Abstract
Previous studies have shown associations between genetic polymorphisms and pain tolerance, but psychological evaluations are seldom measured. The objective of this study was to determine the independent effects of demographic, psychological, and genetic predictors of cold noxious pain tolerance. Healthy subjects (n = 89) completed the Pain Catastrophizing Scale (PCS) and Fear of Pain Questionnaire (FPQ‐III), underwent genotyping for candidate single nucleotide polymorphisms (SNPs), and completed a cold‐pressor test in a 1–2°C water bath for a maximum of 3 minutes. The primary outcome measure was pain tolerance, defined as the maximum duration of time subjects left their nondominant hand in the cold‐water bath. Cox proportional hazards regression indicated that female sex, Asian race, and increasing PCS and FPQ‐III scores were associated with lower pain tolerance. No candidate SNP was significantly associated with pain tolerance. Future genetic studies should include demographic and psychological variables as confounders in experimental pain models.
Interindividual variability of pain perception is not completely understood. Such variation is likely influenced by a combination of complex environmental and biological factors. Previous studies have shown modest heritability of pain tolerance and identified genetic polymorphisms explaining some of this variability.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓Experimental pain models in humans have shown potential associations between genetic polymorphisms and pain measures, but associations between genetic variants and pain tolerance have not been replicated.
Genetic studies simultaneously measure psychological factors, which can also influence sensitivity or confound the relationship between genetic polymorphisms, and pain measurement.
what question did this study address?
✓The objective of this study was to determine the association between demographics, psychological survey data, and selected single nucleotide polymorphisms in TRPA1, COMT, and FAAH genes on tolerance to cold noxious pain using an experimental pain model.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
✓Our study was unable to find a relationship between TRPA1, COMT, and FAAH polymorphisms and inter individual variability in tolerance to cold noxious pain.
Our study highlights the contribution of demographic and psychological variables to pain sensitivity.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓The results of this article suggest that experimental and translational pain models evaluating genetic influences on pain should include demographic and detailed psychological measures.
1, 2, 3 Variants of certain genes encoding target structures within the nociceptive pathway may result in an attenuation or augmentation of specific types of pain. Genetic variation encoding receptors related to temperature sensation have been previously shown to contribute to cold pain sensitivity.4, 5, 6, 7, 8, 9, 10, 11
Genes containing associated variants include the transient receptor potential A subtype 1 gene (TRPA1), the catechol‐o‐methyltransferase gene (COMT), and the fatty acid amide hydrolase gene (FAAH).4, 6, 12, 13, 14, 15 TRPA1 is an ion channel that belongs to a family of transient receptor potential channels, which respond to various sensory stimuli such as pain, and the variant rs11988795 G>A in TRPA1 is known to enhance cold pain perception.12, 16 COMT is an enzyme involved in catecholamine metabolism, influencing concentrations of dopamine and norepinephrine in the brain and affecting mood and mental processes, such as pain perception and catastrophization. Several polymorphisms in COMT, including rs6269 and rs4646312, have been associated with variability in pain sensitivity and perception.6, 17 FAAH, an enzyme that catalyzes the formation of arachidonic acid, regulates fatty acid amide catabolism in the nervous system, which can influence response to pain. FAAH is known to be a key regulator in metabolism of anandamide, a weak cannabinoid receptor activator.18 FAAH polymorphisms, including rs2295633, rs4141964, and rs932816, have also been shown to affect pain perception and susceptibility in experimental pain models.14, 19 Variation in these genes, which are important nodes in nociceptive and pharmacogenetic pathways, are likely important for development of translational pain prediction models due to the previously observed impact on human experimental pain tolerance.
In addition to genetic polymorphisms, patient demographics and psychological factors have a strong influence on pain experiences.1, 3, 20, 21 Differences in patient perceptions of pain have been observed according to demographic factors, including patient race and gender.22, 23, 24 The effect of genetic variants on pain is confounded by these other important factors, which are often not measured in genetic and pharmacogenetic studies.4, 5, 6, 7, 8, 9, 10, 11 The independent effect of genetic variation on the physiological response to pain and their contribution to perception of pain is understudied. Validated scales that measure psychological factors influencing pain perception are available and include the Pain Catastrophizing Scale (PCS) and the Fear of Pain Questionnaire‐III (FPQ‐III).25, 26 Catastrophizing involves an exaggerated negative orientation toward noxious stimuli and has been associated with greater pain intensity during experimental pain models.3, 25, 27 Similarly, the measurement of pain‐related fear using the FPQ‐III was predictive of experimental pain.26, 27 These scales provide important information that could be incorporated into pain models that estimate genetic influences. However, studies using similar experimental pain models often evaluate genetic influences in isolation, without considering these important psychological factors.
The objective of this study was to determine the association between demographics, psychological status, and selected genetic variants on tolerance to cold noxious pain using an experimental pain model. The intent was to determine the relative contribution of each factor by measuring all pertinent predictors in a single experimental pain model.
Methods
Experimental design and subject selection
The present investigation was a cross‐sectional study conducted in a clinical research laboratory. The study was approved by the University of Arizona's Human Subject Protection Program prior to initiation and subject enrollment. Subjects were recruited using advertisements posted on bulletin boards on the University's health sciences campus and via word of mouth. Interested subjects were asked to contact a research coordinator to schedule an initial screening for eligibility and participation in the study. Inclusion criteria were the following: subjects had to be able to consent on their own and be over 18 years of age. Exclusion criteria were: subjects could not have a history of syncope, cardiovascular disease, pulmonary disease, vascular conditions, renal disease, chronic pain, or taking pain medications.
Study protocol and data collection
Subjects were first screened and, if eligible, consent was obtained for study participation. Demographic information was obtained and subjects were measured for height and weight. The experiment consisted of three phases: (i) completion of written surveys; (ii) buccal swabs for genetic analyses; and (iii) a cold‐pressor test. Each phase is described below. The entire visit was estimated to take no more than 1 hour.
Written surveys
The subjects completed two experimentally validated surveys: the PCS25 and the FPQ‐III.26 After a brief explanation by the research staff, the subjects completed the surveys in writing individually. The research staff did not administer the surveys or obtain responses verbally. The PCS consists of 13 questions, which are rated by the subjects on a five‐point scale. The PCS consists of three domains: rumination, magnification, and helplessness. For this study, the sum total score of all domains was used for analyses, as previously described and validated.25, 28, 29 The total score on the PCS can range from 0−52. A higher score indicates a greater degree of catastrophizing.
The FPQ‐III consists of 30 questions, which are also rated by the subjects on a five‐point scale. This questionnaire assesses pain‐related fear of the subjects. The questionnaire is totaled to obtain a score of 30–150. A higher score indicates greater fear related to pain. The FPQ‐III does not have specific domains or categories. The total score was used for analyses. For both surveys, subjects were also dichotomized at the median into high and low fear groups to visually examine differences between the two groups in the survival analysis.
Genetic analyses
Following the surveys, two buccal swabs using a buccal brush were taken for DNA extraction and genetic analysis. The University of Arizona Genetics Core facility provided the brush and collection vials and performed genetic analysis. DNA quantification was performed using PicoGreen (Thermo Fisher Scientific, Waltham, MA). Prevalidated primers and probe sets for TaqMan Allelic Discrimination Assay were obtained (Thermo Fisher Scientific, Waltham, MA). Custom primers and probe sets for TaqMan Allelic Discrimination Assay were generated via Life Technologies File Builder 3.1 software for genotyping. Reactions were run at 10 μL, containing TaqMan Universal PCR Master Mix, No AmpEraseR UNG (Thermo Fisher Scientific, Waltham, MA), 10 ng total DNA, and 1 × Assay Mix. All samples were processed and analyzed on the 7900 Real‐Time PCR System (Thermo Fisher Scientific, Waltham, MA) with cycling conditions (95°C for 10 minutes, 50 cycles of 92°C for 15 seconds, and 60°C for 1 minute) and Genotyper software (SDS system, version 2.3). Single nucleotide polymorphisms (SNPs) within the following genes were evaluated: TRPA1 (rs11988795), COMT (rs4646312, rs6269), and FAAH (rs932816, rs4141964, and rs2295633). Based on previous literature, these six SNPs were considered to be most likely to have an effect on pain perception, using a cold‐pressor test.4, 12, 16 To ensure quality control of genotype data, SNP genotypes were only used in statistical models if the SNP call rate was > 95% and SNPs were within Hardy‐Weinberg equilibrium (P > 0.05).
Cold‐pressor test
After the buccal swabs, subjects completed a cold‐pressor test while seated. A cold‐pressor test was used in order to test replication of existing literature.4 The cold‐pressor test has a robust literature supporting its use. In addition, the cold‐pressor test is easily and inexpensively implemented, safe, and standardized.30 The cold‐pressor test apparatus consisted of a 4‐L circulating water bath, which was maintained between 1 and 2°C. The water in the beaker was stirred continuously with a magnetic stirring rod to maintain a uniform temperature. The subjects were instructed to insert their nondominant hands into the water bath. Although use of the dominant vs. nondominant hand is not expected to affect results, the nondominant hand was used in all subjects to retain consistency in the procedure and reflect the most common approach from the literature.30, 31
The hand was inserted up to 5 cm above the wrist, which was confirmed visually. Subjects were instructed to leave their hand in the water bath for as long as they could. However, the maximum duration was set at 3 minutes at which time they were instructed to remove their hand. Subjects were blinded to this maximum limit. Pain was measured on a verbal numeric rating scale of 0–10 (0 being no pain and 10 being the worst possible pain). Time values were measured using a digital stopwatch and recorded in seconds. The following measurements were taken: (i) pain threshold – time in seconds from hand insertion to a pain score of one or more; (ii) pain tolerance – time in seconds from hand insertion to the time subject voluntarily removes hand; (iii) pain score every 30 seconds; and (iv) pain score at time of removal of hand from water.
Statistical analyses
The primary outcome measured was pain tolerance. Continuous variables such as age, body mass index (BMI), survey scores, pain scores, pain tolerance, and pain threshold were summarized and reported as means and SDs. Categorical variables, such as sex and race, were reported as percentages. Differences between demographic variables according to subjects who did and did not complete the cold‐pressor test were calculated using t‐tests and χ2 tests as appropriate. PCS score was dichotomized at the median, and patients were classified as catastrophizers (higher scores) and noncatastrophizers (lower scores). Similarly, the FPQ‐III was dichotomized at the median, and patients were categorized as high fear (higher scores) or low fear (lower values). The primary outcome variable was censored at 180 seconds, thus, a survival analysis with Cox proportional hazards regression was conducted. Hazards ratios (HRs) were reported for variables that could be associated with the outcome. These were age, sex, race, BMI, PCS score, FPQ‐III score, and SNPs. The HR for the FPQ‐III represents a 25‐point change on the scale. This is an ~20% change in score on this scale. PCS and FPQ‐III scores were dichotomized at the median and Kaplan‐Meier curves were used to visualize differences in pain tolerance. Log‐rank tests were also used to assess differences in pain tolerance between dichotomized groups. SNP influences on pain tolerance were tested in an additive model adjusted for sex, race, and total PCS score. In a sensitivity analysis, SNP influences on pain tolerance were also tested in an additive model adjusted for sex, race, and FPQ‐III score. A polygenic risk score consisting of the combined number of variant alleles for all SNPs was also tested for association with the primary outcome. An alpha of 0.05 was used for all analyses. In order to achieve 80% power at α = 0.05 and an effect of 12% of the variance explained in the multivariate regression model, we required 84 subjects to determine a significant effect of PCS score on an individual's pain sensitivity. Analyses were conducted in STATA 13 (College Station, TX) and R version 3.4.3.
Results
There were 89 subjects who completed the study. The mean age of subjects was 26 ± 7 years, and 58% were women. The most common race/ethnicity was white (n = 45, 51%), followed by Asian (n = 23, 26%), Hispanic (n = 12, 14%), and other (n = 9, 10%). The mean BMI was 25 ± 6 kg/m2. The mean total PCS score was 14 ± 9 and the mean FPQ‐III score was 74 ± 21. Demographic data, survey scores, and results of the cold‐pressor test among subjects who completed and did not complete the cold pressor test are presented in Table 1. Factors associated with completing the maximum duration of the cold‐pressor test were male sex, white race, and lower PCS and FPQ‐III scores. During the cold‐pressor test, the mean pain threshold was 9 ± 7 seconds, and mean pain tolerance was 120 ± 66 seconds. Pain scores measured at 30, 60, 90, 120, 150, and 180 seconds were 4.8 ± 2.1 (n = 78), 6.0 ± 2.1 (n = 63), 5.9 ± 2.0 (n = 53), 5.8 ± 2.2 (n = 51), 5.8 ± 2.2 (n = 47), and 5.9 ± 2.5 (n = 43), respectively. The final pain score at time of hand removal was 7.0 ± 2.3.
Table 1.
Demographic data among subjects who completed and did not complete the cold‐pressor test
| Variablea | Did not complete cold‐pressor test (n = 46)b | Completed cold‐pressor test (n = 43)b | P valuec |
|---|---|---|---|
| Age, years | 27.54 (8.11) | 26.19 (5.49) | 0.361 |
| Female, n (%) | 33 (71.7) | 19 (44.2) | 0.016 |
| BMI, kg/m2 | 24.67 (5.60) | 25.82 (5.82) | 0.348 |
| Race, n (%) | 0.031 | ||
| Asian | 17 (37.0) | 6 (14.0) | |
| Hispanic | 6 (13.0) | 6 (14.0) | |
| Other | 6 (13.0) | 3 (7.0) | |
| White | 17 (37.0) | 28 (65.1) | |
| PCS score | 17.33 (9.73) | 10.19 (6.91) | < 0.001 |
| Rumination | 7.30 (4.05) | 4.47 (3.2) | < 0.001 |
| Magnification | 3.61 (2.58) | 2.16 (1.53) | 0.002 |
| Helplessness | 6.41 (4.34) | 3.56 (3.48) | 0.001 |
| FPQ‐III score | 78.91 (22.65) | 69.18 (17.93) | 0.028 |
| Pain threshold, seconds | 8.30 (6.86) | 9.88 (6.30) | 0.262 |
| Pain tolerance, seconds | 64.80 (45.47) | 180.00 (0.00) | < 0.001 |
BMI, body mass index; FPQ‐III, Fear of Pain Questionnaire‐III; PCS, Pain Catastrophizing Scale.
aValues are mean and SD unless otherwise noted. bSubjects who did not complete cold‐pressor test removed their hand before the 3‐minute mark. Subjects who completed the cold‐pressor test did not remove their hand before the 3‐minute maximum time. c P values are calculated using t‐tests and χ2 tests as appropriate.
In survival analyses, age and BMI were not significantly associated with pain tolerance, whereas women were observed to have lower pain tolerance than men (Table 2). Asians were observed to be more likely to remove their hands more quickly (lower pain tolerance) compared with whites. Increasing PCS and FPQ‐III scores were also associated with decreased pain tolerance. After adjusting for sex and race, PCS score (HR, 1.1 (1.0−1.1), P = 3.35 × 10−4) and FPQ‐III score (HR, 1.6 (1.1–2.4), P = 0.009) were significantly associated with pain tolerance. Although all three domains of the PCS score (rumination, magnification, and helplessness) were independently associated with pain tolerance, rumination was more strongly associated the other two domains (HR, 1.2 (1.1–1.3), P = 2.9 × 10−4). An increasing HR represents lower pain tolerance (i.e., increased hazard of hand removal), and the HR for FPQ‐III represented a 25‐point change on the scale.
Table 2.
Effects of demographic variables and survey responses on pain tolerance
| Variable | HRa | 95% CIa | P valuea |
|---|---|---|---|
| Age, years | 1.0 | 1.0–1.1 | 0.369 |
| Sex, male | 0.4 | 0.2–0.7 | 0.003 |
| BMI, kg/m2 | 1.0 | 0.9–1.0 | 0.218 |
| Race | |||
| White | Reference | – | – |
| Asian | 3.1 | 1.6–6.1 | 0.001 |
| Hispanic | 1.6 | 0.6–4.1 | 0.322 |
| Other | 2.5 | 1.0–6.4 | 0.051 |
| PCS scoreb | 1.1 | 1.0–1.1 | 3.35 × 10−4 |
| Rumination | 1.2 | 1.1–1.3 | 2.9 × 10−4 |
| Magnification | 1.2 | 1.0–1.3 | 0.012 |
| Helplessness | 1.14 | 1.1–1.2 | 7.2 × 10−4 |
| FPQ‐III scorec | 1.6 | 1.1–2.4 | 0.009 |
BMI, body mass index; CI, confidence interval; FPQ‐III, Fear of Pain Questionnaire‐III; HR, hazard ratio; PCS, Pain Catastrophizing Scale.
aHRs, 95% CIs, and P values were calculated with Cox proportional hazards regression conducted with the primary outcome variable censored at 180 seconds. Analyses for age, sex, race, and BMI are unadjusted. bAnalyses for PCS score is adjusted for sex and race. PCS score and domains of the PCS score represent one‐point change on the scale. cAnalysis for FPQ‐III score is adjusted for sex and race. The HR for the FPQ‐III represents a 25‐point change on the scale.
The differences between dichotomized groups in terms of pain tolerance were visualized using Kaplan‐Meier curves. Catastrophizers remove their hand significantly more quickly from the cold water bath (log‐rank P = 4.12 × 10−5; Figure 1). Subjects with high fear were also more likely to remove their hand from the cold water bath more quickly (log‐rank P = 0.028; Figure 2). No statistically significant associations were observed between investigated SNPs and pain tolerance after adjustment for gender, race, and PCS score (Table 3). In the sensitivity analysis, no SNPs were associated with pain tolerance after adjustment for gender, race, and FPQ‐III score. The polygenic risk score, indicating the combined number of variant alleles for all SNPs, was also not associated with pain tolerance (HR, 1.02 (0.68–1.52), P = 0.93).
Figure 1.
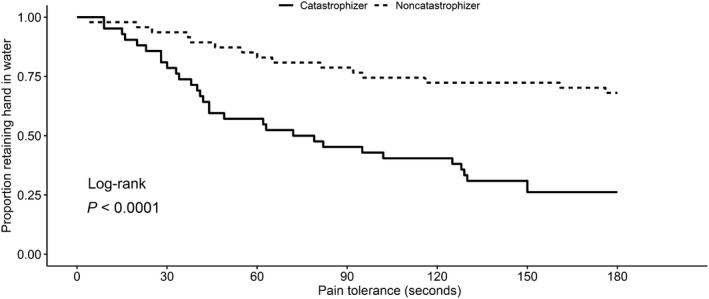
Kaplan‐Meier survival estimates by catastrophization category. The Pain Catastrophizing Scale score was dichotomized at the median, and patients were classified as catastrophizers (higher scores) and noncatastrophizers (lower scores). Survival time is pain tolerance, as indicated by the duration of time in seconds that subjects were able to retain their hand in the water bath.
Figure 2.
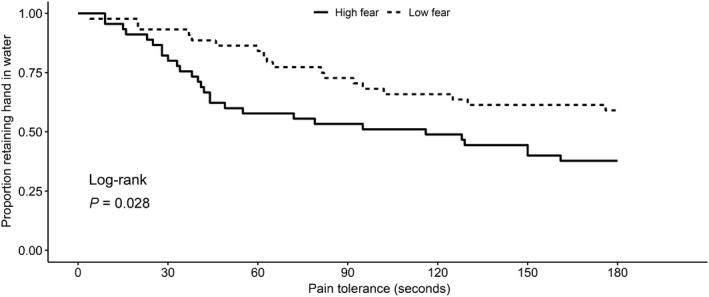
Kaplan‐Meier survival estimates by fear of pain category. The results of the Fear of Pain Questionnaire‐III was dichotomized at the median, and patients were categorized as high fear (higher scores) or low fear (lower values). Survival time is pain tolerance, as indicated by the duration of time in seconds that subjects were able to retain their hand in the water bath.
Table 3.
Effect of single nucleotide polymorphisms on pain tolerance
| SNP | Gene | Allele | MAF | MAF (CEU)a | MAF (CHB)a | MAF (YRI)a | HWE P value | Unadjusted HR (95% CI)b | Unadjusted P value b | Adjusted HR (95% CI) c | Adjusted P valuec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs4646312 | COMT | C | 0.31 | 0.43 | 0.37 | 0.15 | 0.581 | 0.76 (0.48–1.24) | 0.279 | 1.29 (0.75–2.22) | 0.364 |
| rs6269 | COMT | G | 0.31 | 0.44 | 0.37 | 0.36 | 0.756 | 0.78 (0.49–1.24) | 0.297 | 1.27 (0.76–2.14) | 0.364 |
| rs2295633 | FAAH | A | 0.33 | 0.34 | 0.15 | 0.62 | 0.845 | 0.99 (0.63–1.54) | 0.952 | 1.30 (0.78–2.16) | 0.317 |
| rs4141964 | FAAH | T | 0.43 | 0.34 | 0.45 | 0.74 | 0.956 | 1.19 (0.80–1.77) | 0.379 | 1.27 (0.79–2.03) | 0.320 |
| rs932816 | FAAH | A | 0.19 | 0.32 | 0.02 | 0.13 | 0.250 | 0.60 (0.33–1.11) | 0.104 | 0.693 (0.34–1.40) | 0.309 |
| rs11988795 | TRPA1 | T | 0.41 | 0.26 | 0.56 | 0.38 | 0.485 | 1.33 (0.86–2.07) | 0.204 | 1.67 (0.96–2.88) | 0.067 |
95% CI, 95% confidence interval; CEU, Utah residents with Northern and Western European ancestry in the 1000 Genomes project; CHB, Han Chinese in the 1000 Genomes project; COMT, catechol‐o‐methyltransferase; FAAH, fatty acid amide hydrolase; HR, hazard ratio; HWE, Hardy‐Weinberg equilibrium; MAF, minor allele frequency; SNP, single nucleotide polymorphism; TRPA1, transient receptor potential A subtype 1; YRI, Yorubans from Ibadan, Nigeria in the 1000 Genomes project.
aMinor allele frequencies acquired from the 1000 Genomes project acquired from grch37.ensembl.org. bHRs, 95% CIs, and P values were calculated with Cox proportional hazards regression conducted with the primary outcome variable censored at 180 seconds. cHRs, 95% CIs, and P values were calculated with Cox proportional hazards regression conducted with the primary outcome variable censored at 180 seconds. Adjusted SNP analyses are adjusted for sex, race, and Pain Catastrophizing Scale score.
Discussion
The key finding in this study was that demographic and psychological factors were associated with tolerance to pain. These results replicate findings from previous studies evaluating the influence of PCS and FPQ‐III on pain tolerance and perception.25, 26, 27 The effect size for these scales was relatively large with a doubling of the hazard for hand removal for a 20% increase in pain‐related fear or catastrophization. This remained true after adjusting for covariates, such as sex and race, which was also observed to contribute to pain tolerance. We did not replicate previously published findings related to SNPs in TRPA1, COMT, or FAAH and their influence on pain tolerance. Our study suggests that tools to assess psychological aspects of pain, such as the PCS and FPQ‐III, are important in predicting pain tolerance. These results might have important implications for the potential use of such translational tools in the clinical setting, as in preoperative assessment to predict postoperative pain and in predicting the pharmacogenetic impact of SNPs on analgesic response. Similarly, these tools could be studied in patients during admission to the hospital or triage in emergency departments to guide therapy or understand patient perception of their pain. For instance, catastrophizing has been shown to lead to delayed recovery in some pain states.32
Our findings did not replicate the observed associations from previous investigations implicating polymorphisms in TRPA1, COMT, and FAAH in pain tolerance.4, 6, 12, 13, 14, 15 These SNPs were selected based on previous associations with pain sensitivity in similar experimental models and their mechanistic role in nociceptive pathways.4, 6, 12, 13, 14, 15 Kim et al.4 identified multiple SNPs that were associated with pain sensitivity in 735 healthy subjects. The discrepancy between previous results and the present study may be due to the limited sample size of our cohort or to the distinct demographic characteristics of our cohort, which included younger subjects with a high proportion of Asian race. In addition, psychological confounders, which were not measured, may have contributed to pain sensitivity. Our findings are consistent with previous investigations of influence of psychological and demographic variables on genetic associations with pain tolerance.21, 33 Our study adds to existing evidence suggesting that psychological and demographic variables are critical elements of translational pain models, including predictive models of pain responses to analgesic treatment. This study might also be informative for studies of pain response during analgesic treatment. Future studies of pain in many contexts, including drug response, may benefit from thorough investigation of baseline demographic and psychological variables.
The study population primarily included students. Therefore, the demographics of the subjects reflected that of students in the college. The majority of the subjects were female, and a large proportion was Asian. Both of these factors were associated with decreased pain tolerance in our study. Sex‐related differences in experimental pain sensitivity are known and previous studies have also shown increased pain sensitivity in Asians compared with whites.34, 35 We did not find an association between age and tolerance to pain. In a previous investigation, older adults had decreased sensitivity to heat but not cold stimuli compared with middle‐aged adults.36 In our study, the mean age was 26 years, precluding our ability to make conclusions regarding effects of age on pain tolerance.
There are several limitations that are worthy of mention in this study. Our sample size may not have been large enough to find differences in pain tolerance between genotypes. In addition, our study was restricted to a relatively small number of SNPs with putative roles in pain tolerance. Our observations are also based on pain tolerance only as assessed by the cold‐pressor test. Additional SNPs have been associated with pain tolerance and might be included in more comprehensive investigations of genomic influences on pain tolerance.5, 11, 37 Specifically, endogenous opioid receptors likely play a key role in pain sensitivity and inclusion of variation in the opioid receptor mu 1 gene (OPRM1) and the opioid receptor kappa 1 gene (OPRK1) would be informative in future studies.6 Several SNPs have also been shown to influence pain tolerance in a drug‐dependent manner, and these polymorphisms may impact baseline pain perception.8, 9, 10, 38, 39 Notably, the vast majority of these studies did not evaluate psychological indices of pain. As previously mentioned, we were unable to assess the effects of age on pain tolerance. We did find significant associations between pain tolerance and variables, such as sex, race, PCS, and FPQ‐III, excluding the possibility of a type II error for these demographic and psychological variables.
In conclusion, our study identified female gender, Asian race, catastrophizing, and fear of pain as risk factors for decreased tolerance to pain. Our results highlight the relative contribution of demographic and psychological variables to pain sensitivity. Our results suggest that future genetic and pharmacogenetic studies should include demographics and psychological factors as confounders in experimental and clinical pain models.
Funding
No funding was received for this work.
Conflicts of Interest
The authors declared no competing interests for this work.
Author Contributions
A.E.P., C.N., D.M., and J.H.K. wrote the manuscript. A.E.P., E.M.S., and J.H.K. designed the research. A.E.P., K.W., M.L., M.M., and S.E.B. performed the research. A.E.P., H.S., and J.H.K. analyzed the data.
References
- 1. Angst, M.S. et al Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain 153, 1397–1409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lotsch, J. & Geisslinger, G. Current evidence for a modulation of nociception by human genetic polymorphisms. Pain 132, 18–22 (2007). [DOI] [PubMed] [Google Scholar]
- 3. Trost, Z. et al Heritability of pain catastrophizing and associations with experimental pain outcomes: a twin study. Pain 156, 514–520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim, H. , Mittal, D.P. , Iadarola, M.J. & Dionne, R.A. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J. Med. Genet. 43, e40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho, K.W.D. et al Single nucleotide polymorphism in the COL11A2 gene associated with heat pain sensitivity in knee osteoarthritis. Mol. Pain 13, doi: 1744806917724259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen, L.M. , Olesen, A.E. , Sato, H. , Christrup, L.L. & Drewes, A.M. Association between gene polymorphisms and pain sensitivity assessed in a multi‐modal multi‐tissue human experimental model ‐ an explorative study. Basic Clin. Pharmacol. Toxicol. 119, 360–366 (2016). [DOI] [PubMed] [Google Scholar]
- 7. Treister, R. , Pud, D. , Ebstein, R.P. & Eisenberg, E. Dopamine transporter genotype dependent effects of apomorphine on cold pain tolerance in healthy volunteers. PLoS One 8, e63808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treister, R. et al Associations between polymorphisms in dopamine neurotransmitter pathway genes and pain response in healthy humans. Pain 147, 187–193 (2009). [DOI] [PubMed] [Google Scholar]
- 9. Zahari, Z. et al Relationship between CYP2B6*6 and cold pressor pain sensitivity in opioid dependent patients on methadone maintenance therapy (MMT). Drug Alcohol Depend. 165, 143–150 (2016). [DOI] [PubMed] [Google Scholar]
- 10. Zahari, Z. et al ABCB1 polymorphisms and cold pressor pain responses: opioid‐dependent patients on methadone maintenance therapy. Nurs. Res. 66, 134–144 (2017). [DOI] [PubMed] [Google Scholar]
- 11. Zahari, Z. et al Relationship between ABCB1 polymorphisms and cold pain sensitivity among healthy opioid‐naive Malay males. Pain Pract. 17, 930–940 (2017). [DOI] [PubMed] [Google Scholar]
- 12. Schutz, M. et al Consequences of a human TRPA1 genetic variant on the perception of nociceptive and olfactory stimuli. PLoS One 9, e95592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez‐Jauand, M. et al Pain sensitivity in fibromyalgia is associated with catechol‐O‐methyltransferase (COMT) gene. Eur. J. Pain 17, 16–27 (2013). [DOI] [PubMed] [Google Scholar]
- 14. Cajanus, K. et al Effect of endocannabinoid degradation on pain: role of FAAH polymorphisms in experimental and postoperative pain in women treated for breast cancer. Pain 157, 361–369 (2016). [DOI] [PubMed] [Google Scholar]
- 15. Ramesh, D. , D'Agata, A. , Starkweather, A.R. & Young, E.E. Contribution of endocannabinoid gene expression and genotype on low back pain susceptibility and chronicity. Clin. J. Pain 34, 8–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vidal Rodriguez, S. , Castillo Aguilar, I. , Cuesta Villa, L. & de Serrano Saenz Tejada, F . TRPA1 polymorphisms in chronic and complete spinal cord injury patients with neuropathic pain: a pilot study. Spinal Cord Ser. Cases. 3, 17089 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diatchenko, L. et al Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 14, 135–143 (2005). [DOI] [PubMed] [Google Scholar]
- 18. Cravatt, B.F. et al Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 98, 9371–9376 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenbaum, L. et al Contribution of genetic variants to pain susceptibility in Parkinson disease. Eur. J. Pain 16, 1243–1250 (2012). [DOI] [PubMed] [Google Scholar]
- 20. Kim, H. et al Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109, 488–496 (2004). [DOI] [PubMed] [Google Scholar]
- 21. Meloto, C.B. et al Modification of COMT‐dependent pain sensitivity by psychological stress and sex. Pain 157, 858–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamath, A.F. , Horneff, J.G. , Gaffney, V. , Israelite, C.L. & Nelson, C.L. Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. Clin. Orthop. Relat. Res. 468, 3355–3361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aubrun, F. , Salvi, N. , Coriat, P. & Riou, B. Sex‐ and age‐related differences in morphine requirements for postoperative pain relief. Anesthesiology 103, 156–160 (2005). [DOI] [PubMed] [Google Scholar]
- 24. Forsythe, L.P. , Thorn, B. , Day, M. & Shelby, G. Race and sex differences in primary appraisals, catastrophizing, and experimental pain outcomes. J. Pain 12, 563–572 (2011). [DOI] [PubMed] [Google Scholar]
- 25. Sullivan, M.J. , Bishop, S.R. & Pivik, J. The Pain Catastrophizing scale: development and validation. Psychol. Assess. 7, 524–532 (1995). [Google Scholar]
- 26. McNeil, D.W. & Rainwater, A.J. III . Development of the fear of pain questionnaire–III. J. Behav. Med. 21, 389–410 (1998). [DOI] [PubMed] [Google Scholar]
- 27. Hirsh, A.T. , George, S.Z. , Bialosky, J.E. & Robinson, M.E. Fear of pain, pain catastrophizing, and acute pain perception: relative prediction and timing of assessment. J. Pain 9, 806–812 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osman, A. et al Factor structure, reliability, and validity of the Pain Catastrophizing scale. J. Behav. Med. 20, 589–605 (1997). [DOI] [PubMed] [Google Scholar]
- 29. Van Damme, S. , Crombez, G. , Bijttebier, P. , Goubert, L. & Van Houdenhove, B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non‐clinical populations. Pain 96, 319–324 (2002). [DOI] [PubMed] [Google Scholar]
- 30. von Baeyer, C.L. , Piira, T. , Chambers, C.T. , Trapanotto, M. & Zeltzer, L.K. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J. Pain 6, 218–227 (2005). [DOI] [PubMed] [Google Scholar]
- 31. Birnie, K.A. , Petter, M. , Boerner, K.E. , Noel, M. & Chambers, C.T. Contemporary use of the cold pressor task in pediatric pain research: a systematic review of methods. J. Pain 13, 817–826 (2012). [DOI] [PubMed] [Google Scholar]
- 32. Wertli, M.M. et al Catastrophizing ‐ a prognostic factor for outcome in patients with low back pain ‐ a systematic review. Spine J. 14, 2639–2657 (2014). [DOI] [PubMed] [Google Scholar]
- 33. Belfer, I. et al Pain modality‐ and sex‐specific effects of COMT genetic functional variants. Pain 154, 1368–1376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Racine, M. et al A systematic literature review of 10 years of research on sex/gender and pain perception ‐ part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 153, 619–635 (2012). [DOI] [PubMed] [Google Scholar]
- 35. Rowell, L.N. , Mechlin, B. , Ji, E. , Addamo, M. & Girdler, S.S. Asians differ from non‐Hispanic whites in experimental pain sensitivity. Eur. J. Pain 15, 764–771 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riley, J.L. III et al Age and race effects on pain sensitivity and modulation among middle‐aged and older adults. J. Pain 15, 272–282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matic, M. , van den Bosch, G.E. , de Wildt, S.N. , Tibboel, D. & van Schaik, R.H. Genetic variants associated with thermal pain sensitivity in a paediatric population. Pain 157, 2476–2482 (2016). [DOI] [PubMed] [Google Scholar]
- 38. Bruehl, S. et al Associations between KCNJ6 (GIRK2) gene polymorphisms and pain‐related phenotypes. Pain 154, 2853–2859 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zwisler, S.T. et al The antinociceptive effect and adverse drug reactions of oxycodone in human experimental pain in relation to genetic variations in the OPRM1 and ABCB1 genes. Fundam. Clin. Pharmacol. 24, 517–524 (2010). [DOI] [PubMed] [Google Scholar]


