From the moments after our birth1 and throughout our lives, humans serve as reservoirs for extremely complex and dynamic communities of microorganisms of varying origins. These microbes include archaea, bacteria, viruses, bacteriophage, and eukaryotes (uni- and multicellular parasites). Although constituting a potential threat to health and well-being through parasitism, these microbial communities have co-evolved over millions of years with the healthy human host to provide a range of beneficial, and often essential, services. Although still rudimentary, our understanding of the beneficial roles played by the human microbiome has grown appreciably in recent years, as high-throughput, culture-independent technology and related molecular technologies have been adapted to complement the role of traditional microbiologic cultures to facilitate the study of the human ecosystem. Most studies identify individuals within complex populations by focusing on molecular characterization of DNA from bacterial 16S ribosomal genes, a sequence that distinguishes each organism by its phylum, genus, and even species, depending on the length of the sequence. By studying communities in their native habitats, rather than in liquid broth or on Petri plates, we have gained significant insight into the dynamic, multi-factorial interactions that occur among host, pathogen, commensal community, and environment. Under normal, healthy circumstances, these interactions occur across both the integument and mucosal surfaces (eg, airways, intestinal, reproductive tracts, mouth), the surfaces of the human body exposed to the environment that are the primary sites of microbial residence. As thoughtfully reviewed in this issue of Translational Research, the authors consider the microbial ecology of the intestine,2 the lung,3 and the female reproductive tract,4 each of which supports diverse bacterial communities and, more recently appreciated, viruses5 that engage the host on multiple levels. Indeed, even skin has now been exposed to reveal an abundance of microbial species,6–8 well beyond the Staphylococcal and Streptococcal species we were taught to expect, and with heretofore unanticipated effects on host response.9
Of particular relevance are the host and environmental factors that determine the constituents, diversity, and stability of the microbiome (Table I). The microbiome occupies a unique ecological niche at each bodily site. This niche can be described as an “n-dimensional hyper-space” in which multiple factors coalesce to support or limit the selection of its members and to demarcate its boundaries. These factors can include temperature, humidity, oxygen tension, nutrition, osmolarity, host receptors, competition with and resistance to other microbes and their secreted products, and the activity of passive (eg, breast milk in the infant) and locally generated innate and specific immune responses to the organisms. The latter host-derived effects may be modulated by the microbes’ ability to upregulate or downregulate and to evade or subvert host recognition and response. Understanding the determinants of the fitness of the micro-biome could help us facilitate its restoration when necessary.
Table I.
Definitions
| Microbe: Any microscopic life form. Commonly bacteria or archaea, but many eukaryotes are microbes. |
| Microbiome: An assemblage of microbes in a particular time and place. |
| Virome: An assemblage of viruses in a particular time and place. |
| Microbial community: An assemblage of functionally and metabolically interacting microbes. |
| Metagenome: A composite genome from all organisms in a microbiome. |
| Meta-transcriptome: A composite gene expression profile of all organisms in a microbiome. |
| Richness: The number of bacterial species present in a population (alpha diversity, measured by Good’s coverage). |
| Diversity: The “complexity” or relative distribution of different species present in a population, ecosystem, or biome (beta diversity, measured by Morisita-Horn Index). The similarities between distributions can also be determined. |
| Phylotype: A group of individuals characterized by their phylogenetic relationship to each other; a statistically associated shared richness and diversity of specific organisms within an anatomic site, initially based on an evolutionary relationship. |
| Dysbiosis: Disease-associated alteration in the composition of a microbial community. |
| Pathobiont: A member of the microbiota, often antimicrobial-resistant, that can cause disease on perturbation of the otherwise constraining healthy microbiota. |
| Probiotics: Live commensal microbial organisms (eg, Lactobacillus GG, lactobacilli, bifidobacteria, Streptococci, or Saccharomyces boulardii, alone or in combination) administered to enhance or suppress mucosal integrity, inflammation, or immune response. |
| Common Bacterial Phyla: |
| Bacteroides: Obligately anaerobic gram-negative bacteria. Prevalent commensals in human gut (eg, Bacteroides fragilis). |
| Firmicutes: Very diverse phylum of low G-C gram-positive bacteria, including staphylococci, streptococci, bacillii, and clostridia. Prevalent commensals in human gut. |
| Proteobacteria: Very diverse phylum of gram-negative bacteria, including enterobacteriaceae (eg, Escherichia coli). |
| Actinobacteria: High G-C gram-positive bacteria, including mycobacteria and corynebacteria. |
The microbiome at each site serves the needs of the host as well as its own. For example, the trillions of microbial cells and hundreds of species that colonize the intestine have evolved metabolic pathways capable of extracting energy from mammalian dietary inputs. Rather than drawing down the energy content that is available for absorption by the intestine, the enteric microbiome, particularly members of the bacterial phyla Firmicutes (ie, low G+C gram-positive organisms such as Clostridia) and the gram-negative Bacteroidetes, transform otherwise indigestible material, such as complex plant polysaccharides, into fermentation products that are more readily metabolized by mammals. In this way, intestinal microbes provide significant additional calories and thereby extend the human genomic capacity to harvest energy from foodstuffs.
Moreover, just as a lush lawn of grass with thick roots in rich soil precludes invasion by dandelions and weeds, an intact microbiome limits colonization and clinical infection with pathogenic, and particularly antimicrobial resistant, organisms. Antibiotics disrupt the integrity and diversity of the microbial “lawn,” predisposing the patient to Clostridium difficile, enterococci, methicillin-resistant Staphylococcus aureus, andgram-negative infections.10,11 The predisposition to infection conferred by antibiotics may well extend beyond interrupting the competitive inhibition of the pathogens by the normal commensal species to include their effects on the epithelium, metabolism, regulation of inflammasomes,11,12 and immune status.13 Indeed, among mice challenged with influenza, antibacterial treatment significantly limited their ability to generate specific antiviral antibodies, CD41 and CD8+ T-cell and interferon-α responses, and to control viral replication compared with those in untreated control animals,14 suggesting a role for bacteria in generating immune responses.
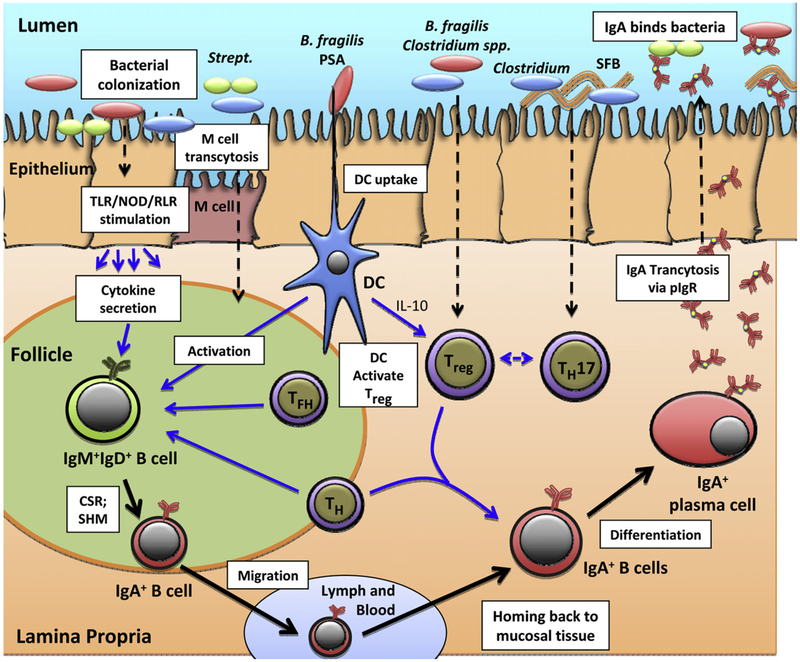
One of the most intriguing aspects of our understanding of the role and regulation of the microbiome is its interaction with the immune system. In animal models, the acquisition of intestinal microbiota drives immune development and maturation from birth, but maintenance of intestinal homeostasis between immune competence and tolerance is also critical to the proper control of inflammation and progression to disease states. These interactions are subserved by the juxtaposition of a range of complementary cell types in anatomically distinct areas, such as the surface epithelium, inductive sites (eg, isolated or aggregated germinal centers), and the more diffuse effector sites in the lamina propria (Fig 1). Initial immune interactions with microbes are mediated by innate immune surface and intracellular pattern recognition receptors, such as toll-like receptors, NOD-like receptors, and retinoic acid-inducible gene-1–like receptors. For example, a deficiency of the toll-like receptor 5 gene, which encodes a protein that recognizes bacterial flagellin, is associated with a change in gut microbiota and significant metabolic perturbations in the murine host, changes that can conveyed to a wild-type host by transfer of stool.15
Fig 1.
Specific colonizing bacteria elicit innate immune responses and development of T cells and IgA-producing cells in the intestine. (1) Bacteria colonize the lumen and interact with epithelial cells. Bacteria can be transported directly through the epithelium by Microfold cells. Binding and uptake of bacteria or their products by epithelial cells can activate innate receptors (toll-like receptor, NOD, retinoic acid-inducible gene-1–like receptors) and stimulate cytokine secretion from the basolateral surface. DCs extending through the epithelium can sample antigens, such as polysaccharide A of Bacteroides fragilis, and become activated. B. fragilis and Clostridium spp. can enhance differentiation of Treg and segmented filamentous bacteria in the development of T-helper 17 cells. (2) In the lymphoid follicle or germinal center, naïve IgD+IgM+B cells are activated by bacterial antigens. In association with epithelial-derived soluble factors, these cells are committed to undergo class switch recombination to IgA and somatic hypermutation under the influence of DCs, follicular helper CD4+ T cells (T follicular helper cells), and T-helper cells. These committed B cells then leave the follicle, transit through the lymph and blood, and return or “home” predominantly to the lamina propria effector sites from which they originated. With support from Treg and T-helper cells, the returning B cells in the lamina propria differentiate into IgA-producing plasma cells. (3) The polymeric IgA produced binds to polymeric IgA receptors on the basolateral surface of epithelial cells and is transported into the lumen to bind bacteria and their antigens to limit adherence to, activation of, and transport through epithelial cells. CSR, class switch recombination; DC, dendritic cell; Ig, immunoglobulin; M cell, Microfold cell; pIgR, polymeric immunoglobulin A receptor; PSA, polysaccharide A; RLR, retinoic acid-inducible gene-1–like receptor; SFB, segmented filamentous bacteria; SHM, somatic hypermutation; TFH, T follicular helper cells; TH, T-helper cells; TLR, toll-like receptor; Treg, T-regulatory cells.
Commensal bacteria can elicit selective immunologic effects (Fig 1). In the mouse intestine, segmented fila mentous bacteria induce inflammatory T-helper 17 cells that protect against bacterial and fungal infections.16 Conversely, Bacteroides fragilis and members of the Clostridium groups IV and XIVa induce development of immunomodulatory FoxP3+ T-regulatory cells (Treg) in germ-free mice.17,18 Mucosal Treg cells, unlike thymic Treg cells, have T-cell receptors that are specific for the antigens of commensal bacteria, suggesting that local exposure to commensal intestinal bacterial antigens drives this development.19
Mucosal dendritic cells (DCs) support induction of immune tolerance by secretion of interleukin-10 that drives the differentiation of Treg cells. Escherichia coli or Bacillus subtilis can support differentiation of monocytes into DCs.20 DCs can extend through the epithelium to bind microbial glycans in the lumen via DC-SIGN and other receptors. Intestinal DCs can present bacterial polysaccharide A of B fragilis to activate CD4+T cells and cytokine secretion.21
Mucosal DCs also initiate the commitment of mucosal B cells to immunoglobulin (Ig)A, including gA specific for the colonizing bacteria.22,23 Treg cells, with T follicular helper cells, promote the differentiation of mucosal B cells to IgA-producing plasma cells. Depletion of Treg cells causes reduction in mucosal IgA production,24 as does reversal of bacterial colonization.22 These data indicate a functional link among commensal bacteria, T cells, and induction and maintenance of protective antibodies at the mucosa. Despite the elegant and specific work in mouse models, efforts to establish such direct links between the microbiome and human immune function in children and adults are in progress25 but are confounded by the complexity of the human model.
Perturbations of the delicate balance between immune tolerance/ignorance and activation in human disease states are associated with disturbances in the compositions of commensal communities (ie, “dysbiosis”). Altered distributions of various microbial groups have been described with obesity,26,27 type 1 diabetes,28 childhood asthma,29 inflammatory bowel disease,30 colorectal cancer,31 cardiovascular disease,32 and human immunodeficiency virus transmission.33,34 Indeed, microbial products and metabolites from mucosal sites are found circulating in the blood35 with potential systemic effects.36 However, determining the extent to which the microbiome influences the human syndrome and the syndrome influences the microbiome requires robust, creative, and reproducible study design and analysis.
This issue of Translational Research offers 4 incisive reviews of our current understanding of the human microbiome, focusing on the gastrointestinal tract, lung, female reproductive tract, and virome. Although each review presents a glimpse into a unique ecological niche, taken collectively, these articles convey the great challenges and potentially greater rewards to clinical practice of elucidating the mechanisms by which the microbiome affects human health.
In the first article, Dave et al2 introduce the most complex, yet best studied microbiome, that of the gastrointestinal tract. Each section of the digestive tract, from oral cavity to rectum, provides a different physiochemical environment that is colonized by unique kinds and quantities of attendant microorganisms. Work on animal models, most notably germ-free mice, has revealed a plethora of beneficial services that a healthy microbiome can deliver to its host,37–39 including inducing the development and maintenance of immune homeostasis40 and provision of key nutrients.41 As the authors describe, the microbiome of the gastrointestinal tract is potentially amenable to a range of means of directed perturbation with the goal of treating or preventing disease through provision of selected probiotics (Table II), prebiotics, and antibiotics. Evidence of the clinical efficacy of probiotics and prebiotics is currently lacking in many instances. However, better understanding of the functional significance of particular members of a microbiome could lead to more rational selection of potential probiotic or prebiotic agents. For instance, if a lack of clostridial species disrupts immune homeostasis and thus contributes to inflammatory bowel disease,30,42 it is perhaps not surprising that consumption of bifidobacteria or lactobacilli (common probiotics) would not ameliorate disease. If a canonical set of intestinal microbial constituents could be determined, successful resolution of serious chronic recurrent infection with C difficile might be more amenable to therapy with a microbial pill, rather than the often successful but cumbersome fecal transplant from a donor.43
Table II.
Conditions for which probiotics have been considered as prevention or therapy
| Irritable bowel syndrome |
| Inflammatory bowel disease |
| Necrotizing enterocolitis |
| Helicobacter pylori-associated gastritis/ulcer disease |
| Periodontitis |
| Prematurity |
| Travelers’ diarrhea |
| Acute diarrhea |
| HIV disease progression |
| Liver disease and hepatic encephalopathy |
| Atopic dermatitis/eczema |
| Bacterial vaginosis |
Abbreviations: HIV, human immunodeficiency virus.
In the second in the series, Beck et al3 address a number of relevant logistic aspects in designing experimental systems to simulate or inform human biology. They carefully consider the attention to detail required to generate meaningful data on the lung microbiome and its implications, including standardization between investigators, the need for appropriate statistical methods, the pitfalls of small data sets, the need for longitudinal studies to define the stability of the microbiome, and the potential for confounding by use of different methods. In addition to the risk of contamination between anatomic sites, they propose that true differences in microbial populations are present within microenvironments within any tissue, such as the lung, just as geographic differences will be present between patient groups. Their consideration of the relevance and challenge of discriminating between live and dead organisms with molecular methods is paralleled by an ongoing controversy as to whether noncultivable species identified by sequencing are relevant as potential pathogens compared with those readily grown in culture. On a pathophysiologic level, they advance and support the paradigm that the microbiome may modulate immune development, immune defense, allergy, inflammation, and immune tolerance, each of which may be mediated by specific organisms and mechanisms. Beck et al3 do not see the lung in isolation. Rather, they draw connections between its microbiome and immunologic responsiveness with that of the intestine and propose both microbiologic and immunologic links between these otherwise distinct anatomic sites.
With an ecological perspective, Forney et al4 focus less on the specific bacteria but more on their metabolic products as determinants of the organisms, whether commensals or pathogens, present in the vagina. Indeed, the lactic acid and associated low pH produced most often, but not exclusively, by Lactobacillus spp. are highlighted as a primary determinant of the microbiota present or absent in the vagina. An intriguing aspect, perhaps unique to the vaginal microbiome, is the influence of hormonal variation from early to adult life, throughout the menstrual cycle, and with menopause on the “vaginal microbial ecosystem.” These changes are reflected in the relative availability of glycogen locally and thus the presence of organisms capable of fermenting glycogen to lactic acid. The terms “richness,” referring to the number of species present, and “diversity,” which also considers the distribution of these species (Table I) are relevant to their discussion of how to characterize the vaginal microbiome in different conditions. Indeed, geographically and ethnically distinct women in each population at each anatomic site and at each time have both shared and distinct microbial constituents and environmental conditions, which are also affected by medications, contraceptives, antibiotics, lubricants, intercourse, and other behaviors. So, what is the “normal” microbial ecology of the vagina, how stable and resilient is it over time, and how can investigators distinguish effects on and from the host of these microbial populations and their products? In this context the authors highlight that the vaginal microbiota and resultant ecosystem should not be considered to be in a “commensal” relationship in which the organisms derive food from but provide no benefit to the host. Rather, the relationship is one of “mutualism” in which the organisms also provide protection against colonization and infection of the host by potentially pathogenic organisms. This perspective is reinforced by the clinical scenario in which development of symptomatic vaginal infections with, for example, Candida species follows treatment of urinary tract infections with antibacterial agents that modify the vaginal microbiome.
In the last article in the series, Wylie et al5 tackle the human virome, the collection of eukaryotic viruses and bacteriophage (bacterial viruses) that constitute a relatively little studied, yet likely critical, component of the human-microbe axis. Unlike with bacteria, the lack of a common genomic element in viruses (eg, a 16S rRNA gene) greatly complicates novel viral discovery by necessitating viral enrichment schemes or deep sequencing. Nevertheless, recent viral metagenomic surveys performed in a variety of human samples and disease contexts have revealed a staggering diversity of viral and bacteriophage genomes in even healthy individuals, many heretofore uncharacterized. Most provocatively, the identification of myriad human and bacterial genes spliced into viral and bacteriophage genomes predicts a substantial and ongoing flux of genetic information between all members of the human supra-organism.
Overall, the reviews in this volume provide a well-considered overview of the technical hurdles raised by metagenomic studies, such as which samples to survey, how best to procure and prepare specimens, how deeply to interrogate a microbial community, and how to analyze the data and compare populations. More important, the authors also confront the theoretic challenge of ascribing etiologic significance to the human microbiome in disease processes. Perturbations in the composition of the human microbiome have been noted in many diseases, yet we typically have only circumstantial evidence that the loss or gain of a particular group of microorganisms actually contributes to disease progression.44 This problem is not unique to studying the human microbiome, but to the fact that “dysbiosis” typically is observed in chronic, multifactorial diseases such as inflammatory bowel disease or obesity, and sometimes in healthy subjects. In these situations, the challenge is to envision how Koch’s postulates allow one to prove convincingly that a microbiome or metagenome causes disease or immune effects in humans, as has been elegantly and convincingly proven in mice.44–47 Rather, the tenants of risk factor epidemiology can be invoked to bolster the inferential case in humans. Even the author of the Bradford-Hill Criteria for assessing evidence of causation47 recognized that the “criteria,” including strength of association, consistency in different venues, specificity of effect, temporality, biological gradient, and plausibility, were not indisputable evidence for or against cause and effect. In humans, we must build the case from several perspectives, such as that advanced for smoking causing lung cancer or Helicobacter pylori causing gastric cancer.
Despite the impressive technologies that are being applied to the human microbiome, to date, translation of findings from bench to bedside has proven arduous. Studies involving human subjects require disentangling multiple, highly interlinked factors thought to contribute to disease. We are striving to move beyond “associated with” and “may be related to” in our efforts to place these data in a meaningful clinical and causal context. However, the reviews in this volume clearly and encouragingly indicate that the bedside is informing the bench. Indeed, the goal of translational research is to translate, to use and implement results generated in vitro and in vivo in animals into enhanced understanding of clinical human health and disease, and to introduce and test more effective interventions to prevent or treat disease with behavior, diet, medications, vaccines, or modulatory pre- and probiotics (Table II). The continuing development of metagenomic technology for culture-independent interrogation of the human microbiome and its integrated analysis will undoubtedly help us decipher the complex and unique interactions between ourselves and our microbial world within.
Acknowledgments
This work was supported by the Mucosal and Vaccine Research Colorado Program; National Institutes of Health Grants R01HD059527, R21AI083615, and R21HG005964; the Dean’s Strategic Research Committee grant to Mucosal and Vaccine Research Colorado Program; and the Veterans Affairs Research Service.
REFERENCES
- 1.Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dave M, Higgins PD, Middha S, Rioux KP. The human gut micro-biome: current knowledge, challenges and future directions. Transl Res 2012. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res 2012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forney L, Hickey R, Zhou X, Pierson J, Ravel J. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 2012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wylie KM, Weinstock GM, Storch GA. Emerging view of the human virome. Transl Res 2012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. The human nasal microbiota and Staphylococcus aureus carriage. PLos ONE 2010;5:e10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science 2009;324: 1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci U S A 2007;104:2927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 2009;15:1377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosh PK, McDonald LC. Infection control in the multidrug-resistant era: tending the human microbiome. Clin Infect Dis 2012;54:707–13. [DOI] [PubMed] [Google Scholar]
- 11.Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med 2012. April 22 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012;13:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 2011;9:233–43. [DOI] [PubMed] [Google Scholar]
- 14.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011;108:5354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010;328:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Round JL, Mazmanian SK. Inducible Foxp31 regulatory T-cell development by a commensal bacterium of the intestinal micro-biota. Proc Natl Acad Sci U S A 2010;107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011;331: 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011;478:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong C, Matos I, Choi JH, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(1) dendritic cells for immune T cell areas. Cell 2010;143:416–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005;122:107–18. [DOI] [PubMed] [Google Scholar]
- 22.Hapfelmeier S, Lawson MAE, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 2010;328:1705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004;303: 1662–5. [DOI] [PubMed] [Google Scholar]
- 24.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal micro-biota. Proc Natl Acad Sci U S A 2009;106:19256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sjogren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy 2009;39:1842–51. [DOI] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–131. [DOI] [PubMed] [Google Scholar]
- 27.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathis D, Benoist C. The influence of the microbiota on type-1 diabetes: on the threshold of a leap forward in our understanding. Immunol Rev 2012;245:239–49. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 2007;167:821–7. [DOI] [PubMed] [Google Scholar]
- 30.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008;22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank DN, Manigart O, Leroy V, et al. Altered vaginal microbiota are associated with perinatal mother-to-child HIV transmission in African women from Burkina Faso. J Acquir Immune Defic Syndr 2012. February 16 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 2009;106:3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010;16:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev 2005;206:260–76. [DOI] [PubMed] [Google Scholar]
- 38.Sartor RB. Intestinal microflora in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol 2001;17: 324–30. [DOI] [PubMed] [Google Scholar]
- 39.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 2007;19:59–69. [DOI] [PubMed] [Google Scholar]
- 40.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009;9:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 2002;22:283–307. [DOI] [PubMed] [Google Scholar]
- 42.Atarashi K, Tanoue T, Honda K. Induction of lamina propria Th17 cells by intestinal commensal bacteria. Vaccine 2010;28:8036–8. [DOI] [PubMed] [Google Scholar]
- 43.Khoruts A, Sadowsky MJ. Therapeutic transplantation of the distal gut microbiota. Mucosal Immunol 2011;4:4–7. [DOI] [PubMed] [Google Scholar]
- 44.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol 2011;19:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans AS. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med 1976;49:175–95. [PMC free article] [PubMed] [Google Scholar]
- 46.Fredricks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev 1996;9:18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]