Abstract
The efficacy of proton pump inhibitor (PPI) medications is highly dependent on plasma concentrations, which varies considerably due to cytochrome P450 (CYP2C19) genetic variation. We conducted a pragmatic, pilot study of CYP2C19 genotype‐guided pediatric dosing of PPI medications. Children aged 5–17 years old with gastric‐acid‐related conditions were randomized to receive either conventional dosing of a PPI or genotype‐guided dosing for a total of 12 weeks. Sixty children (30 in each arm) were enrolled and had comparable baseline characteristics. The mean daily omeprazole equivalent dose prescribed to participants across metabolizer phenotype groups was significantly different in the genotype‐guided dosing arm (P < 0.001), but not in the conventional dosing arm. Prescribers waited for the genotype result before prescribing the PPI medication for 90% of the participants in the genotype‐guided dosing arm. The number of participants who reported an infection was marginally lower in genotype‐guided dosing vs. conventional dosing (20% vs. 44%; P = 0.07). Sinonasal symptoms were higher in the conventional dosing arm as compared with genotype‐guided dosing arm: (2.6 (2.0, 3.4) vs. 1.8 (1.0, 2.3), P = 0.031). CYP2C19 genotype‐guided PPI therapy is feasible in a clinical pediatric setting, well accepted by providers, resulted in differential PPI dosing, and may reduce PPI‐associated infections. A future large scale randomized clinical trial of CYP2C19 genotype‐guided pediatric dosing of PPI medications in children is warranted.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
It is well known that efficacy (and perhaps safety) of PPIs is related to plasma drug concentrations, which are influenced by genetic variability in CYP2C19. It has been suggested to dose PPI by CYP2C19 genotype but it is unknown if it is feasible in pediatric specialty clinics.
what question did this study address?
This study sets out to determine if pharmacogenetic guided dosing of PPIs is feasible and if it leads to differential dosing of PPIs.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study found that the CYP2C19‐guided approach to dosing PPIs was feasible in children, led to differential dosing of PPIs, was accepted by providers, and reduced occurrence of infection without compromising efficacy.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
A large scale randomized clinical trial in children is warranted and may lead to widespread clinical implementation of CYP2C19‐genotype guided PPI dosing. This can be applicable for both children and adults as the pharmacokinetics of PPIs are similar between the two populations.
Gastroesophageal reflux disease (GERD) and related disorders are highly prevalent in both adults and children, 10–20% and 7%, respectively.1, 2 The efficacy of PPIs for GERD is well established in the pediatric population3 and current guidelines recommend a 4–12 week course of empiric proton pump inhibitor (PPI) medications for treatment of typical symptoms.4 However, there is emerging evidence that the use of PPIs has been associated with potentially serious adverse events, including respiratory infections,5, 6, 7, 8, 9, 10, 11, 12 gastric infections,8, 13, 14, 15 fractures,16, 17 as well as acute and chronic kidney disease.18, 19
PPI efficacy is highly dependent on plasma concentrations, which vary considerably owing in part to cytochrome P450 (CYP2C19) genetic variation in both pediatric,20, 21, 22 and adult populations.23, 24, 25, 26, 27 No function and increased function CYP2C19 alleles reduce and increase PPI hepatic clearance, respectively. This results in higher and lower concentrations, respectively, as compared with normal function alleles20, 21, 22, 23, 24, 25, 26, 27, 28 following administration of equal doses. Carriage of one or two no function alleles defines intermediate metabolizer (IM) and poor metabolizer (PM) phenotypes, respectively, carriage of one or two increased function alleles defines rapid metabolizer (RM) and ultrarapid metabolizer (UM) phenotypes, respectively, and carriage of no variant alleles defines the normal metabolizer (NM) phenotype.29 Across populations, 33% will carry one or more increased function alleles and be at risk for decreased PPI concentrations, whereas 30% will carry one or more decreased function alleles and be at risk for increased PPI concentrations.30, 31
Although PPIs are believed to be highly effective and safe, numerous studies have reported lower blood levels and higher treatment failures in RM and UM phenotypes compared with NM phenotypes.30, 32, 33, 34 Additionally, some studies have linked PPI‐associated respiratory infections to CYP2C19 PM phenotype.6, 35, 36 Indeed, several authors have recommended genotype‐guided PPI dosing to avoid treatment failures and adverse events.30, 37, 38 Implementing pharmacogenetic testing for certain gene‐drug pairs has been challenging, especially in a pediatric setting.39 The objective of the present study was to conduct a pilot study of genotype‐guided dosing of PPIs based on CYP2C19 metabolizer phenotype in pediatric patients to evaluate the feasibility for a larger multisite clinical trial. To determine implementation fidelity, we investigated the prescribed PPI dose as a measure of acceptance of the genotype‐guided recommendation. We hypothesized that clinical implementation of CYP2C19 genotype‐guided therapy in pediatric gastroenterology specialty clinics was feasible and would lead to differential dosing of PPIs.
Methods
Setting
This pilot was implemented in select clinics across the Nemours Children's Health System in Florida. The locations were the pediatric gastroenterology practice clinics at Nemours Children's Hospital in Orlando and Lake Mary, Florida, and Nemours Children's Specialty Care in Jacksonville, Florida.
Study Population
We planned a target sample size of 60 children over 6 months for our pilot study. Although a formal power calculation was not done, we believed this was a reasonable sample size to test the fidelity of the clinical implementation, as we expected, about 18 participants in the genotype‐guided dosing (GGD) arm to have an actionable genotype leading to a dosing recommendation. Children aged 5–17 years old with GERD, or other gastric‐acid related conditions (e.g., esophagitis, gastritis, and duodenitis), in which initiating PPI treatment was warranted, or whose symptoms were poorly controlled on existing PPI treatment, were eligible to participate. Study exclusion criteria included lack of access to the internet, extensive esophageal or gastric surgery, major chronic conditions that, in the opinion of the provider, would interfere with participation, and an inability to communicate in English. Participation required written parental informed permission for all ages and, in addition, assent was required from children age 7 years and older. Study procedures and data collection were approved by the Nemours Children's Health System Institutional Review Board, and all procedures were in accordance with the ethical standards of the Declaration of Helsinki. The study is registered on ClinicalTrials.gov (trial no. NCT02930824).
Genotyping
Participants were randomized to receive a PPI medication using either standard weight‐based conventional dosing (CD) per the US Food and Drug Administration (FDA) approved product label (https://www.accessdata.fda.gov/scripts/cder/daf/), or CYP2C19 GGD for a total of 12 weeks. Figure 1 depicts the study flow, including the dosing recommendations in the GGD arm. The allocation sequence for randomization was generated using the website Randomization.com (http://www.randomization.com). All participants provided a saliva sample using Oragene OGR‐575 saliva collection kits from DNA Genotek, and some also provided a buccal sample at baseline pending on their clinic site. The genotype results for participants in the CD arm were blinded to participants, their parents, and prescribers until the end of the study. Genotyping at the Orlando clinic site was done with a buccal sample by the Spartan RX (Spartan Bioscience, Ottawa, Ontario, Canada) system. Spartan RX is approved by the FDA as a qualitative in vitro diagnostic test to identify the presence of CYP2C19 *2, *3 and *17 alleles. One sample is run at a time within 1‐hour of collection, and takes ~1 hour to provide results. CYP2C19 genotyping on the Spartan RX system was validated using external standards and met all Clinical Laboratory Improvement Amendments requirements at Nemours Children's Hospital in Orlando. For the participants whose samples were collected when Spartan RX was not available (e.g., sample already running, Spartan RX not available on site), or had inconclusive results, their saliva samples were sent to a Clinical Laboratory Improvement Amendments‐licensed laboratory at the Nemours Alfred I. DuPont for Children Hospital. Phenotypes were assigned from the genotype, PMs had two no function alleles (*2 or *3), IMs carried a *1 allele and one no function allele, NMs had the *1/*1 or *2/*17 diplotype, RMs were *1/*17, and UMs were *17/*17.
Figure 1.
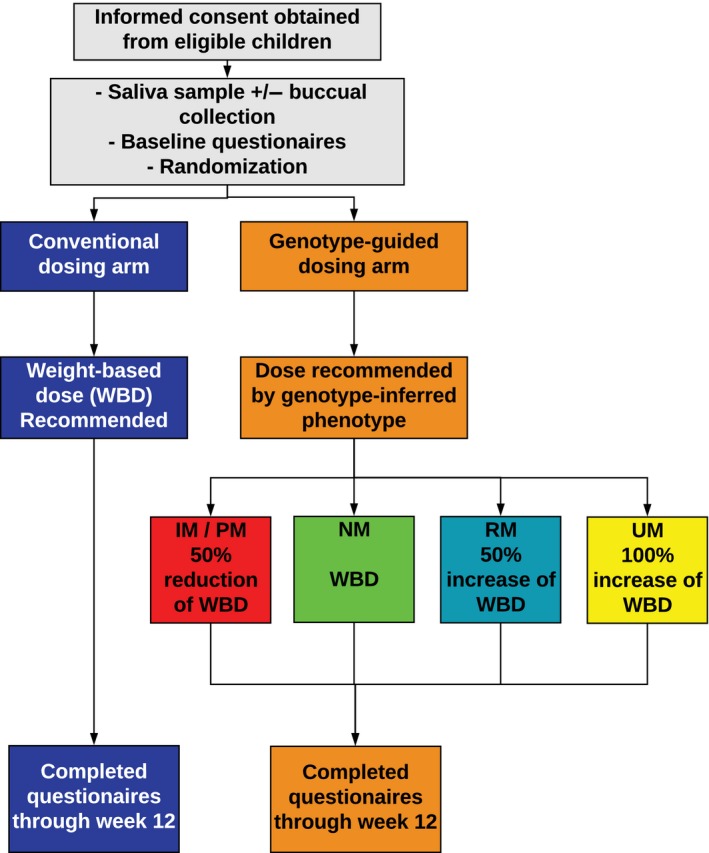
Study flow diagram. Children were eligible for the study if they were age 5‐17 years old and presented with symptoms of gastroesophageal reflux disease, or other gastric‐acid related conditions, where initiating a proton pump inhibitor (PPI) medication was warranted, or were on a subtherapeutic PPI. Once informed consent was obtained, participants provided a saliva sample and if enrolled from the Orlando site they also provided a buccal sample for genotyping, completed baseline questionnaires, and then were randomized 1:1 into either the conventional dosing arm or genotype‐guided dosing arm. Prescribers received a recommendation for the participant's PPI dose, once the participant started the PPI they completed questionnaires on a weekly basis throughout the study. IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; UM, ultrarapid metabolizer.
Dosing recommendations
Once the diplotype results were returned, the prescriber received the results and a per‐protocol dosing recommendation for the PPI via email from the study coordinator for participants in the GGD arm. The dosing recommendations were specific for CYP2C19 phenotype and based on previous recommendations published by our group,37 which are identical for the entire class of PPIs, and supported by primary literature.20, 24, 26 Specifically, for participants classified as PMs and IMs, a dose recommendation of decreasing the PPI dose by 50% was provided. For the participants classified as RMs and UMs, a dose recommendation of increasing the PPI dose by 50% and 100%, respectively, was provided. Participants in the CD treatment arm received weight‐based dosing recommendations that were based on the FDA approved label.
Data collection
All data were collected and managed using Research Electronic Data Capture, an electronic data capture tool hosted at Nemours Children's Hospital.40 Participants and their parents provided their baseline characteristics (e.g., age, gender, race, and ethnicity), medical diagnoses, and medication use at the baseline visit. Implementation metrics collected throughout the study included the date the DNA sample was collected, genotype results and date returned, recommended PPI dose, as well as PPI prescription information (date written, specific PPI, and dose). These measures allowed for calculation of genotype results turnaround time, length of time between genotype results and PPI prescription, as well as the number of recommendations accepted.
Pilot data on clinical outcomes, including safety and efficacy, were assessed by having participants and their parents complete questionnaires at baseline and throughout the study. There were four questionnaires in total, two assessed safety and two assessed efficacy. Occurrence of adverse events over the 12 weeks was captured by the Safety Questionnaire (SafetyQ), which was to be completed on a weekly basis by the parents. The SafetyQ, which has been used in a previous clinical trial,7 asked about the occurrence of seven different respiratory symptoms since their last visit (i.e., upper respiratory infection, sore throat, strep throat, bronchitis, pneumonia, ear infection, and acute sinusitis). In addition, a validated patient‐reported outcome, the SN‐5: Pediatric Sinonasal Symptom Survey41 was to be completed monthly by the parents. The SN‐5 metrics included sinus infection, nasal obstruction, allergy symptoms, emotional distress, activity limitations, and overall quality of life. The composite score takes the average of all aforementioned metrics excluding quality of life. The parents were asked how often each of these metrics was a problem for their child in the last 4 weeks. Efficacy of PPI therapy was evaluated using by two validated patient‐reported outcome questionnaires, the GERD Assessment of Symptoms in Pediatrics Questionnaire (Gasp‐Q)42 and Pediatric Quality of Life Inventory (PedsQL) Gastrointestinal Symptoms Module,43, 44 which were both completed weekly by the participants. The Gasp‐Q inquired about the severity and frequency of belly pain, chest pain, difficulty swallowing, choking, burping, nausea, pain after eating, night pain, and vomiting. The gastrointestinal problems included in the PedsQL were stomach pain and hurt, stomach upset, food and drink limits, trouble swallowing, heartburn and reflux, gas and bloating, constipation, diarrhea, and worry. The date the participants started their PPI therapy was confirmed with the family by the research coordinator and was used by Research Electronic Data Capture to automatically send participants a secure link to complete the questionnaires at the appropriate time points (e.g., after 1 week of PPI therapy).
Data analysis
The majority of the data analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC). We analyzed baseline characteristics and implementation measures between the two arms using χ2/Fishers Exact, or T test for categorical and continuous data, respectively.
To address our hypothesis of differential PPI doses, we analyzed the mean dose of PPI prescribed across phenotypes within each arm using Kruskal‐Wallis analysis and within each phenotype between arms using a Wilcoxon Rank Sum test. The PPI and dose prescribed for all participants was converted into omeprazole equivalents for analyses. The equivalents were based on the potency of the PPI as described by Kirchheiner and colleagues.45 The SafetyQ was analyzed by a Kaplan‐Meier analysis using GraphPad Prism (GraphPad Software, La Jolla, CA). We included all the results from participants’ who took their PPI for any duration but excluded those who reported an infection on the same day they started their PPI therapy. This analysis included only the questions inquiring about upper respiratory infection (URI), sore throat, and acute sinusitis, to determine if we could replicate previous published findings.6 For the SN‐5, which was completed at weeks 4, 8, and 12, we compared the patient‐reported scores reported at week 4 (or next available results) between the CD and GGD groups utilizing a Wilcoxon Rank Sum Test. We adjusted these end points for covariates, including the baseline score, race, and baseline medications using logistic regression. The scores from the efficacy questionnaires were also analyzed with a Wilcoxon Rank Sum Test, but the change in score from baseline to the week 4 ± 1‐week questionnaire was analyzed between arms. If a participant did not complete a follow‐up efficacy questionnaire during their third to fifth week of PPI therapy, or a SN‐5 questionnaire after week 4, their questionnaire results were excluded from the analyses. The SN‐5, Gasp‐Q, and PedsQL used the week 4 time point for analyses, as it has been shown that the greatest symptomatic improvement occurs in the first 2–4 weeks of PPI therapy,4 and because the greatest number of questionnaires were completed at week 4, with a limited number of completed questionnaires past this time point.
Results
Enrollment took place from March 9, 2017, to August 17, 2017. Approximately 71 children were approached for enrollment, 2 declined to participate and 9 were non‐English speaking and, thus, not eligible. Sixty participants were enrolled and randomized in a 1:1 allocation to CD (n = 30) or GGD (n = 30). Participants were followed for 12 weeks, with the last follow‐up occurring on October 23, 2017. Table 1 summarizes the baseline characteristics. Over half of the participants were female (56%), most were white (78%), and only a small portion reported taking a PPI at the start of the study (20%). Due to the limited number of participants treated with a PPI at entry (n = 12), they were treated the same as all other participants in the analyses. The mean age was 12 ± 3 years old. The CD and GGD arms were not significantly different from one another with regard to medical diagnoses, medication use, or baseline heartburn symptoms. One child in the GGD arm opted to not initiate PPI therapy and, thus, was not prescribed a PPI due to symptom improvement.
Table 1.
Baseline characteristics
| Conventional dosing n = 30 | Genotype‐guided dosing n = 30 | P value | |
|---|---|---|---|
| Age, mean ± SD | 12.5 ± 3.6 | 12.0 ± 3.7 | 0.66 |
| Gender, n (%) | |||
| Female | 17 (57) | 17 (57) | 1.00 |
| Race, n (%) | |||
| White | 27 (90) | 20 (67) | 0.10 |
| African American | 1 (3) | 3 (10) | |
| Other | 2 (7) | 7 (23) | |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 6 (20) | 7 (25) | 0.64 |
| Body composition, mean ± SD | |||
| Weight (kg) | 50.5 ± 20 | 49.8 ± 20 | 0.90 |
| BMI | 21.1 ± 4.8 | 20.5 ± 4.8 | 0.61 |
| BMI Z score | 0.6 ± 1.1 | 0.3 ± 1.7 | 0.46 |
| Medical information, n (%) | |||
| Prior GER diagnosis | 8 (27) | 10 (33) | 0.78 |
| Asthma | 5 (17) | 6 (20) | 0.79 |
| Bronchitis | 6 (20) | 4 (13) | 0.49 |
| Sinusitis | 7 (23) | 3 (10) | 0.30 |
| Pneumonia | 3 (10) | 1 (3) | 0.61 |
| Hay fever | 3(10) | 3 (10) | 1.00 |
| Seasonal allergies | 5 (17) | 3 (10) | 0.45 |
| Frequency of heartburn symptoms, n (%) | |||
| Daily | 8 (27) | 10 (33) | 0.80 |
| 2–6x per week | 15 (50) | 11 (37) | |
| 1–4x per month | 3 (10) | 5 (17) | |
| Never | 4 (13) | 4 (13) | |
| Medication use, n (%) | |||
| OTC antacida | 13 (43) | 12 (40) | 0.58 |
| H2RA | 7 (23) | 13 (47) | 0.09 |
| PPI | 8 (27) | 4 (13) | 0.20 |
| OME, mean ± SD | 19 ± 4 | 30 ± 20 | 0.34 |
| Omeprazole | 5 (62) | 3 (75) | 0.79 |
| Pantoprazole | 1 (12) | 0 (0) | |
| Lansoprazole | 1 (12) | 0 (0) | |
| Esomeprazole | 0 (0) | 1 (25) | |
| Unknown | 1 (12) | 0 (0) | |
BMI, body mass index; GER, gastroesophageal reflux; H2RA, histamine receptor antagonist; OTC, over‐the‐counter; OME, omeprazole equivalent dose in milligrams; PPI, proton pump inhibitor.
Examples of OTC include: sodium bicarbonate, magnesium hydroxide, aluminum hydroxide, calcium carbonate, and bismuth subsalicylate.
The number of participant questionnaires included in the questionnaire analyses varies, as it depends on the number of questionnaires completed. Although automatic reminders for the questionnaires were sent out to the families, they were not always completed. Overall, there was a 51% completion rate for all four questionnaires. In certain instances, participants completed some of the questionnaires for a particular week but not all of the questionnaires that were asked to be completed for that particular week. Two participants from the CD arm and four children from the GGD arm were lost to follow‐up, they were included in the SafetyQ, using their last day of contact, but were excluded from the analyses of the other questionnaires due to lack of questionnaire response data. For the Gasp‐Q, 40 participant's responses were analyzed (67%) (CD, n = 22; GGD, n = 18), 41 for the PedsQL (68%) (CD, n = 22; GGD, n = 19), and 34 for the SN‐5 (57%; CD, n = 18; GGD, n = 16).
Implementation metrics
The details of the implementation metrics are listed in Table 2. Regardless of the method of genotyping, prescribers waited for the genotype result before prescribing the PPI medication for 90% (n = 26/29) of the participants in the GGD arm. The remaining three participants received their PPI prescription prior to genotype results, two of whom received the prescription within 1 day of sample collection. The third participant received the prescription ~2 weeks after sample collection due to delayed genotype results. Twenty‐three samples were tested on Spartan RX, which had a success rate of 74% (n = 17), and genotype results returned the same day. The samples not genotyped successfully in Spartan RX either had an inconclusive result (n = 2) or were collected when the Spartan RX was not available (n = 4). The participants enrolled at the sites without a Spartan RX (n = 37), Lake Mary and Jacksonville, had their saliva sample genotyped at the Nemours Alfred I. DuPont Hospital for Children Laboratory. The turnaround time from sample collection to result for these samples was a median (interquartile range (IQR)) of 8.0 (7–12) days.
Table 2.
Implementation metrics
| CD n = 30 | GGD n = 30 | P value | |
|---|---|---|---|
| Genotyping procedure, n (%) | |||
| Spartan Rx | 11 (37) | 6 (20) | 1.00 |
| Laboratory developed test | 19 (63) | 24 (80) | 0.72 |
| TAT, median (IQR), days | 8 (7–13) | 10.5 (8–14) | 0.27 |
| PPI prescription | — | n = 29 a | — |
| Rx written after genotype results returned, n (%) | — | 26 (90) | — |
| Number of days after, median (IQR) | — | 1.5 (1–4) | — |
| Accepted recommendation, n (%) | — | 27 (93) | — |
CD, conventional dosing; GGD, genotype‐guided dosing; IQR, interquartile range; PPI, proton pump inhibitor; TAT, turnaround time.
One individual opted not to participate prior to PPI being prescribed.
The mean dose of PPI, expressed as omeprazole equivalents, prescribed to participants in both groups, distributed by phenotype is shown in Figure 2. The mean daily PPI doses prescribed to participants across phenotype groups in the GGD arm were significantly different (P < 0.001), although they were not different in the CD arm (P = 0.86). Further, the mean dose prescribed to participants who were RMs, IMs, or PMs, were significantly different between the CD and GGD arms (all P < 0.05), but not for NMs (P = 0.16). All active enrolled participants in the GGD arm received a recommendation for a specific PPI dose according to their phenotype. The majority of participants in the GGD arm (93% n = 27/29) were prescribed the recommended dose of PPI, according to their phenotype. The two participants who were not prescribed the recommended dose had RM and NM phenotypes, and were prescribed a lower and higher dose, respectively, which were deemed clinically necessary by the prescriber.
Figure 2.
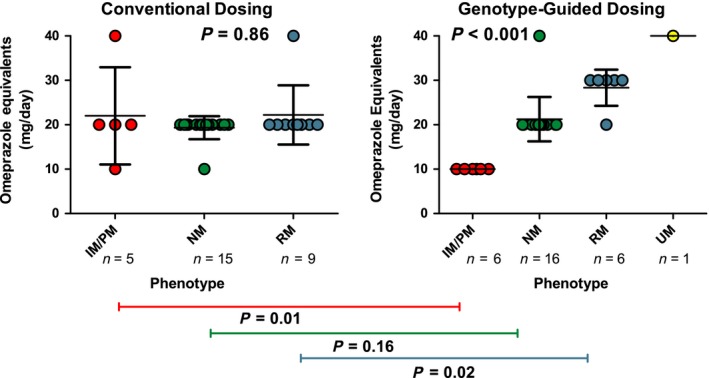
Mean dose of proton pump inhibitor (PPI) prescribed. The mean doses of PPI prescribed were compared across phenotypes within each arm using Kruskal‐Wallis analysis, we observed that the dose was significantly different between the phenotype groups in the genotype‐guided dosing arm (P < 0.001), but not in the conventional dosing arm (P = 0.86). The doses were also compared within each phenotype between arms using a Wilcoxon Rank Sum test, and we observed that the dose was significantly different between the two arms in both the intermediate metabolizers/poor metabolizers (IMs/PMs) and rapid metabolizers (RMs), but not in the normal metabolizers (NMs; P values 0.01, 0.02, and 0.016, respectively). The specific PPI prescribed was up to the discretion of the prescriber, majority were omeprazole (n = 51), followed by esomeprazole (n = 4), lansoprazole (n = 3), and pantoprazole (n = 1). All are expressed as omeprazole equivalents for comparison. CYP2C19 *2/*17 genotype was treated as a NM. UM, ultrarapid metabolizer.
Clinical outcomes
Figure 3 compares the occurrence of URI, sore throat, and acute sinusitis in participants in the GGD and CD arms as reported from the Safety Questionnaire over the 12‐week period. The number of participants who reported an infection was numerically lower in GGD vs. CD, but the difference did not achieve statistical significance (20% vs. 44%; hazard ratio = 2.4; 95% confidence interval = 0.9–6.3; P = 0.07)
Figure 3.
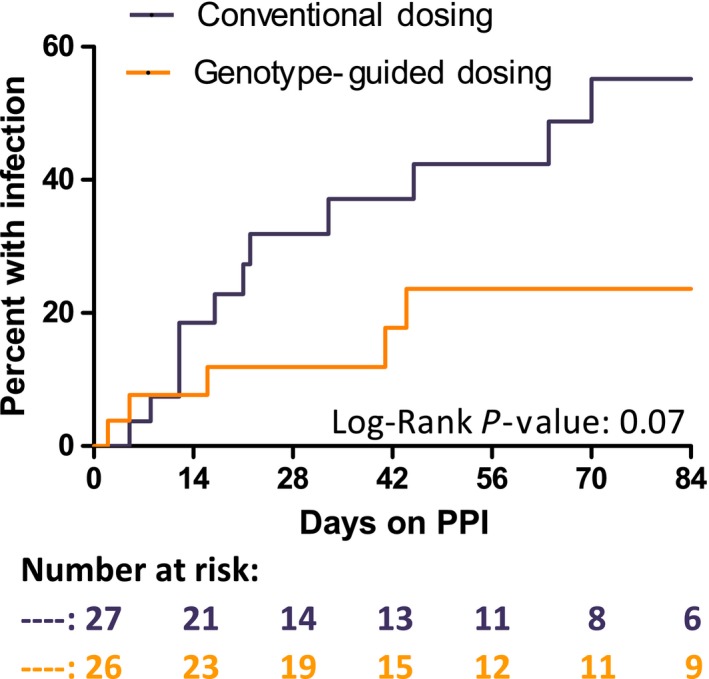
Kaplan‐Meier curve for infections reported in the Safety Questionnaire. Occurrence of upper respiratory infection, sore throat, or acute sinusitis over the 12‐week period or last date of follow‐up with participant was included (total n = 53). Participants who did not start a proton pump inhibitor (PPI; n = 5) or who reported an infection on the same day as they started PPI therapy (n = 2) were excluded from analysis. The conventional dosing (CD) arm included 27 participants with 12 events and the genotype‐guided dosing (GGD) arm included 26 participants with 5 events. The mean infection‐free time was 70 days for the CD arm and undefined for the GGD arm. The unadjusted hazard ratio was 2.42 with a 95% confidence interval of 0.93–6.29.
Table 3 describes the scores reported from the SN‐5. After an average of 35 days on PPI therapy, participants in the CD arm reported a higher frequency of sinonasal symptoms as compared with those in the GGD arm. Specifically, allergy symptoms were more common in the CD arm (median (IQR) 3.0 (2.0–4.0) vs. 1.0 (1.0–4.0); P = 0.006). The total SN‐5 score, which is a composite of sinus infection, nasal obstruction, allergy symptoms, emotional distress, and activity limitations, was higher in the CD arm as compared with the GGD arm: (2.6 (2.0–3.4) vs. 1.8 (1.0–2.3); P = 0.031). A sensitivity analysis of participants only on omeprazole revealed similar findings (Table S1).
Table 3.
Pediatric sinonasal symptom survey (SN‐5)
| SN‐5 components, median (IQR) | Conventional dosing n = 18 | Genotype‐guided dosing n = 16 | P value (unadjusted) | P valuea |
|---|---|---|---|---|
| Sinus infection | 2.0 (1.0–3.0) | 1.0 (1.0–2.5) | 0.201 | 0.721 |
| Nasal obstruction | 2.5 (1.0–4.0) | 1.0 (1.0–2.5) | 0.170 | 0.327 |
| Allergy symptoms | 3.0 (2.0–4.0) | 1.0 (1.0–2.0) | 0.007 | 0.006 |
| Emotional distress | 2.5 (1.0–5.0) | 1.5 (1.0–3.0) | 0.198 | 0.528 |
| Activity limitations | 1.0 (1.0–3.0) | 1.0 (1.0–1.0) | 0.092 | 0.244 |
| SN‐5 score | 2.6 (2.0–3.4) | 1.8 (1.0–2.3) | 0.012 | 0.031 |
IQR, interquartile range.
Adjusted for baseline score, race, baseline proton pump inhibitor use, baseline histamine receptor antagonist use.
There were no differences in the change of GERD symptoms from baseline to follow‐up (median of 39 days) for PedsQL and Gasp‐Q between the CD and GGD arms (Tables S2 and S3); the median (IQR) composite score for PedsQL was 5.4 (−1.7‐13.4) vs. 8.2 (−3.0‐21.1) and for Gasp‐Q, was −26.0 (−35.0‐10.0) vs. −24.0 (−32.0‐−9.0), suggesting efficacy of PPI was similar.
Discussion
Our study represents the first pragmatic trial of CYP2C19 GGD vs. CD of PPI medication therapy in children. PPI prescribing was at the discretion of the provider and we had few inclusion and exclusion criteria in an effort to mimic day‐to‐day clinical practice. We demonstrated feasibility of GGD for PPIs in specialized pediatric practices as over 90% of the participants in the GGD arm were prescribed the recommended PPI dose. Our hypothesis that the implementation would lead to differential dosing of PPIs proved to be true, indicating clinical acceptance of medication dosing recommendations in the GGD arm. We observed that the PPI dose was similar among participants with different CYP2C19 phenotypes in the CD arm but was significantly different among participants with different CYP2C19 phenotypes in the GGD arm (P < 0.001). This was to be expected as the prescribers were blinded to the CYP2C19 genotype for participants in the CD arm, and, thus, projected to be treated identically. Additionally, the PPI dose was different in both IMs/PMs and RMs between the two arms (P = 0.01 and 0.02, respectively), but not in NMs (P = 0.16), suggesting fidelity of the clinical implementation. The recommended dose for NMs in the GGD arm was the same weight‐based dosing recommendation as all of the participants in the CD arm, thus, lack of differences for NMs doses between arms was expected. Not only did the prescribers accept the recommended dose of PPI, but they, along with the parents, were willing to wait for the genotype results prior to prescribing the PPI. This was surprising as participants presented with GERD symptoms at the time of enrollment. The knowledge that parents are willing to wait for genotype results before starting their child on medication is valuable information, especially if same‐day genotyping is not available. In our pilot, same‐day genotype results were available for 17 participants, but for those who had to wait, the turnaround time was at least 1 week, with a median (IQR) of 8 (7–12) days. Acceptance of GGD of PPIs and willingness to wait may have been enhanced by the on‐site genotyping. Future studies should consider including on‐site rapid turnaround genotype testing.
Despite the fact that participants were prescribed different PPI doses (e.g., lower doses in IMs and PMs), the data do not suggest differences in overall efficacy between the GGD and CD arms. The results from both efficacy questionnaires, PedsQL and GASP‐Q, indicated the change in symptoms from baseline to follow‐up were similar between arms. The outcomes were analyzed after a median of 39 days on PPI therapy, which coincides with the expected PPI efficacy time frame of ~4 weeks.4
These pilot data also suggest the potential for improved safety with GGD of PPIs, as there were fewer adverse effects in the GGD arm compared with the CD arm. We detected a significant difference in the total score of the SN‐5 questionnaire between arms (Table 2). Parents of participants in the CD arm reported a higher frequency of sneezing, itchy nose/eyes, need to rub nose/eyes, or watery eyes, as compared with those in the GGD arm. These symptoms were grouped together as allergy symptoms, but given that we observed a higher frequency of URI, sore throat, and acute sinusitis in the CD arm, reported in the safety questionnaire (Figure 3), we believe that these allergy symptoms may reflect infection, rather than seasonal allergies. Our results align with others who found an association with URI and PPI use.6, 7, 46 Specifically, Lima et al.6 found the association between URI and PPI use is exaggerated when genetic variation in CYP2C19 is present. The finding was statistically significant for PMs (odds ratio of 2.5 for URI and 2.9 for sore throat, P value 0.046 and 0.016, respectively) and trending for NMs (odds ratio of 1.6 for URI and 2.0 for sore throat, P value of 0.15 and 0.024, respectively). PPIs are typically considered to be safe medications, although more recently they have been associated with the occurrence of infections.47 It has been suggested that the increase in gastric pH, which alleviates symptoms, also removes the protective barrier of the stomach and alters the gastric flora. In turn, this allows for bacteria overgrowth and dysbiosis, which may be aspirated or ingested, leading to infections.48, 49 It is possible that standard doses of PPIs have higher risk than is generally recognized, especially in PMs, and this should be carefully considered when prescribing. Our results indicate it may be possible to reduce the adverse effects of PPIs by prescribing a dose recommended by CYP2C19 phenotype. Individuals with low or high CYP2C19 enzyme activity (e.g., PMs or UMs, respectively) who receive a lower or higher PPI dose would be exposed to a similar plasma drug concentration as an individual who is an NM who received a standard dose. Thus, individualizing the dose of PPI by genotype ensures that individuals are exposed to similar plasma drug concentrations. This may result in a more consistent therapeutic response across patients. Our pilot data suggest genotype‐guided PPI dosing in children may minimize the occurrence of infections without compromising efficacy.
Our study has several limitations. First, this was a pilot study with a relatively small sample size and we were not powered to detect differences in clinical outcomes. Nevertheless, the doses of PPIs were different among CYP2C19 phenotypes and we were able to observe a trend toward improved safety without a loss in efficacy. This study is important as it is the first pilot and feasibility trial of its kind in children of CYP2C19 genotype‐guided dosing of PPIs. To note, we focused on children ages 5–17 years, whose CYP2C19 activity may more closely mimic adult CYP2C19 activity, although Koukouritaki et al.50 suggest that the majority of CYP2C19 expression occurs by 5 months of age and, thus, we may be able to extrapolate our data to younger populations. Large scale randomized clinical trials are warranted to fully evaluate the impact of a genotype‐guided approach on clinical outcomes. Additionally, as this study design was pragmatic, there were no pharmacokinetic analyses done. We realize this is necessary to confirm similar plasma concentrations across phenotype groups with modified PPI dosing and future studies should address this. In addition, due to the pragmatic design, any PPI medication could have been prescribed. The majority was omeprazole, but others were also used. We are aware that each PPI differs in the fraction of metabolism due to CYP2C19, however, due to limited data, all PPIs were treated the same in regard to recommendations. If sufficient data are produced, these recommendations may be PPI‐specific in future studies. Next, there was a high number of missing questionnaires by participants and their parents, either due to participants being lost to follow‐up or questionnaires not being completed as scheduled. However, this problem was equally distributed between both study groups. Lesson learned for future clinical trials is that questionnaire fatigue is an important consideration and the focus should be on minimally necessary data collection (e.g., baseline, week 4, and week 12). Another limitation of our study is the possible lack of reported use of over‐the‐counter antacid medications. Our knowledge of concomitant medication use throughout the study was limited to what was prescribed in the electronic health record. In the future, medication reconciliation, especially with nonprescription antacids, should be completed at the same time questionnaires are completed.
In conclusion, our novel pilot study of CYP2C19 genotype‐guided dosing of PPI medications in children demonstrates that pharmacogenetic‐guided PPI therapy is feasible and results in differential dosing by phenotype. The dose adjustments may reduce adverse infectious outcomes without compromising efficacy. A future, large scale randomized clinical trial in children is warranted.
Funding
National Institute of Health IGNITE Network (NIH grant U01 HG007269), Spartan RX provided use of machine at no charge.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
E.J.C., K.B., J.A.J., J.J.L., and J.P.F. wrote the manuscript. K.B., E.B.M., J.A.J., J.J.L., and J.P.F. designed the research. H.A., N.C., J.D., J.E., D.E.G., R.G., P.P., S.T., and J.P.F. performed the research. E.J.C. and Y.G. analyzed the data.
Supporting information
Table S1. Sensitivity analysis of SN‐5 (participants prescribed omeprazole only).
Table S2. Change in Gasp‐Q scores.
Table S3. Change in PedsQL scores.
Acknowledgments
We would like to acknowledge our research coordinators, Shannon Henry and Jenny Batalla and our data curator, Suzanne McCahan for their hard work. We would also like to acknowledge funding from the National Institutes of Health IGNITE Network (NIH grant U01 HG007269 to J.A.J.).
References
- 1. Dent, J. , El‐Serag, H.B. , Wallander, M.A. & Johansson, S. Epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 54, 710–717 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson, S.P. , Chen, E.H. , Syniar, G.M. & Christoffel, K.K. Prevalence of symptoms of gastroesophageal reflux during childhood: a pediatric practice‐based survey. Pediatric Practice Research Group. Arch. Pediatr. Adolesc. Med. 154, 150–154 (2000). [DOI] [PubMed] [Google Scholar]
- 3. Ward, R.M. & Kearns, G.L. Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr. Drugs 15, 119–131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenplas, Y. et al Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 49, 498–547 (2009). [DOI] [PubMed] [Google Scholar]
- 5. Orenstein, S.R. , Hassall, E. , Furmaga‐Jablonska, W. , Atkinson, S. & Raanan, M. Multicenter, double‐blind, randomized, placebo‐controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J. Pediatr. 154, 514–520.e514 (2009). [DOI] [PubMed] [Google Scholar]
- 6. Lima, J.J. et al Association of CYP2C19 polymorphisms and lansoprazole‐associated respiratory adverse effects in children. J. Pediatr. 163, 686–691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holbrook, J.T. et al Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 307, 373–381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canani, R.B. et al Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community‐acquired pneumonia in children. Pediatrics 117, e817–e820 (2006). [DOI] [PubMed] [Google Scholar]
- 9. Lambert, A.A. et al Risk of community‐acquired pneumonia with outpatient proton‐pump inhibitor therapy: a systematic review and meta‐analysis. PLoS One 10, e0128004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez, L.A. , Ruigomez, A. , Wallander, M.A. & Johansson, S. Acid‐suppressive drugs and community‐acquired pneumonia. Epidemiology 20, 800–806 (2009). [DOI] [PubMed] [Google Scholar]
- 11. Hermos, J.A. et al Risk of community‐acquired pneumonia in veteran patients to whom proton pump inhibitors were dispensed. Clin. Infect. Dis. 54, 33–42 (2012). [DOI] [PubMed] [Google Scholar]
- 12. Eurich, D.T. , Sadowski, C.A. , Simpson, S.H. , Marrie, T.J. & Majumdar, S.R. Recurrent community‐acquired pneumonia in patients starting acid‐suppressing drugs. Am. J. Med. 123, 47–53 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Kwok, C.S. et al Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta‐analysis. Am. J. Gastroenterol. 107, 1011–1019 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Bavishi, C. & Dupont, H.L. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment. Pharmacol. Ther. 34, 1269–1281 (2011). [DOI] [PubMed] [Google Scholar]
- 15. Turco, R. et al Proton pump inhibitors as a risk factor for paediatric Clostridium difficile infection. Aliment. Pharmacol. Ther. 31, 754–759 (2010). [DOI] [PubMed] [Google Scholar]
- 16. Zhou, B. , Huang, Y. , Li, H. , Sun, W. & Liu, J. Proton‐pump inhibitors and risk of fractures: an update meta‐analysis. Osteoporos. Int. 27, 339–347 (2016). [DOI] [PubMed] [Google Scholar]
- 17. Freedberg, D.E. et al Use of proton pump inhibitors is associated with fractures in young adults: a population‐based study. Osteoporos. Int. 26, 2501–2507 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazarus, B. et al Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern. Med. 176, 238–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antoniou, T. et al Proton pump inhibitors and the risk of acute kidney injury in older patients: a population‐based cohort study. CMAJ Open. 3, E166–E171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kearns, G.L. , Leeder, J.S. & Gaedigk, A. Impact of the CYP2C19*17 allele on the pharmacokinetics of omeprazole and pantoprazole in children: evidence for a differential effect. Drug Metab. Dispos. 38, 894–897 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kearns, G.L. et al Single‐dose pharmacokinetics of oral and intravenous pantoprazole in children and adolescents. J. Clin. Pharmacol. 48, 1356–1365 (2008). [DOI] [PubMed] [Google Scholar]
- 22. Ward, R.M. et al Single‐dose, multiple‐dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD). Eur. J. Clin. Pharmacol. 66, 555–561 (2010). [DOI] [PubMed] [Google Scholar]
- 23. Furuta, T. , Shirai, N. , Xiao, F. , Ohashi, K. & Ishizaki, T. Effect of high‐dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clin. Pharmacol. Ther. 70, 484–492 (2001). [DOI] [PubMed] [Google Scholar]
- 24. Qiao, H.L. et al Pharmacokinetics of three proton pump inhibitors in Chinese subjects in relation to the CYP2C19 genotype. Eur. J. Clin. Pharmacol. 62, 107–112 (2006). [DOI] [PubMed] [Google Scholar]
- 25. Ieiri, I. et al Comparison of the kinetic disposition of and serum gastrin change by lansoprazole versus rabeprazole during an 8‐day dosing scheme in relation to CYP2C19 polymorphism. Eur. J. Clin. Pharmacol. 57, 485–492 (2001). [DOI] [PubMed] [Google Scholar]
- 26. Shirai, N. et al Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment. Pharmacol. Ther. 15, 1929–1937 (2001). [DOI] [PubMed] [Google Scholar]
- 27. Lou, H.Y. , Chang, C.C. , Sheu, M.T. , Chen, Y.C. & Ho, H.O. Optimal dose regimens of esomeprazole for gastric acid suppression with minimal influence of the CYP2C19 polymorphism. Eur. J. Clin. Pharmacol. 65, 55–64 (2009). [DOI] [PubMed] [Google Scholar]
- 28. Furuta, T. et al CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin. Pharmacol. Ther. 65, 552–561 (1999). [DOI] [PubMed] [Google Scholar]
- 29. Caudle, K.E. et al Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 19, 215–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El Rouby, N. , Lima, J.J. & Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 14, 447–460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martis, S. et al Multi‐ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J. 13, 369–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franciosi, J.P. et al Association between CYP2C19 extensive metabolizer phenotype and childhood anti‐reflux surgery following failed proton pump inhibitor medication treatment. Eur. J. Pediatr. 177, 69–77 (2018). [DOI] [PubMed] [Google Scholar]
- 33. Franciosi, J.P. et al Association between CYP2C19*17 alleles and pH probe testing outcomes in children with symptomatic gastroesophageal reflux. J. Clin. Pharmacol. 58, 89–96 (2018). [DOI] [PubMed] [Google Scholar]
- 34. Ichikawa, H. , Sugimoto, M. , Sugimoto, K. , Andoh, A. & Furuta, T. Rapid metabolizer genotype of CYP2C19 is a risk factor of being refractory to proton pump inhibitor therapy for reflux esophagitis. J. Gastroenterol. Hepatol. 31, 716–726 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Aka, I. et al Clinical pharmacogenetics of cytochrome P450‐associated drugs in children. J. Pers. Med. 7, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernal, C.J. et al CYP2C19 variants and proton pump inhibitor associated infections. Pediatr. Acad. Soc. Ann. Meet. https://www.xcdsystem.com/pas/program/2018/index.cfm?pgid=156&print=1&printmode=1 (2018). [Google Scholar]
- 37. Lima, J.J. & Franciosi, J.P. Pharmacogenomic testing: the case for CYP2C19 proton pump inhibitor gene‐drug pairs. Pharmacogenomics 15, 1405–1416 (2014). [DOI] [PubMed] [Google Scholar]
- 38. Swen, J.J. et al Pharmacogenetics: from bench to byte–an update of guidelines. Clin. Pharmacol. Ther. 89, 662–673 (2011). [DOI] [PubMed] [Google Scholar]
- 39. Sing, C.W. , Cheung, C.L. & Wong, I.C. Pharmacogenomics–how close/far are we to practising individualized medicine for children? Br. J. Clin. Pharmacol. 79, 419–428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris, P.A. et al Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kay, D.J. & Rosenfeld, R.M. Quality of life for children with persistent sinonasal symptoms. Otolaryngol. Head Neck Surg. 128, 17–26 (2003). [DOI] [PubMed] [Google Scholar]
- 42. Deal, L. et al Age‐specific questionnaires distinguish GERD symptom frequency and severity in infants and young children: development and initial validation. J. Pediatr. Gastroenterol. Nutr. 41, 178–185 (2005). [DOI] [PubMed] [Google Scholar]
- 43. Varni, J.W. , Kay, M.T. , Limbers, C.A. , Franciosi, J.P. & Pohl, J.F. PedsQL gastrointestinal symptoms module item development: qualitative methods. J. Pediatr. Gastroenterol. Nutr. 54, 664–671 (2012). [DOI] [PubMed] [Google Scholar]
- 44. Varni, J.W. et al PedsQL gastrointestinal symptoms module: feasibility, reliability, and validity. J. Pediatr. Gastroenterol. Nutr. 59, 347–355 (2014). [DOI] [PubMed] [Google Scholar]
- 45. Kirchheiner, J. et al Relative potency of proton‐pump inhibitors‐comparison of effects on intragastric pH. Eur. J. Clin. Pharmacol. 65, 19–31 (2009). [DOI] [PubMed] [Google Scholar]
- 46. Winter, H. et al Efficacy and safety of pantoprazole delayed‐release granules for oral suspension in a placebo‐controlled treatment‐withdrawal study in infants 1‐11 months old with symptomatic GERD. J. Pediatr. Gastroenterol. Nutr. 50, 609–618 (2010). [DOI] [PubMed] [Google Scholar]
- 47. De Bruyne, P. & Ito, S. Toxicity of long‐term use of proton pump inhibitors in children. Arch. Dis. Child. 103, 78–82 (2018). [DOI] [PubMed] [Google Scholar]
- 48. Williams, C. & McColl, K.E. Review article: proton pump inhibitors and bacterial overgrowth. Aliment. Pharmacol. Ther. 23, 3–10 (2006). [DOI] [PubMed] [Google Scholar]
- 49. Clooney, A.G. et al A comparison of the gut microbiome between long‐term users and non‐users of proton pump inhibitors. Aliment. Pharmacol. Ther. 43, 974–984 (2016). [DOI] [PubMed] [Google Scholar]
- 50. Koukouritaki, S.B. et al Developmental expression of human hepatic CYP2C9 and CYP2C19. J. Pharmacol. Exp. Ther. 308, 965–974 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sensitivity analysis of SN‐5 (participants prescribed omeprazole only).
Table S2. Change in Gasp‐Q scores.
Table S3. Change in PedsQL scores.


