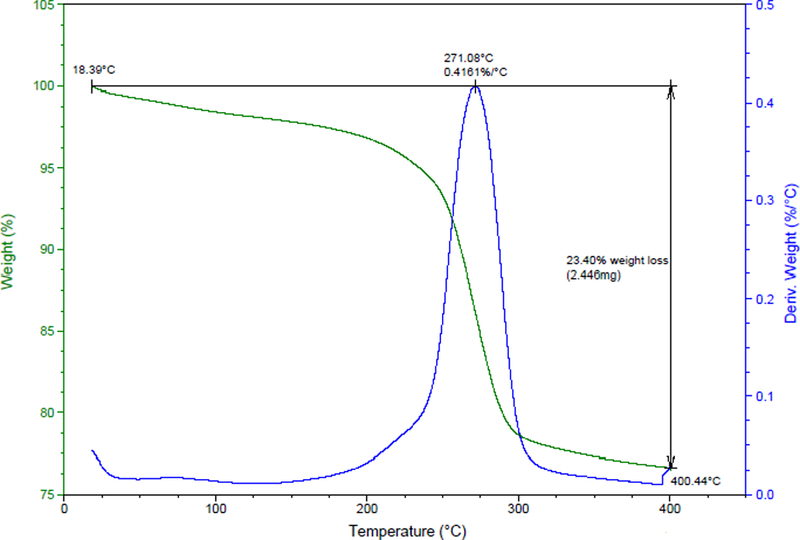
TGA weight percentage and rate of weight loss curves from the zinc carbonate basic test article. The average weight loss was 23.3%.
Because zinc carbonate basic degrades to ZnO upon heating, theoretical weight loss due to loss of water and carbon dioxide for Equation 1 is 25.9%. However, the observed weight loss was 23.3% (0.43% relative standard deviation), suggesting that nonvolatile impurities may be present in the test lot. In addition, the temperature of maximum weight loss (approximately 270 °C) in our investigation was slightly greater than that observed in similar studies (Said et al. 1990), thus supporting the presence of non-volatile impurities. When these data are taken collectively with XRD observations, ZnO is a plausible minor component of the test lot of zinc carbonate basic. It is also important to note that there was an absence of a peak between 100 °C and 200 °C, which signifies that the test chemical was not hydrated (Dobrydnev, Molodtsova, and Kizim 2014).
X-ray Fluorescence
The zinc carbonate basic test article was subjected to a preliminary XRF scan to determine whether elements with atomic weights ranging from sodium to uranium were present. From these scans, zinc, calcium, magnesium, and sulfur were observed, and the test article was re-analyzed using optimal instrumental conditions for these elements. Peaks were observed for calcium and sulfur by using the optimal cellulose filter condition for these elements (Figure 4a) and for magnesium and sulfur by using the optimal no filter condition for these elements (Figure 4b). Large peaks on these scans are attributed to X-ray source (rhodium). Sulfur is a plausible impurity in the zinc carbonate test article because zinc sulfate compounds can be used in the synthesis of zinc carbonate hydroxides (Cao et al. 2009, Du, Liu, and Chen 2009) and because zinc carbonates are effective sulfide scavengers (Cameron 2005). Calcium and magnesium are further discussed in a following section.
Figure 4.
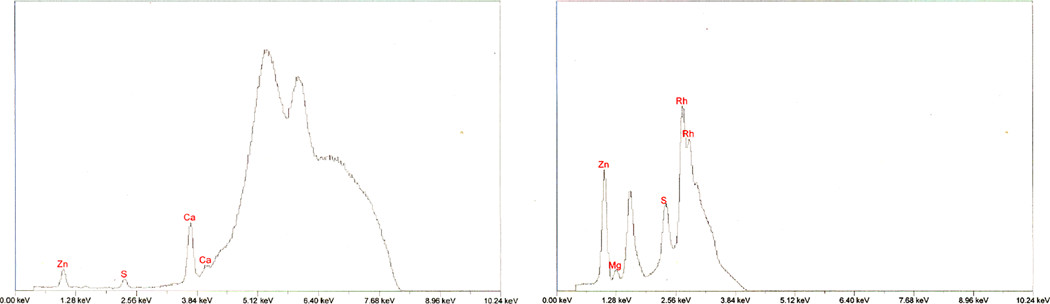
(a) XRF spectrum for the zinc carbonate basic test article analyzed with cellulose filter. (b) XRF spectrum for zinc carbonate basic test article analyzed with no filter. The large Rh peaks are artifacts of the X-ray source.
Inductively Coupled Plasma–Optical Emission Spectrometry
Concentrations of zinc and a panel of selected elements were determined in triplicate preparations of the zinc carbonate basic test article. The ICP-OES results and data from the test chemical vendor’s certificate of analysis are summarized in Table 3. Quantitation limits for each element were conservatively defined as the concentration of the lowest calibration standard included in the regression, expressed as weight percent. Measurable levels were observed for calcium, magnesium, and zinc. Importantly, heavy metals, including arsenic, cadmium, chromium, mercury, lead, and thallium were not detected. Collected data were generally consistent with values reported in the COA. Because it is reported on the vendor’s COA at a 0.5% w/w level, calcium is a plausible impurity at its measured level (0.0916% w/w). Although magnesium was not reported on the vendor’s COA, the element was detected in the test chemical lot at a relatively high level (1.32% w/w). The determined concentration of zinc (56.6% w/w) was slightly different from the vendor reported value (58% w/w), suggesting other zinc-based compounds (i.e., ZnO, additional zinc carbonate phases) and other elements (i.e., calcium, sulfur and magnesium) may be present in the test article lot.
Table 3.
ICP-OES analyses of zinc carbonate basic test article for zinc and selected metals.
| Element | Determined Concentrationa (% w/w) |
COA Concentrationb (% w/w) |
|---|---|---|
| Arsenic | <0.005 | Not applicablec |
| Calcium | 0.0916 (relative standard deviation of 6.3%) |
0.5 |
| Cadmium | <0.005 | 0.005 |
| Cobalt | <0.005 | 0.005 |
| Chromium | <0.005 | Not applicable |
| Copper | <0.005 | 0.005 |
| Iron | <0.005 | 0.02 |
| Mercury | <0.005 | Not applicable |
| Magnesiu | 1.32 (relative standard deviation of 3.0%) |
Not applicable |
| Manganese | <0.005 | Not applicable |
| Nickel | <0.01 | 0.005 |
| Lead | <0.005 | 0.005 |
| Palladium | <0.005 | Not applicable |
| Platinum | <0.01 | Not applicable |
| Antimony | <0.005 | Not applicable |
| Tin | <0.005 | Not applicable |
| Thallium | <0.01 | Not applicable |
| Zinc | 56.6 (relative standard deviation of 0.89%) |
58 |
a Average of triplicate preparations; percent relative standard deviation shown, where applicable.
b Concentration reported on the vendor’s certificate of analysis.
c Not reported on vendor’s certificate of analysis.
Elemental Analysis, Ion Analysis, and Water Determination
Duplicate analyses of the zinc carbonate basic test article were conducted to determine the carbon, hydrogen, and water content. The results from the analyses were as follows: 3.5% w/w (0.80% relative standard deviation) for carbon, 1.2% w/w (5.3% relative standard deviation) for hydrogen, and 2.5% w/w (7.3% relative standard deviation) for water. These results confirm the presence of carbon and hydrogen at appreciable levels and that the test chemical lot did not contain a significant amount of moisture (0.30% by weight loss and 2.52% by Karl Fischer titration). Chromatographic analysis revealed the presence of minor ionic impurities, including chloride (429 µg/g), sulfate (1,871 µg/g), and sodium (698 µg/g), which were consistent with the test article lot’s certificate of analysis. Although observed values for carbon and hydrogen compared reasonably well to theoretical values for these elements (4.4% for carbon and 1.1% for hydrogen) from the zinc carbonate basic test lot structure ([ZnCO3]2·[Zn(OH)2]3), they did not match exactly. This discrepancy may be an artifact of impurities (i.e., ZnO, calcium, magnesium, sulfur) and/or the presence of additional phases in the Zn-C-O-H family.
Stability of Zinc Carbonate Basic
A second objective of this investigation was to assess the stability of the zinc carbonate basic test article by analyzing aliquots of the bulk compound stored under ambient temperature conditions used during toxicological studies of dietary zinc and aliquots stored frozen (nominal −20°C) by using several techniques. XRD, FTIR (Figure 5a and b, respectively), and TGA ambient temperature and frozen reference data were essentially identical, and photomicrographs did not show any visual evidence of degradation. In addition, the zinc emission response measured by ICP-OES for aliquots of the test chemical stored at ambient temperature and at 60 °C for 15-days were within ± 1% of each other. Taken collectively, these data were used to establish that the test chemical is stable when stored at room temperature.
Figure 5.
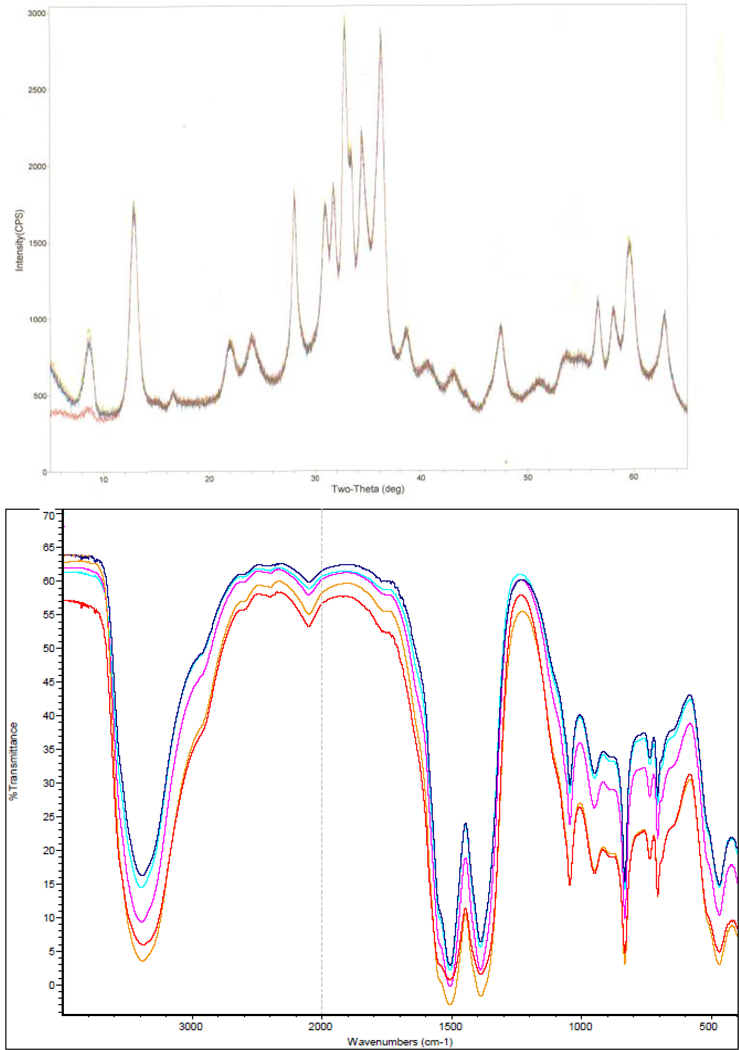
(a) Equivalent XRD diffraction patterns from multiple aliquots (n = 3) of the zinc carbonate basic test article stored at ambient temperature and stored frozen as a reference (n = 2). (b) Equivalent infrared spectra from multiple aliquots (n = 3) of the zinc carbonate basic test article stored at ambient temperature and stored frozen as a reference (n = 2).
CONCLUSIONS
The primary goal of this work was to characterize a zinc carbonate basic test article before its use in a toxicity and carcinogenicity study examining the role of zinc excess and deficiency in the diet. Even though zinc carbonate basic compounds are used in a variety of industrial processes, knowledge about their chemistry remains fragmentary and incomplete. Multiple synthetic routes employing a wide range of reagents and preparation procedures have been reported, and multiple naturally occurring phases of these compounds are also known to exist. Further, there is inconsistent nomenclature for both synthetic and naturally occurring zinc carbonate basic compounds in vendor catalogs, scientific literature references, and XRD reference cards.
Because of the complexity of zinc carbonate basic compounds, it was necessary to employ multiple analytical techniques to characterize the test article lot. XRD was used to obtain structural information about the zinc carbonate basic test article. Although there were reference patterns for naturally occurring zinc carbonate basic phases, none could be used to confirm the definitive test compound structure. Further, the zinc carbonate basic test article’s diffraction pattern indicated the presence of a significant amount of amorphous material, suggesting that multiple Zn-C-O-H phases may be present. Therefore, the database was further queried for possible partial matches and the presence of other possible phases. In addition to zinc carbonate basic, these included zinc oxide, zinc hydroxide, zinc carbonate hydroxide hydrate, smithsonite, and hydrozincite. When diffraction patterns of zinc hydroxide, zinc carbonate hydroxide hydrate, smithonsite, and hydrozincite were compared to the pattern from the zinc carbonate basic test article, in each case there were a number of unmatched peaks. However, all peaks for zinc oxide corresponded to matching peaks from the zinc carbonate basic test article, but relative peak height distributions (intensities) of the most abundant peaks did not correlate, suggesting that zinc oxide was present in the sample, but as a possible minor component. To further aid in characterization efforts, zinc oxide and a zinc carbonate hydroxide with a nominal chemical formula identical to the zinc carbonate basic test article were employed for comparison. XRD diffraction patterns (peak position and intensity) for the zinc carbonate basic test article and zinc carbonate hydroxide were nearly identical, with minor differences corresponding to peaks associated with ZnO.
Infrared spectra of the zinc carbonate basic test lot and zinc carbonate hydroxide are nearly indistinguishable and closely matched the library spectrum of zinc carbonate basic. In addition, the strong Zn-O stretch (approximately 450 cm−1) observed in the ZnO spectrum was also clearly visible in the zinc carbonate basic test article spectrum. This is further evidence that ZnO is a plausible minor component of the test article lot.
TGA showed weight loss on heating of 23.3%. This observed value was less than the theoretical weight loss values of 25.9% from the decarbonation and dihydroxylation of the zinc carbonate basic test article with a formula [ZnCO3]2·[Zn(OH)2]3 and 26.3% of zinc carbonate hydroxide hydrate. These data suggest the likely presence of non-volatile minor components, such as zinc oxide. It is also important to note the absence of a peak between 100 °C and 200 °C, which signifies that the test chemical was likely not a hydrated compound, such as zinc carbonate hydroxide hydrate.
XRF spectra of the zinc carbonate basic test article confirmed presence of zinc and identified calcium, magnesium, and sulfur impurities. Quantitative analysis by ICP-OES also confirmed the presence of calcium, magnesium, and zinc in a panel of trace elements quantitatively analyzed by ICP-OES. Observed values (w/w) were 0.0916% for calcium, 1.32% for magnesium, and 56.6% w/w for zinc. Importantly, heavy metals (e.g., arsenic, cadmium, chromium, mercury, lead, and thallium) that could adversely impact a toxicological investigation were below quantitation limits. The determined concentrations of zinc (56.6% observed versus 59.6% theoretical), carbon (3.5% observed versus 4.4% theoretical), and hydrogen (1.2% observed versus 1.1% theoretical) were slightly different than the theoretical values for zinc carbonate basic, suggesting the presence of other potential compounds in the Zn-C-H-O family.
Taken collectively, data suggested that the test article was an amorphous compound in the Zn-C-H-O family. Although there are several zinc compounds within this family, the preponderance of the data given above suggests that the test article was most likely composed of zinc carbonate basic with zinc oxide present as a minor component, resulting in a zinc content of 56.6%. In addition, the test article was stable when stored under the ambient conditions recommended by the vendor supporting the use of it as a source of dietary zinc in a study investigating the zinc deficiency and excess in rodents.
ACKNOWLEDGMENT
This work was funded in full by the National Institute of Environmental Health Sciences, National Institute of Health, Contract Number: N01-ES-65554.
REFERENCES
- Agarwal R, Virmani D, Jaipal M, Gupta S, Sankar MJ, Bhatia S, Agarwal A, Devgan V, Deorari A, Paul VK, and Toteja GS. 2013. Poor zinc status in early infancy among both low and normal birth weight infants and their mothers in Delhi. Neonatology 103 (1): 54–9. 10.1159/000342227. [DOI] [PubMed] [Google Scholar]
- Alhawi T, Rehan M, York D, and Lai X. 2015. Synthesis of zinc carbonate hydroxide nanoparticles using microemulsion process. Procedia Engineering 102: 346–355. [Google Scholar]
- Alloway BJ 2009. Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31 (5): 537–48. 10.1007/s10653-009-9255-4. [DOI] [PubMed] [Google Scholar]
- Anžlovar Alojz, Marinšek Marjan Orel Zorica Crnjak, and Žigon Majda. 2015. Basic zinc carbonate as a precursor in the solvothermal synthesis of nano-zinc oxide. Materials & Design 86: 347–353. 10.1016/j.matdes.2015.07.087. [DOI] [Google Scholar]
- Barch DH, and Fox CC. 1987. Dietary zinc deficiency increases the methylbenzylnitrosamine-induced formation of O6-methylguanine in the esophageal DNA of the rat. Carcinogenesis 8: 1461–1464. [DOI] [PubMed] [Google Scholar]
- Barney GH, Orgebin-Crist MC, and Macapinlac MP. 1968. Genesis of esophageal parakeratosis and histologic changes in the testes of the zinc-deficient rat and their reversal by zinc repletion. Journal of Nutrition 95: 526–534. [DOI] [PubMed] [Google Scholar]
- Bitenc M, Marinšek M, and Crnjak Orel Z. 2008. Preparation and characterization of zinc hydroxide carbonate and porous zinc oxide particles. Journal of the European Ceramic Society 28 (15): 2915–2921. 10.1016/j.jeurceramsoc.2008.05.003. [DOI] [Google Scholar]
- Brewer George J. 2014. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Frontiers in Aging Neuroscience 6: 92 10.3389/fnagi.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun ER, Greist A, Tricot G, and Hoffman R. 1990. Excessive zinc ingestion: A reversible cause of sideroblastic anemia and bone marrow depression. Journal of the American Medical Association 264: 1441–1443. [DOI] [PubMed] [Google Scholar]
- Bruno RS, Song Y, Leonard SW, Mustacich DJ, Taylor AW, Traber MG, and Ho E. 2007. Dietary zinc restriction in rats alters antioxidant status and increases plasma F2 isoprostanes. J Nutr Biochem 18 (8): 509–18. 10.1016/j.jnutbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Cameron C 2005. Water-based fluids approach synthetic capabilities. Offshore 65 (3): 44. [Google Scholar]
- Cao Z, Zhang Z, Wang F, and Wang G. 2009. Synthesis and UV shielding properties of zinc oxide ultrafine particles modified with silica and trimethyl siloxane. Colloids and Surfaces A: Physiochemical and Engineering Aspects 340 (1–3): 161–167. [Google Scholar]
- CDC. 1983. Illness associated with elevated levels of zinc in fruit punch-New Mexico. Morbidity and Mortality Weekly Report 32: 257–258. [PubMed] [Google Scholar]
- Chasapis CT, Loutsidou AC, Spiliopoulou CA, and Stefanidou ME. 2012. Zinc and human health: An update. Arch Toxicol 86 (4): 521–34. 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- Corbo MD, and Lam J. 2013. Zinc deficiency and its management in the pediatric population: A literature review and proposed etiologic classification. J Am Acad Dermatol 69 (4): 616–624. 10.1016/j.jaad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Della Lucia CM, Santos LL, Rodrigues KC, Rodrigues VC, Martino HS, and Sant’Ana HM. 2014. Bioavailability of zinc in Wistar rats fed with rice fortified with zinc oxide. Nutrients 6 (6): 2279–89. 10.3390/nu6062279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Swenerton H, and Hurley LS. 1971. Testicular and esophageal lesions in zinc-deficient rats and their reversibility. Journal of Nutrition 101: 77–84. [DOI] [PubMed] [Google Scholar]
- Dobrydnev SV, Molodtsova MY, and Kizim NF. 2014. Synthesis and study of basic zinc carbonate. Russian Journal of Inorganic Chemistry 59 (8): 798–800. 10.1134/S0036023614080038. [DOI] [Google Scholar]
- Domingo JL, Llobet JM, Paternain JL, and Corbella J. 1998. Acute zinc intoxication: Comparison of the antidotal efficacy of several chelating agents. Veterinary and Human Toxicology 30: 224–228. [PubMed] [Google Scholar]
- Du S, Liu H, and Chen Y. 2009. Large-scale preparation of porous ultrathin Ga-doped ZnO nanoneedles from 3D basic zinc carbonate superstructures. Nanotechnology 20 (8): 085611 10.1088/0957-4484/20/8/085611. [DOI] [PubMed] [Google Scholar]
- Du Shangfeng, Tian Yajun, Liu Haidi, Liu Jian, and Chen Yunfa. 2006. Calcination effects on the properties of gallium-doped zinc oxide powders. Journal of the American Ceramic Society 89 (8): 2440–2443. 10.1111/j.1551-2916.2006.01093.x. [DOI] [Google Scholar]
- Dumrongwongsiri O, Suthutvoravut U, Chatvutinun S, Phoonlabdacha P, Sangcakul A, Siripinyanond A, Thiengmanee U, and Chongviriyaphan N. 2015. Maternal zinc status is associated with breast milk zinc concentration and zinc status in breastfed infants aged 4–6 months. Asia Pac J Clin Nutr 24 (2): 273–80. 10.6133/apjcn.2015.24.2.06. [DOI] [PubMed] [Google Scholar]
- Feng Xun, Li ZhongJun, Wang Peiyuan, and Zhou Yifeng. 2005. Preparation and gas-sensitivity of ultra-fine zinc-oxide powders from roasted zinc-blended. Journal of Materials Science 40 (24): 6597–6600. 10.1007/s10853-005-2148-8. [DOI] [Google Scholar]
- Fischer PWF, Giroux A, and L’Abbé MR. 1984. Effect of zinc supplementation on copper status in adult man. American Journal of Clinical Nutrition 40: 743–746. [DOI] [PubMed] [Google Scholar]
- Frost RL, and Hales MC. 2007. Syntheisis and vibrational spectroscopic characterization of synthetic hyrdrozincite and smithsonite. Polyhedron 26 (17): 4955–4962. [Google Scholar]
- Ho E 2004. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 15 (10): 572–8. 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Impa Somayanda M., Gramlich Anja, Tandy Susan, Schulin Rainer, Frossard Emmanuel, and Johnson-Beebout Sarah E.. 2013. Internal Zn allocation influences Zn deficiency tolerance and grain Zn loading in rice (Oryza sativa L.). Frontiers in Plant Science 4: 534 10.3389/fpls.2013.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambor JL 1963. Studies of basic copper and zinc carbonates: I-synthetic zinc carbonates and their relationship to hyrdozincite. Canadian Mineralogist: 92–109.
- Kanari N, Mishra D, Gaballah I, and Dupré B. 2004. Thermal decomposition of zinc carbonate hydroxide. Thermochimica Acta 410 (1–2): 93–100. 10.1016/S0040-6031(03)00396-4. [DOI] [Google Scholar]
- Khalid N, Ahmed A, Bhatti MS, Randhawa MA, Ahmad A, and Rafaqat R. 2014. A question mark on zinc deficiency in 185 million people in Pakistan--possible way out. Crit Rev Food Sci Nutr 54 (9): 1222–40. 10.1080/10408398.2011.630541. [DOI] [PubMed] [Google Scholar]
- Koga N, and Tanaka H. 2005. Thermal decomposition of copper(II) and zinc carbonate hydroxides by means of TG-MS. Journal of Thermal Analysis and Calorimetry 82 (3): 725–729. 10.1007/s10973-005-0956-3. [DOI] [Google Scholar]
- Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, and Wu Y. 2014. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clinical Nutrition 33: 415–420. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Chan WC, Fong LYY, and Newberne PM. 1976. Zinc levels in serum, hair and tumors from patients with esophageal cancer. Nutrition Reports International 15: 635–643. [Google Scholar]
- Lindenmayer GW, Stoltzfus RJ, and Prendergast AJ. 2014. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv Nutr 5 (1): 1–6. 10.3945/an.113.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R 1978. Bleeding gastric erosion after oral zinc sulphate. British Medical Journal 1 (6115): 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musić S, Dragčević Đ, and Popović S. 2007. Influence of synthesis route on the formation of ZnO particles and their morphologies. Journal of Alloys and Compounds 429: 242–249. [Google Scholar]
- Newberne PM, Schrager TF, and Broitman S. 1997. Esophageal carcinogenesis in the rat: Zinc deficiency and alcohol effects on tumor induction. Pathobiology 65: 39–45. [DOI] [PubMed] [Google Scholar]
- Nyquist RA, and Kagel RO. 1971. Infrared spectra of inorganic compounds (3800 −45 cm-1) Orlando, FL: Academic Press. [Google Scholar]
- Okutani Tadao, Yamakawa Kazuto, Sakuragawa Akio, and Gotoh Ryozoh. 1993. Determination of a micro amount of sulfide by ion chromatography with amperometric detection after coprecipitation with basic zinc carbonate. Analytical Sciences 9 (5): 731–734. 10.2116/analsci.9.731. [DOI] [Google Scholar]
- Porter KG, McMaster D, Elmes ME, and Love AHG. 1977. Anaemia and low serum-copper during zinc therapy. Lancet October 8: 774. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Brewer GJ, Schoomaker EB, and Rabbani P. 1978. Hypocupremia induced by zinc therapy in adults. Journal of the American Medical Association 240: 2166–2168. [PubMed] [Google Scholar]
- Raqib R, Hossain MB, Kelleher SL, Stephensen CB, and Lonnerdal B. 2007. Zinc supplementation of pregnant rats with adequate zinc nutriture supresses immune functions in their offspring. Journal of Nutrition 137 (4): 1037–1042. [DOI] [PubMed] [Google Scholar]
- Sadeek Sadeek A., and Refat Moamen S.. 2005. Synthesis, infrared spectra and thermal investigation of gold(III) and zinc(II) urea complexes. A new procedure for the synthesis of basic zinc carbonate. Journal of Coordination Chemistry 58 (18): 1727–1734. 10.1080/00958970500262254. [DOI] [Google Scholar]
- Said AA, Hassan EA, Abd El-Salaam KM, and Mohamed MM. 1990. Influence of iron ion additions on the thermal decomposition of basic zinc carbonate. Journal of Thermal Analysis 36 (4): 1331–1345. 10.1007/bf01914056. [DOI] [Google Scholar]
- Salzman MB, Smith EM, and Koo C. 2002. Excessive oral zinc supplementation. Journal of Pediatric Hematology/Oncology 24: 582–584. [DOI] [PubMed] [Google Scholar]
- Samman S, and Roberts DCK. 1987. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. Medical Journal of Australia 146: 246–249. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Murakami M, and Nishide T. 1996. Thermal analysis of basic zinc carbonate. Part 1. Carbonation process of zinc oxide powders at 8 and 13°C. Thermochimica Acta 273: 95–102. 10.1016/0040-6031(95)02631-2. [DOI] [Google Scholar]
- Schrager TF, Busby WF Jr, Goldman ME, and Newberne PM. 1986. Enhancement of methylbenzylnitrosamine-induced esophageal carcinogenesis in zinc-deficient rats: Effects on incorporation of [3H]thymidine into DNA of esophageal epithelium and liver. Carcinogenesis 7: 1121–1126. [DOI] [PubMed] [Google Scholar]
- Stoilova D, Koleva V, and Vassileva V. 2002a. Infrared study of some synthetic phases of malachite (Cu2(OH)2CO3)-hydrozincite (Zn5(OH)6(CO3)2) series. Spectrochim Acta A Mol Biomol Spectrosc 58 (9):2051–9. [DOI] [PubMed] [Google Scholar]
- Stoilova D, Koleva V, and Vassileva V. 2002b. Infrared Study of Some Synthetic Phases of Malachite (Cu2(OH)2CO3) - Hydrozincite (Zn5(OH)6(CO3)2) Series. Spectrochimica Acta Part A 58: 2051–2059. [DOI] [PubMed] [Google Scholar]
- Swenerton H, and Hurley LS. 1968. Severe zinc deficiency in male and female rats. Journal of Nutrition 95: 8–18. [DOI] [PubMed] [Google Scholar]
- Tahmasebi Boroujeni S, Naghdi N, Shahbazi M, Farrokhi A, Bagherzadeh F, Kazemnejad A, and Javadian M. 2009. The effect of severe zinc deficiency and zinc supplement on spatial learning and memory. Biol Trace Elem Res 130 (1): 48–61. 10.1007/s12011-008-8312-7. [DOI] [PubMed] [Google Scholar]
- Tassabehji NM, Corniola RS, Alshingiti A, and Levenson CW. 2008. Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav 95 (3): 365–9. 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Wahab R, Ansari SG, Kim YS, Dar MA, and Shin HS. 2007. Synthesis and Characterization of Hydrozincite and its Conversion into Zinc Oxide Nanoparticles. Journal of Alloys and Compounds 461: 66–71. [Google Scholar]
- Wahab Rizwan, Ansari SG, Kim Young Soon, Dar MA, and Shin Hyung-Shik. 2008. Synthesis and characterization of hydrozincite and its conversion into zinc oxide nanoparticles. Journal of Alloys and Compounds 461 (1–2): 66–71. 10.1016/j.jallcom.2007.07.029. [DOI] [Google Scholar]
- Wang H, Hu YF, Hao JH, Chen YH, Su PY, Wang Y, Yu Z, Fu L, Xu YY, Zhang C, Tao FB, and Xu DX. 2015. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction:A population-based birth cohort study. Sci Rep 5: 11262 10.1038/srep11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadrick MK, Kenney MA, and Winterfeldt EA. 1989. Iron, copper, and zinc status: Response to supplementation with zinc or zinc and iron in adult females. American Journal of Clinical Nutrition 49: 145–150. [DOI] [PubMed] [Google Scholar]
- Yamada S, Tsukumo E, and Koga N. 2009. Influences of evolved gases on the thermal decomposition of zinc carbonate hydroxide evaluated by controlled rate evolved gas analysis coupled With TG. Journal of Thermal Analysis and Calorimetry 95 (2): 489–493. 10.1007/s10973-008-9272-z. [DOI] [Google Scholar]
- Yan Michelle, Song Yang, Wong Carmen P., Hardin Karin, and Ho Emily. 2008. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. Journal of Nutrition 138 (4): 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Jun, Wang Shurong, Xu Mijuan, Wang Yan, Zhu Baolin, Zhang Shoumin, Huang Weiping, and Wu Shihua. 2009. Hierarchically porous ZnO architectures for gas sensor application. Crystal Growth & Design 9 (8): 3532–3537. 10.1021/cg900269a. [DOI] [Google Scholar]
- Zhang S, Chi L, and Li X. 2002. Preparation of ZnO nanoparticles by precipitation/mechanochemical method. International Journal of Nanoscience 01 (05n06): 563–567. 10.1142/S0219581X0200067X. [DOI] [Google Scholar]
- Zhang S, Fortier H, and Dahn JR. 2004. Characterization of zinc carbonate hydroxides synthesized by precipitation from zinc acetate and potassium carbonate solutions. Materials Research Bulletin 39 (12): 1939–1948. 10.1016/j.materresbull.2004.05.023. [DOI] [Google Scholar]


