Abstract
Hepatocellular carcinoma (HCC) is increasing as a cause of liver-related mortality largely due to the growing burden of nonalcoholic steatohepatitis (NASH). The mechanisms of HCC development in nonalcoholic fatty liver disease (NAFLD) are incompletely understood. We initially identified apoptosis antagonizing transcription factor (AATF) to be associated with HCC in a mouse model of NASH that develops HCC without the addition of specific carcinogens. AATF also called che-1, is a transcriptional factor that is highly conserved among eukaryotes. AATF is known to be a central mediator of the cellular responses as it promotes cell proliferation and also survival by inducing cell cycle arrest, autophagy, DNA repair and inhibition of apoptosis. However, the role of AATF in NASH and HCC remains unknown. Here, we provide evidence for AATF as a contributory factor for HCC in NAFLD. AATF overexpression was further verified in human NASH and HCC and multiple human HCC cell lines. TNFα, known to be increased in NASH, induced AATF expression. Promoter analysis of AATF revealed a SREBP-1c binding site; inhibition of SREBP-1 by using specific inhibitors as well as siRNA decreased TNFα-induced AATF expression. AATF interacted with STAT3 to increase MCP-1 expression. AATF knockdown decreased cell proliferation, migration, invasion, colony formation and anchorage-dependent growth in HCC cell lines. Xenograft of QGY-7703 HCC cells with AATF stably knocked down in to NSG mice demonstrated reduced tumorigenesis and metastases.
Conclusion:
AATF drives NAFLD and hepatocarcinogenesis, offering a potential target for therapeutic intervention.
Keywords: TNF-α, tumorigenesis, metastasis, MCP-1, STAT3
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related deaths worldwide (1,2). Non-alcoholic fatty liver disease (NAFLD) that manifest as non-alcoholic fatty liver (NAFL) or non-alcoholic steatohepatitis (NASH) is recognized as a common underlying risk factor for HCC and liver transplantation (3,4,5). The incidence of HCC related to NAFLD is increasing with the growing epidemic of obesity, type 2 diabetes mellitus and metabolic syndrome (6,7). While NAFLD-related cirrhosis is a known risk factor for HCC, recent clinical studies have also shown that a third of NAFLD-associated HCC occurred even prior to development of cirrhosis (8,9). These data underscore the need to better understand the molecular mechanisms underlying the development of HCC in NAFLD.
We first identified the apoptosis antagonizing transcriptional factor (AATF) to be overexpressed while performing an unbiased transcriptomic analysis of HCC in a diet-induced animal model of NAFLD (DIAMOND) which recapitulates many key features of the human disease and develops HCC without requiring additional carcinogen administration (10). AATF, also called che-1, is a transcriptional factor that is highly conserved among eukaryotes (11,12). AATF is known to promote cell proliferation and also survival by inducing cell cycle arrest, autophagy, DNA repair and inhibition of apoptosis (13–17). Recent studies have shown that AATF is up regulated in breast and lung cancer and leukemia (18–20). The role of AATF in HCC in the setting of NAFLD and the associated molecular mechanisms were not known.
The overall objective of the current study was to validate AATF as a potential driver of HCC in NAFLD. The specific objectives of the study were to: (1) confirm that AATF expression was increased in human and mouse tissue with NASH and HCC, (2) define the mechanisms for increased AATF expression in NAFLD, (3) define the impact of AATF on key oncogenic properties of HCC, and (4) identify the oncogenic signaling pathways activated by AATF in NAFLD and HCC. These provide novel data on AATF expression in NASH and insights in to how it augments HCC progression. Together, these findings demonstrate the functional significance of AATF in NAFLD and HCC, offering a viable potential target for therapeutic intervention.
Materials and Methods
Cell Culture.
HepG2 and Hepa1–6 (obtained from ATCC), Huh7 and QGY-7703 cells [kind donation from Dr. Devanand Sarkar (21)] were cultured in Dulbecco’s modified eagle’s medium (DMEM; Invitrogen, Carlsbad, CA). THLE-3 cells (obtained from ATCC) were cultured in Bronchial epithelial cell growth medium (BEGM) along with 5 ng/ml EGF and 70 ng/ml phosphoethanolamine on precoated plates. All the media were supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA) and 100 units/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA), and the cells were incubated at 37°C in 5% CO2.
Animal Model.
The animals were housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
DIAMOND mice:
A unique isogenic mouse strain derived from C57BL/6J and 129S1/SvImJ background (B6/129) was maintained with inbreeding as described previously (10). Male mice (8–10 weeks of age) were fed ad libitum a high fat diet, high carbohydrate diet (Western diet, WD) with 42% kcal from fat and containing 0.1% cholesterol (Harlan TD. 88137) with a high fructose-glucose solution (SW, 23.1 g/L d-fructose +18.9 g/L d-glucose). Control mice were fed a standard chow diet (CD, Harlan TD. 7012) with normal water (NW). All mice were maintained on a 12 hour light/dark cycle in a 21–24°C facility and were euthanized at varying time points following initiation of dietary intervention.
NSG Mice:
For the in vivo tumorigenesis and metastasis study, we used NOD scid gamma (NSG) female mice (8–10 weeks) from Jackson laboratory. All mice were maintained on a 12 hour light/dark cycle with ad libitum access to water and normal chow diet.
Human Subjects.
Human normal, NASH or HCC liver tissue (frozen, tissue blocks or slides) were obtained through the Liver Tissue Cell Distribution System (LTCDS), Minneapolis, Minnesota and Cooperative Human Tissue Network (CHTN)-western division.
Human serum samples were obtained at the author’s institution. Subjects with NAFLD and HCC were recruited from fatty liver disease and primary care clinics. All subjects provided informed consent, and the study was approved by the Virginia Commonwealth University institutional review board.
Human Angiogenesis Array.
Cells were cultured to 75–80% confluence and the media was changed to serum free media for 24 hours. Supernatants of cells cultured in serum free media (conditioned media) were analyzed for the expression of angiogenesis associated molecules using human angiogenesis antibody array kit following the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Migration Assay.
Cells were seeded into 24 well culture plate to 70–80% confluence forming a monolayer. Gently a scratch was made using a 1 ml pipette tip across the center of the well and another scratch perpendicular to the first line creating a cross in each well. The media was replaced with fresh media after scratching to remove the detached cells. After 24 hours, the cells were observed under the microscope. The images were then analyzed and the gap distance was measured using ImageJ software.
Invasion Assay.
Invasion assay was performed using BD BioCoat Matrigel Invasion Chamber (BD Biosciences, Franklin Lakes, NJ) following the manufacturer’s instructions. Pre warmed serum free media was added to the matrigel for 2 hours at room temperature for rehydration. 5×104 cells were seeded in the upper chamber in serum free medium while the wells of the lower chamber were filled with medium containing 5% FBS. Following incubation for 22 hours at 37°C, the cells on the upper surface of the filters were removed by wiping with a cotton swab and the cells attached on the lower surface of the filters were fixed and stained with Diff-Quik stain (IMEB Inc., San Marcos, CA). The membranes were photographed using a microscope and invasion was determined by counting the cells using ImageJ software.
Anchorage-independent Growth Assay.
Cells were seeded at 105 cells/plate in 6 cm dishes with culture media containing 0.4% noble agar (Sigma-Aldrich, St. Louis, MO) over a 0.8% agar base layer and cultured at 37°C incubator with 5% CO2 for 2 weeks. The colonies formed were counted manually under the microscope and photographed.
Colony Formation Assay.
Cells were seeded in 6 cm dishes at a density of 1000 cells per plate and cultured for about 2 weeks until the colonies are visible. The cells were fixed in formaldehyde and stained with 10% Giemsa (Sigma-Aldrich, St. Louis, MO). After rinsing with water, the plated were air dried, visualized under the microscope and photographed. The images were analyzed using ImageJ software and colonies counted.
Mice Xenograft studies.
Subcutaneous xenografts were established in the flanks of NSG mice using 6.5×105 control and AATF knockdown QGY-7703 cells. Body weight and tumor volume were measured twice weekly. The mice were euthanized after 4 weeks.
For the metastasis study, 1×106 cells (control and AATF knockdown QGY-7703 cells) were intravenously injected through the tail vein in NSG mice. After 4 weeks, the lungs and liver were isolated and analyzed.
Statistical Analysis.
Data are presented as the means ± SEM. Statistical significance was analyzed using 2-tailed unpaired Student’s t test. GraphPad Prism software (version 6) was used for all statistical analysis and p values ˂ 0.05 were considered significant.
Results
Upregulation of AATF in NAFLD and HCC
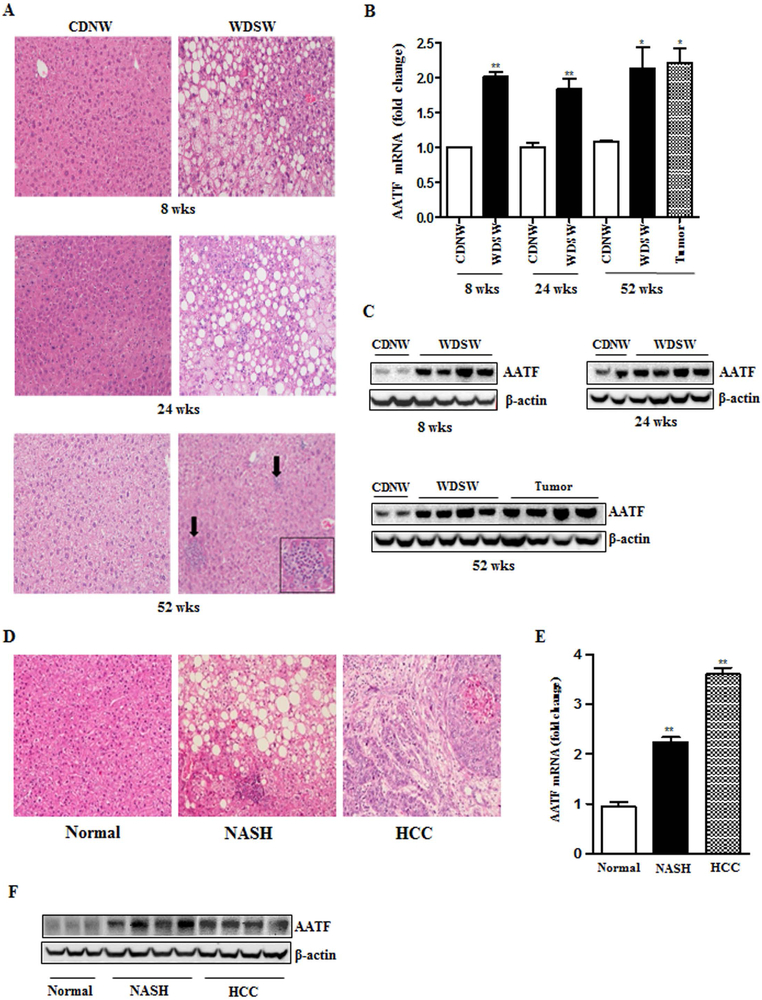
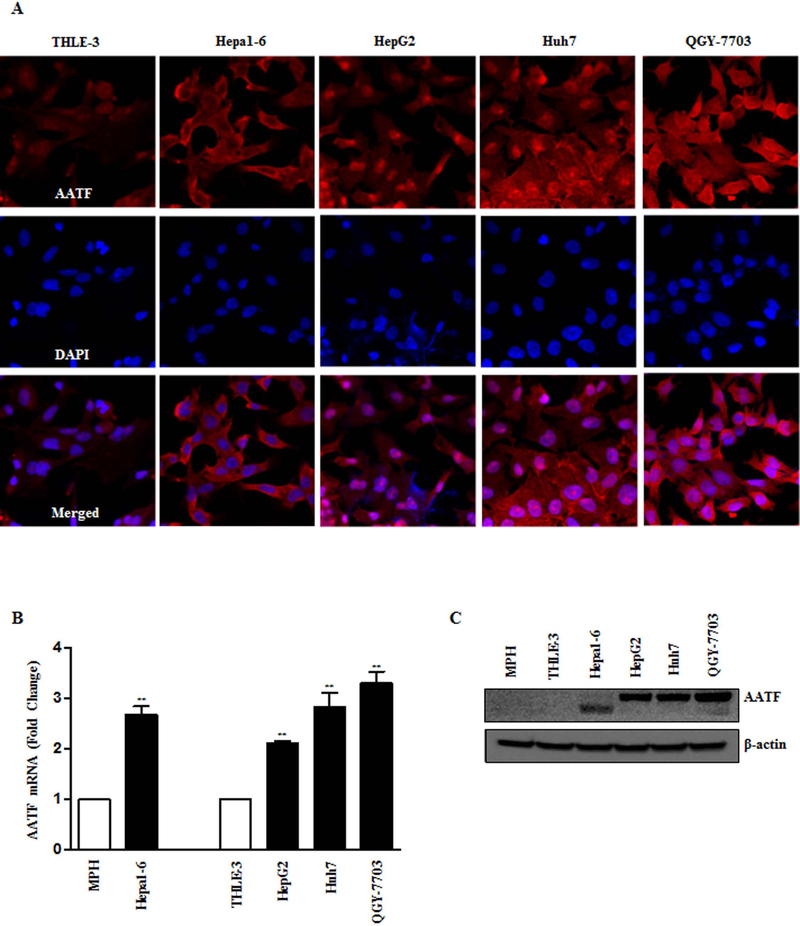
AATF mRNA and protein were measured in the liver of DIAMOND mice, at the stages of fatty liver (8 weeks), steatohepatitis with stage 1–2 fibrosis (24 weeks) and advanced fibrosis and HCC (52 weeks) after initiation of western diet sugar water (WDSW) compared to chow diet normal water (CDNW) (Fig. 1A and Supplementary Fig. 1A and B). AATF expression was significantly higher in DIAMOND mice fed with WDSW compared to CDNW (Fig. 1B, 1C and Supplementary Fig. 2A). Similar results were obtained in human subjects, wherein AATF expression was upregulated in NASH and HCC patients compared to healthy normal controls (Fig. 1D-F and Supplementary Fig. 2B). Consistent with these observations, we also found that AATF expression was robustly upregulated in human and mouse HCC cells when compared to normal hepatocytes and normal immortalized human liver cells (Fig. 2A-C). AATF expression was also detected in the nucleus (Fig. 2A and Supplementary Fig. 2C). Taken together, these results suggest that AATF is significantly overexpressed in NAFLD and HCC both in humans and mice.
Figure 1. Expression of AATF in DIAMOND mice and human liver tissue.
(A) Representative Hematoxylin and Eosin (H&E) stained liver sections of DIAMOND mice fed with CDNW or WDSW for 8, 24 and 52 weeks. (B and C) AATF protein and mRNA expression in the liver tissues of DIAMOND mice fed with CDNW or WDSW for 8, 24 and 52 weeks. (D) Representative Hematoxylin and Eosin (H&E) stained liver sections of human normal, NASH and HCC subjects. (E and F) AATF protein and mRNA expression (n=18–20) in the liver tissues of human normal, NASH and HCC subjects. Data are expressed as the mean ± SEM (n= 6–8) (*p<0.05; **p<0.001).
Figure 2. Expression of AATF in mouse and human HCC cells.
(A-C) Analysis of AATF expression by immunofluorescence (A), q-PCR (B) and western blot (C) in normal and HCC cells of mouse and human origin. Data are expressed as the mean ± SEM of three experiments (*p<0.05; **p<0.001).
AATF is upregulated by TNF-α via SREBP1
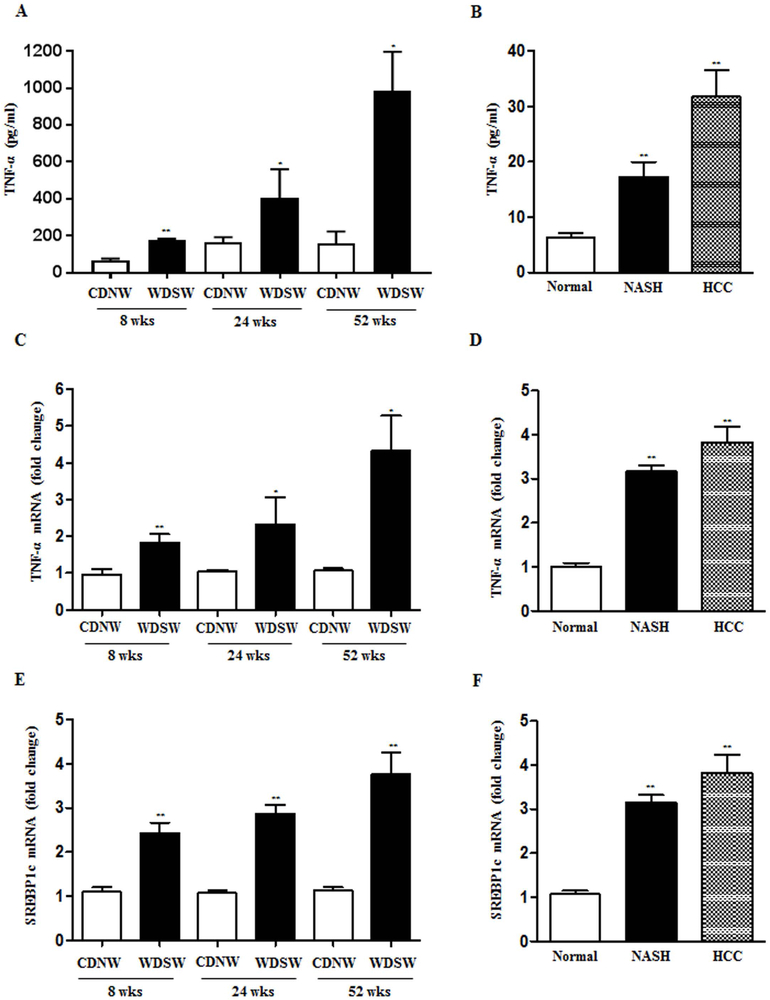
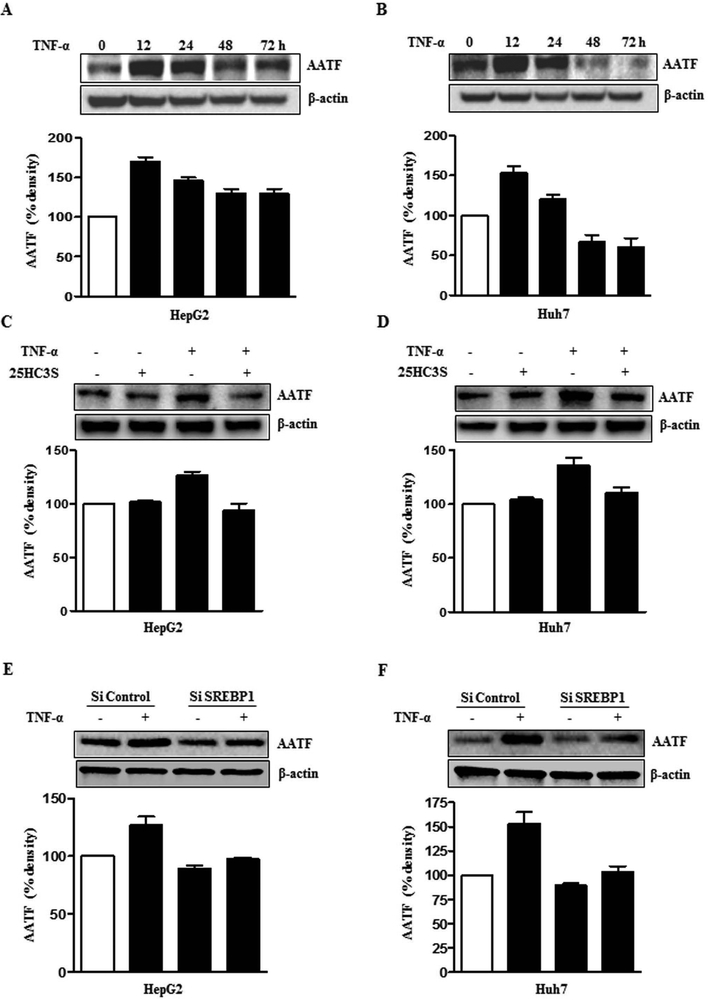
To examine the mechanisms underlying the upregulation of AATF in NAFLD, we sought to explore whether it is mediated by cytokines that contribute to obesity-associated inflammation. Since tumor necrosis factor (TNF)-α is known to play a key role among the proinflammatory cytokines in the pathogenesis of NASH (22), the levels of TNF-α was measured in the DIAMOND mice. Serum TNF-α levels in mice fed with WDSW were significantly higher than those with CDNW (Fig. 3A). In addition, liver tissue TNF-α mRNA levels was also found be higher in WDSW fed DIAMOND mice (Fig. 3C). Similarly, serum TNF-α levels and liver tissue TNF-α mRNA were higher in human subjects with NASH and HCC compared to healthy normal controls (Fig. 3B and 3D). The potential role of TNF-α in driving AATF expression was tested by treatment of HepG2 and Huh7 cells with a relevant dose (10 ng/ml) of TNF-α. These results demonstrated that TNF-α increased AATF expression in a time-dependent manner (Fig. 4A, 4B and Supplementary Fig. 3A).
Figure 3. Upregulation of TNF-α and SREBP1c in DIAMOND mice and human NASH and HCC subjects.
(A and C) ELISA and qPCR detection of TNF-α in DIAMOND mice fed with CDNW or WDSW at 8, 24 and 52 weeks. (B and D) ELISA and qPCR detection of TNF-α in human normal, NASH and HCC subjects. (E and F) SREBP1c mRNA expression in DIAMOND mice (E) and human normal, NASH and HCC subjects (F). Data are expressed as the mean ± SEM (mice, n= 8–10, human samples, n= 18–20) (*p<0.05; **p<0.001).
Figure 4. Upregulation of AATF expression by TNF-α via SREBP1.
(A and B) AATF protein expression in Hep2 and Huh7 cells upon TNF-α treatment. (C and D) AATF protein expression upon TNF-α treatment in the presence or absence of 25HC3S in HepG2 and Huh7 cells. (E and F) AATF protein expression in HepG2 and Huh7 cells transfected with control SiRNA or SREBP1 SiRNA with or without TNF-α treatment. Data are expressed as the mean ± SEM of three experiments (*p<0.05; **p<0.001).
To further investigate the transcriptional regulation of AATF in NAFLD, we performed AATF promoter analysis using the TRANSFAC database and identified the binding site of sterol regulatory element binding protein-1 (SREBP1), a master regulator of lipogenesis that is well known to be associated with NAFLD (23–25). We first confirmed that the expression of SREBP1-c was increased in DIAMOND mice following initiation of a western diet along with ad lib administration of sugar water (Fig. 3E). Similarly, SREBP1-c was upregulated in liver tissues of human NASH and HCC subjects compared to normal (Fig. 3F). Consistent with these results, the expression of SREBP1-c was upregulated upon TNF-α treatment in human HepG2 and Huh7 cells (Supplementary Fig. 3B).
Multiple approaches were then taken to determine whether SREBP1 played a role in the increase in AATF expression induced by TNF-α. First, a SiRNA approach was taken to silence SREBP1 (Supplementary Fig. 3C) and then a pharmacological approach to inhibit SREBP1 activation by 25HC3S (26) was taken in two separate human HCC cell lines (HepG2 and Huh7). Both approaches suppressed AATF expression upon TNF-α treatment (Fig. 4C-F). Furthermore, transfection of human AATF wild type or SREBP1c binding site deleted luciferase promoter construct in HepG2 and Huh7 cells confirmed the role of SREBP1c in AATF transactivation (Supplementary Fig. 4A and 4B). Collectively, these data demonstrates that the obesity-induced proinflammatory cytokine, TNF-α upregulates AATF expression through SREBP1.
AATF influences key oncogenic properties of human HCC cells in vitro and in vivo
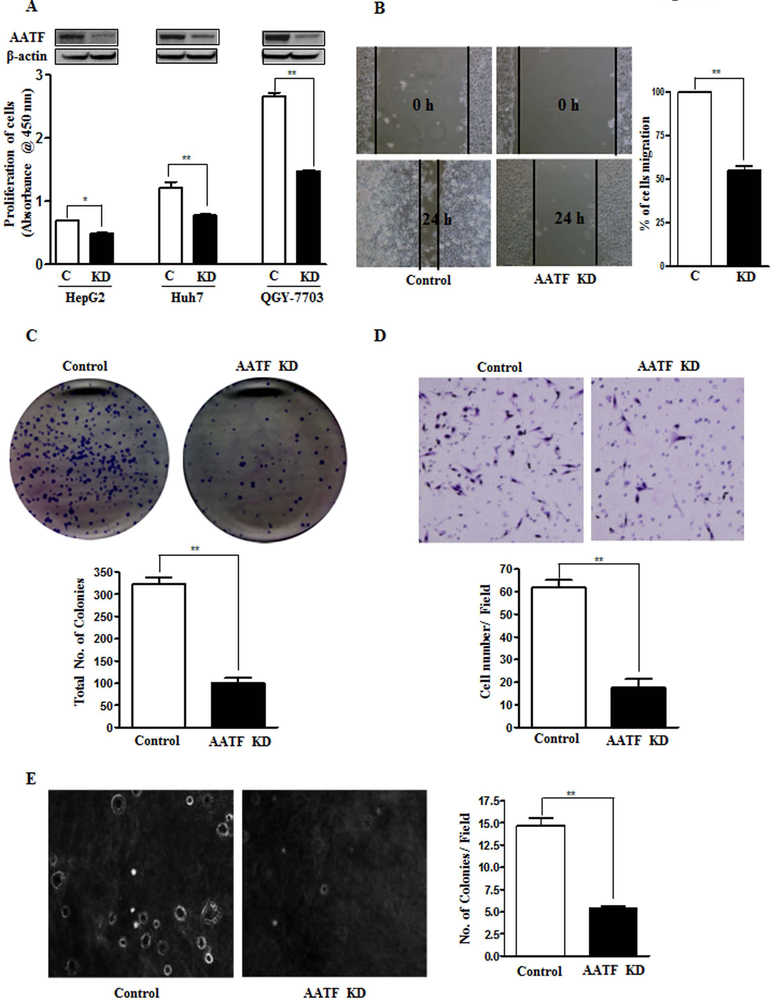
Compared to other HCC cell lines, QGY-7703 cells express high levels of AATF (Fig. 2). The pathophysiological effects of AATF was determined assessing the impact of its loss of function. This was accomplished by first establishing stable QGY-7703 cell lines (Supplementary Fig. 5A-C). We hypothesized that knockdown of AATF would affect the tumorigenic properties of QGY-7703 cells, a highly aggressive human hepatocellular carcinoma cell line (27). Consistent with this hypothesis, we found that knockdown of AATF inhibited the proliferative activity, migration, colony formation ability, matrigel invasion ability, and anchorage-independent growth in soft agar of QGY-7703 cells in vitro (Fig. 5A-E).
Figure 5. Knockdown of AATF inhibits proliferation, migration, anchorage-independent growth, invasion and colony formation of QGY-7703 cells.
(A) Cell proliferation was determined by the WST-1 assay in control and AATF knockdown cells of HepG2, Huh7 and QGY-7703. (Inset- AATF expression by western blot). (B-E) Representative images of migration assay (B), colony formation assay (C), invasion assay (D) and anchorage-independent growth assay (E) performed using control and AATF KD clones of QGY-7703 cells.
Data are expressed as the mean ± SEM of three experiments (*p<0.05; **p<0.001).
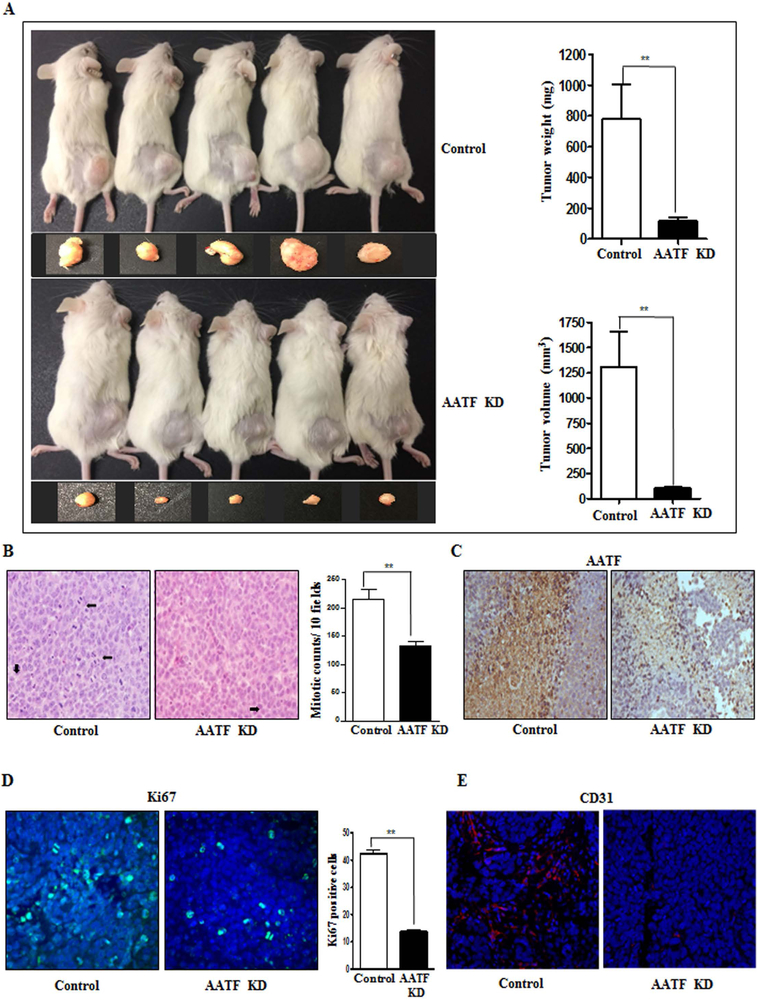
We further validated the oncogenicity of AATF by using the xenograft model. In line with the in vitro data, AATF knockdown clones of QGY-7703 cells formed significantly smaller tumors when implanted subcutaneously in the flanks of NSG mice compared to control QGY-7703 cells (Fig. 6A). Analysis of tumor sections revealed low mitotic counts, low AATF expression, low proliferative index (Ki67 expression) and reduced angiogenesis (CD31 expression) in AATF knockdown compared to control (Fig. 6B-E).
Figure 6. Knockdown of AATF decreases tumorigenesis of QGY-7703 cells in NSG mice.
(A) Representative excised tumors from NSG mice implanted with control or AATF knockdown clones of QGY-7703 cells and graphical representation of tumor weight and volume. (B) Representative images of mitotic cells in the tumor sections of control and AATF knockdown QGY-7703 cells. (C-E) Immunohistochemistry analysis of AATF (C), Ki67 (D) and CD31 (E) in the tumor sections of control and AATF Knockdown clones. Data are expressed as the mean ± SEM of n= 10–12 per group (*p<0.05; **p<0.001).
AATF affects the metastasizing potential of human HCC cells
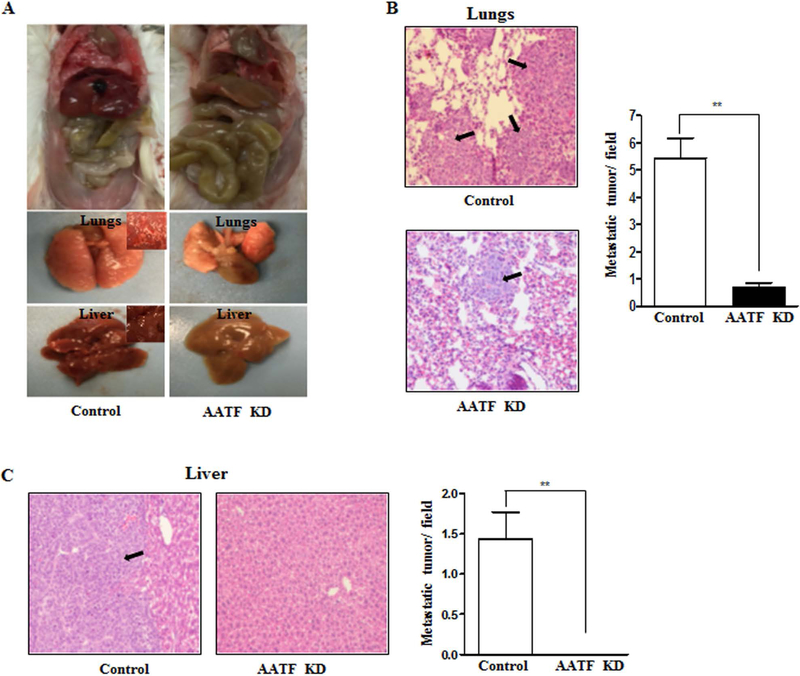
The effect of AATF on the metastasizing potential of QGY-7703 cells was next evaluated using the tail vein metastasis assay. Intravenous injection of control QGY-7703 cells resulted in the formation of tumors in the lungs and liver of NSG mice, whereas minimal or no metastasis was observed in AATF knockdown QGY-7703 cells (Fig. 7A). Staining of the lungs and liver identified solid nodules in the NSG mice injected with control QGY-7703 cells, whereas only few isolated metastatic nodules were seen in the lungs and none in the liver of AATF knockdown QGY-7703 cells injected mice (Fig. 7B and 7C). Taken together, these findings indicate that AATF promotes growth and invasion of HCC cells.
Figure 7. Knockdown of AATF inhibits metastasis of QGY-7703 cells in NSG mice.
(A) Representative images of the lungs and liver of NSG mice after tail vein metastasis assay. (B and C) H&E stained sections and graphical representation of metastatic tumors in the lungs and liver of NSG mice injected with control or AATF KD clones of QGY-7703 cells. Data are expressed as the mean ± SEM of n= 10–12 per group (*p<0.05; **p<0.001).
AATF induces MCP-1 expression through STAT3
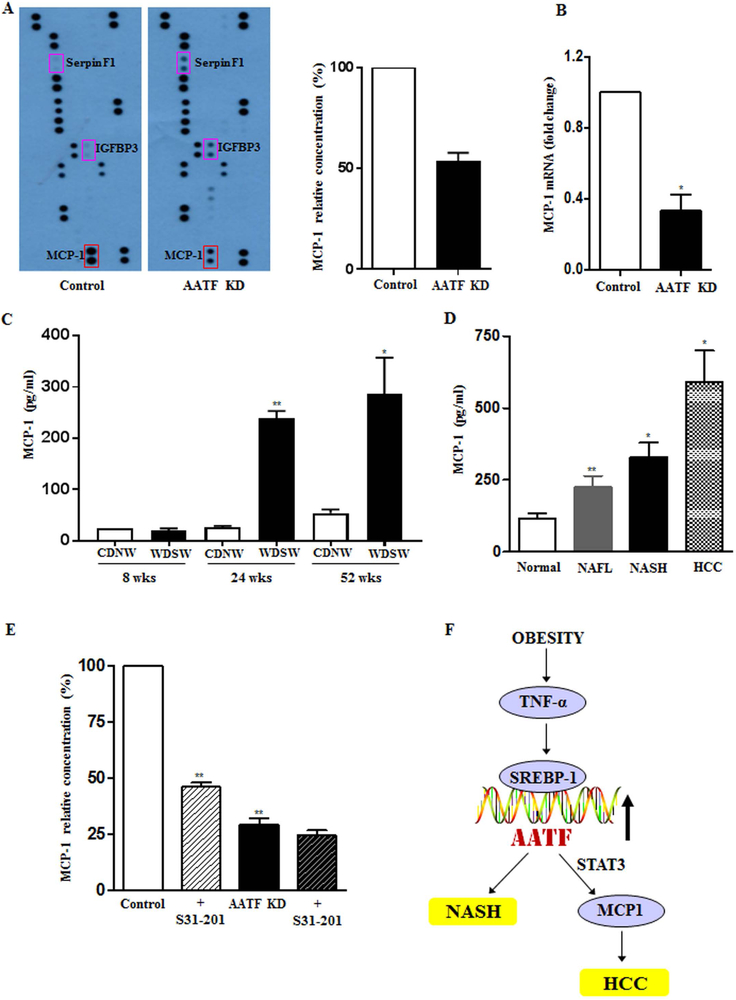
Next, we sought to explore the potential mechanisms of AATF could affect NASH and also oncogenesis. Two separate approaches were taken. First, since there was no a priori hypothesis to pursue, an unbiased approach was taken by evaluation of the effects of supernatants from control- and AATF knockdown QGY-7703 cells in a human angiogenesis array. The results demonstrated that the concentration of monocyte chemoattractant protein 1 (MCP-1), a chemokine that plays an important role in various inflammatory diseases including cancer (28, 29) and which was found to be high in control QGY-7703 cells, was significantly reduced in AATF KD QGY-7703 cells (Fig. 8A). This was accompanied by reduced MCP-1 mRNA expression in the AATF KD QGY-7703 cells (Fig. 8B).
Figure 8. MCP-1, the downstream target of AATF is regulated by AATF-STAT3 complex.
(A) Profiling of angiogenesis related proteins in the conditioned media of control and AATF KD clones by human angiogenesis antibody array. (B) mRNA expression of MCP-1 in control and AATF KD clones of QGY-7703 cells. (C and D) ELISA detection of MCP-1 in DIAMOND mice fed with CDNW or WDSW at 8, 24 and 52 weeks and in human normal, NAFL, NASH and HCC subjects. (E) MCP1 measurement by ELISA in the supernatant of control and AATF KD clones treated in the presence or absence of S31–201 (STAT3 inhibitor). (F) Mechanism of AATF-mediated regulation in the pathogenesis of NAFLD and HCC. Data are expressed as the mean ± SEM (n= 6–8) (*p<0.05; **p<0.001).
To corroborate these in vitro data in vivo, MCP-1 levels were measured in the plasma of DIAMOND mice at various time points after initiation of a high fat diet with ad lib administration of glucose/fructose as previously described (10). MCP-1 levels rose after 24 weeks corresponding to development of steatohepatitis with stage 2 fibrosis and then remained high up to the last time point measured (52 weeks) (Fig. 8C and supplementary figure 6A). Of note, the earliest tumors were noted from 32 weeks onwards in this model corresponding to steatohepatitis with bridging fibrosis. These data were further corroborated in human subjects with NASH and HCC compared to control subjects (Fig. 8D and supplementary Fig 6B). Additionally, Serpin F1 and insulin-like growth factor-binding protein 3 (IGFBP3), known for their antitumorigenic effects (30,31) were found to be high in AATF KD compared to control QGY-7703 cells (Fig. 8A). Similar results were obtained in the AATF depleted tumors compared to control (Supplementary Fig. 7A-C).
Next, we explored the interaction of AATF with STAT3, a known oncogenic pathway in HCC development. Of note, STAT3 is a known transcriptional modulator of MCP-1, wherein STAT3 binds directly to MCP-1 promoter and induces its expression (32, 33). We tested the hypothesis that AATF increases MCP-1, a cytokine that is known to play a role in NASH by activation of STAT3. Control QGY-7703 cells had high levels of STAT3 (Supplementary Fig. 6C) and also secreted high levels of MCP-1 compared to AATF Knock down cells (Fig. 8A). MCP-1 levels were measured in the presence or absence of STAT3 inhibitor. Upon inhibition of STAT3 by the inhibitor S31–201 (50 µM), there was a decrease in MCP-1 secretion (Fig. 8E). Immunoprecipitation analysis also confirmed the nuclear interaction of AATF and STAT3 in QGY-7703 cells (Supplementary Fig. 6C). Overall, the data indicate that AATF via STAT3 enhances the expression of MCP-1, a cytokine that is implicated in disease progression in NASH (34).
Discussion
HCC is the most prevalent primary malignancy of liver with high rates of mortality (35). NAFLD has emerged as the leading etiology of HCC and accounts for almost 50% of all HCC reported in the USA in claims-based data sets (36,37). Furthermore, NAFLD related HCC is associated with a shorter survival compared to HCC related to other etiologies and NAFLD is already the second leading etiology for HCC requiring liver transplantation (38,39). These sobering data highlight the need to better understand the complex process of NAFLD-associated hepatocarcinogenesis and identify key players that could be targeted for therapy. In this study, we have elucidated the unexplored regulatory role of AATF in promoting NAFLD progression and HCC (Fig. 8F).
The first novel finding is that AATF expression is increased in NAFLD, especially NASH as well as NASH-associated HCC. The data are concordant both in tissue obtained from humans, human HCC cell lines and in the DIAMOND model. The DIAMOND model develops HCC after 32 weeks following initiation of a high fat sugar water diet and has a transcriptomic signature which is concordant with human HCC (10). In this model, AATF expression increased early at a stage when NAFL was present and did not increase further with disease progression (Fig. 1). This could have several implications both for NASH and the development of HCC.
NASH is associated with endoplasmic reticulum stress and dysregulated activation of the unfolded protein response, which in turn perpetuates inflammatory signaling and activation of pro-apoptotic signaling (40,41). Furthermore, lipotoxic stress, ambient inflammatory cytokine exposure and activation of innate immune pathways all further promote cell death and inflammation (42,43). The increase in AATF in the liver may play a role in preventing massive hepatocellular injury by its known anti-apoptotic effects. On the other hand, increased MCP-1 expression, which is known to be present in NASH (34), is expected to promote inflammation. This was not however the focus of this study and the role of AATF in modulating the phenotype of NAFLD i.e. as a determinant of development of NAFL vs NASH and as a modulator of disease activity awaits experimental elucidation.
The current study also identified SREBP1 as a key activator of AATF in the setting of NAFLD. This data is consistent with the observation that AATF expression increases early in the course of the disease at a time when SREBP1 activation is already increased. SREBP1 is activated by hyperinsulinemia (25) and AATF is thus a novel mechanism by which hyperinsulinemia may modulate the NAFLD phenotype and hepatocarcinogenesis. It would also be expected that therapeutic agents that decrease SREBP1 activity would also reduce the risk of HCC development via decrease in AATF. The present study sets the stage for confirmation of this hypothesis in appropriately designed animal and human studies.
Perhaps the strongest evidence to support a role for AATF in hepatocarcinogenesis is the demonstration of decreased tumor burden and metastasis in the xenograft model (Fig. 6 and Fig. 7). This is further corroborated by confirmation of its impact on the key oncogenic properties of the QGY-7703 cells such as increased cell proliferation, mitosis, decreased apoptosis, cell migration, angiogenesis and anchorage-independent growth. These provide a rationale to continue to develop AATF as a target for the prevention and treatment of HCC in NAFLD.
The current study also provides initial insights in to the mechanisms by which AATF may promote hepatocarcinogenesis. It was already known that it promoted cell cycle progression and prevented apoptosis, specifically apoptosis induced by DAP-like kinase (44). This study demonstrates AATF interaction with STAT3 to promote MCP-1 expression, a novel pathway which potentially explains how AATF promotes HCC. There may also be other NAFLD associated HCC pathways and a key future direction will be to evaluate if targeting AATF alone is sufficient for HCC prevention and if the current ongoing long-term pivotal clinical trials blocking CCR2-CCR5, the receptors for MCP-1 lead to decreased HCC formation (45).
As with many novel observations, new questions arise that need to be answered. The impact of AATF knockdown at various phases of NAFLD (NAFL vs NASH with early or advanced fibrosis) on HCC development needs to be determined. The specificity of the AATF pathway for NAFLD-associated HCC also warrants evaluation. Further work on the oncogenic signaling networks in HCC and how these are impacted by AATF (Supplementary fig. 2D) is another key area worthy of future investigation. While it is clear that much additional work is needed before the observations of this study can be translated in to human trials, the present study provide a novel target for intervention and direction for studies to reduce the burden of HCC due to NAFLD.
Supplementary Material
Acknowledgements
We would like to thank Dr. Bin Hu, VCU Massey Cancer Center (MCC) for his excellent technical assistance and Dr. Jennifer Koblinski, Director of Cancer Mouse Models Core facility (CMMC) for guidance with xenograft study. The VCU Massey Cancer Center Cancer Mouse Model Shared Resource is supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. Confocal microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059. We thank Dr. Shunlin Ren for providing us with the chemical, 25HC3S and Dr. Devanand Sarkar for the QGY-7703 and huh7 cells. Human normal, NASH or HCC liver tissue samples were obtained through the Liver Tissue Cell Distribution System, Minneapolis, Minnesota, funded by NIH Contract #HHSN276201200017C and Cooperative Human Tissue Network (CHTN)-western division, a National Cancer Institute supported resource.
Grant Support: This work was supported by NIH grants RO1 DK 081450 and T32 DK 007150–38 to AJS.
Abbreviations:
- AATF
apoptosis antagonizing transcription factor
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- HCC
hepatocellular carcinoma
- TNF-α
tumor necrosis factor-α
- MCP-1
monocyte chemoattractant protein- 1
- STAT3
signal transducer and activator of transcription 3
- mRNA
messenger ribonucleic acid
- DIAMOND
diet-induced animal model of non-alcoholic fatty liver disease
- CDNW
chow diet normal water
- WDSW
western diet sugar water
- SREBP1-c
sterol regulatory element binding transcription factor 1-c
- siRNA
small interfering RNA
- NSG
NOD (non-obese diabetic) scid gamma mice
- CD31
cluster of differentiation 31
- KD
knockdown
- C
control
- IGFBP3
insulin-like growth factor binding protein 3
- DLK
DAP (death associated protein) like kinase.
Footnotes
Competing Financial Interest: The following authors have no potential conflict of interest in regards to this manuscript.
Divya Prasanna Kumar, Prasanna Santhekadur, Cora Uram-Tuculescu, Mulugeta Seneshaw
Faridoddin Mirshahi has stock in Sanyal Biotechnologies
Arun J. Sanyal: Dr. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect. He has served as a consultant to AbbVie, Astra Zeneca, Nitto Denko, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Fibrogen, Jannsen, Gilead, Boehringer, Lilly, Zafgen, Novartis, Novo Nordisk and Pfizer. He has served as an unpaid consultant to Exhalenz, Intercept, Echosens, Immuron, Galectin, Fractyl, Northsea Pharma, Gencia, Syntlogic, Affimune, Chemomab, Nordic Bioscience Zydus and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn K, London W. The Global Epidemiology of Hepatocellular Carcinoma: Present and Future. Clin Liver Dis. 2011; 15: 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White DL, Kanwal F, El-Serag H. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012; 10: 1342–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013; 10: 656–665. [DOI] [PubMed] [Google Scholar]
- 5.Charrez B, Qiao L, Hebbard L. Hepatocellular carcinoma and non-alcoholic steatohepatitis: The state of play. World J. Gastroenterol. 2016; 22: 2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidell JC. Obesity, insulin resistance and diabetes — a worldwide epidemic. Br. J. Nutr. 2000; 83: 10–13. [DOI] [PubMed] [Google Scholar]
- 7.Byrne CD, Targher G. NAFLD: A multisystem disease. J. Hepatol. 2015; 62: S47–S64. [DOI] [PubMed] [Google Scholar]
- 8.Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J. Gastroenterol. 2009; 44: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 9.Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer. 2011; 128: 2436–2443. [DOI] [PubMed] [Google Scholar]
- 10.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer Amon. J. Hepatol. 2016; 65: 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindfors K, Halttunen T, Huotari P, Nupponen N, Vihinen M, Visakorpi T, et al. Identification of novel transcription factor-like gene from human intestinal cells. Biochem. Biophys. Res. Commun. 2000; 276: 660–666. [DOI] [PubMed] [Google Scholar]
- 12.Fanciulli M, Bruno T, Di Padova M, De Angelis R, Iezzi S, Iacobini C, et al. Identification of a novel partner of RNA polymerase II subunit 11, Che-1, which interacts with and affects the growth suppression function of Rb. FASEB J. 2000; 14: 904–912. [DOI] [PubMed] [Google Scholar]
- 13.Bruno T, De Angelis R, De Nicola F, Barbato C, Di Padova M, Corbi N, et al. Che-1 affects cell growth by interfering with the recruitment of HDAC1 by Rb. Cancer Cell. 2002; 2: 387–399. [DOI] [PubMed] [Google Scholar]
- 14.Bruno T, Iezzi S, De Nicola F, Di Padova M, Desantis a, Scarsella M, et al. Che-1 activates XIAP expression in response to DNA damage. Cell Death Differ. 2008; 15: 515–520. [DOI] [PubMed] [Google Scholar]
- 15.Desantis A, Bruno T, Catena V, De Nicola F, Goeman F, Iezzi S, et al. Che-1-induced inhibition of mTOR pathway enables stress-induced autophagy. EMBO J. 2015; 34: 1214–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desantis A, Bruno T, Catena V, De Nicola F, Goeman F, Iezzi S, et al. Che-1 modulates the decision between cell cycle arrest and apoptosis by its binding to p53. Cell Death Dis. 2015; 6: e1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbato C, Corbi N, Canu N, Fanciulli M, Serafino A, Ciotti M, et al. Rb binding protein Che-1 interacts with Tau in cerebellar granule neurons. Modulation during neuronal apoptosis. Mol. Cell. Neurosci. 2003; 24: 1038–1050. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M Apoptosis-antagonizing transcription factor (AATF) gene silencing: role in induction of apoptosis and down-regulation of estrogen receptor in breast cancer cells. Biotechnol. Lett. 2013; 35: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 19.Welcker D, Jain M, Khurshid S, Jokić M, Höhne M, Schmitt A, et al. AATF suppresses apoptosis, promotes proliferation and is critical for Kras-driven lung cancer. Oncogene. 2018; 1503–1518. [DOI] [PubMed] [Google Scholar]
- 20.Kaul D, Mehrotra A. Functional characterization of AATF transcriptome in human leukemic cells. Mol. Cell. Biochem. 2007; 297: 215–220. [DOI] [PubMed] [Google Scholar]
- 21.Wang JB Establishment and some characteristics of a hepatoma cell line (QGY-7703). Zhonghua Zhong Liu Za Zhi. 1981; 3: 241–244. [PubMed] [Google Scholar]
- 22.Lang CH, Dobrescu C, and Bagby GJ (1992). Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992; 130: 43–52. [DOI] [PubMed] [Google Scholar]
- 23.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002; 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferré P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007; 68: 72–82. [DOI] [PubMed] [Google Scholar]
- 25.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008; 21: 507–511. [PubMed] [Google Scholar]
- 26.Ren S, Li X, Rodriguez-Agudo D, Gil G, Hylemon P, Pandak WM. Sulfated oxysterol, 25HC3S, is a potent regulator of lipid metabolism in human hepatocytes. Biochem. Biophys. Res. Commun. 2007; 360: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su ZZ, Luo ZY, Guo LP, Li JZ, Liu YL. Inhibitory effect of parvovirus H-1 on cultured human tumour cells or transformed cells. Sc. Sin. B. 1998; 31: 69–80. [PubMed] [Google Scholar]
- 28.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009; 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016; 7: 28697–28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999; 285: 245–248. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Choi DS, Lee OH, Oh SH, Lippman SM, Lee HY. Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1 / 2 activation and Egr-1 – mediated transcriptional events. Blood. 2015; 118:2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z, Neupane M, Zhou HR, Wu D, Chang CC, Moustaid-Moussa N, et al. Leptin differentially regulate STAT3 activation in ob/ob mouse adipose mesenchymal stem cells. Nutr. Metab. (Lond). 2012; 9: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potula HS, Wang D, Quyen DV, Singh NK, Kundumani-Sridharan V, Karpurapu M, et al. Src-dependent STAT-3-mediated expression of monocyte chemoattractant protein-1 is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. J. Biol. Chem. 2009; 284: 31142–31155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haukeland JW, Damas JK, Konopski Z, Lobeg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006; 44: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 35.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology. 2010; 51: 1820–1832. [DOI] [PubMed] [Google Scholar]
- 36.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002; 36: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 37.Baffy G Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: Epidemiology, Pathogenesis, and Prevention. J. Clin. Transl. Hepatol. 2013; 1: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pais R, Baritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P et al. NAFLD and liver transplantation: Current burden and expected challenges. J. Hepatol. 2016; 65: 1245–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 40.Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014; 20:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henkel A, Green RM. The unfolded protein response in fatty liver disease. Semin. Liver Dis. 2013; 33: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirsova P, Ibrabim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J. Lipid Res. 2016; 57: 1758–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016; 61: 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page G, Lödige I, Kögel D, Scheidtmann KH. AATF, a novel transcription factor that interacts with Dlk/ZIP kinase and interferes with apoptosis. FEBS Lett. 1999; 462: 187–191. [DOI] [PubMed] [Google Scholar]
- 45.Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, et al. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp. Clin. Trials. 2016; 47: 356–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.