Abstract
Purpose
Report 5-year outcomes of patients receiving anti-vascular endothelial growth factor (VEGF) for the treatment of macular oedema secondary to retinal vein occlusion (RVO.
Methods
Retrospective review of eyes with RVO which initiated anti-VEGF treatment. Data including age, gender, visual acuity (VA) and injection numbers were obtained from medical records. Optical coherence tomography scans were graded for presence or absence of macular oedema and central foveal thickness (CFT). Macular perfusion was assessed on fundus fluorescein angiography by masked graders.
Results
68 eyes (31 branch RVO, BRVO; 35 central RVO, CRVO and 2 hemi-RVO) with 5 years of follow-up after initiation of anti-VEGF treatment. Mean change in VA at 5 years was + 9.6 ± 21.6 letters among CRVO eyes and + 14.2 ± 15.6 letters among eyes with BRVO (p=0.001). Vision of 20/40 or better was achieved in 65 % of treated eyes. The proportion of eyes with a three-line improvement of vision (15 letters) at 5 years was 22 %. Mean CFT decreased by 257.6 ± 249.8 µm in eyes with CRVO and 145.6 ± 143.3 µm in eyes with BRVO.
Conclusion
The results confirm good long-term outcomes can be achieved with anti-VEGF therapy for RVO.
Keywords: retina, treatment medical
Key messages.
What is already known about this subject?
The efficacy and safety of anti-vascular endothelial growth factor (VEGF) therapies for the treatment of macular oedema secondary to retinal vein occlusion has long been established; however, clinical trials only present up to 2 years of outcomes.
What are the new findings?
The present study assesses the long-term efficacy of anti-VEGF therapy over 5 years in a real-world setting. The number of anti-VEGF injections did not decrease from 2 to 5 years in contrast to eyes with diabetic macular oedema.
How might these results change the focus of research or clinical practice?
Anti-VEGF therapy is a substantial burden to patients; however, the long-term gains demonstrated reassure all to the substantial gains that can be achieved although with persistent anti-VEGF therapy.
Introduction
Macular oedema secondary to retinal vein occlusions (RVO) can cause significant vision loss.1 Vascular endothelial growth factor (VEGF) inhibitors have become the mainstay of treatment for cystoid macular oedema (CMO) due to RVO.2 RVO is an obstruction of the retinal venous system and can be classified into two primary categories depending on the location of obstruction: central RVO (CRVO) involving the entire central retinal vein, and branch RVO (BRVO) when only one branch of the central vein is affected.3 Additionally, hemi-RVO (HRVO) may be considered either as a subtype of CRVO as it involves the anterior part of the central retinal vein, or as a BRVO, since it is the first branch of the central retinal vein.4
Both CRVO and BRVO are associated with a decreased vision-related quality of life as evaluated by the National Eye Institute visual function questionnaire.5 6 Current treatment of RVO is aimed at the treatment of macular oedema, which is the leading cause of vision loss.7 In RVO, elevated secretion of VEGF leads to elevated vascular permeability and vasodilation.8–10 Long-lasting macular oedema usually produces secondary retinal pigment epithelial (RPE) changes, which themselves result in poor visual acuity.1 11 12
Intravitreal (IVT) injections of VEGF inhibitors such as bevacizumab, ranibizumab and aflibercept have displayed superior outcomes compared with the natural history of the disease.13–15
Significant improvements in visual acuity (VA) and macular oedema among patients with RVO receiving VEGF inhibitors have been demonstrated in randomised clinical studies including COPERNICUS, GALILEO, BRAVO, CRUISE and VIBRANT.16–19 However, there is very limited long-term data for outcomes of treatment for RVO. The CRUISE study was a 12-month study of 392 eyes comparing two doses of ranibizumab (0.3 and 0.5 mg) compared with sham in CRVO. At month 12, the mean gain in best-corrected VA (BCVA) was 13.9 letters in both the 0.5 and 0.3 mg groups.17 BRAVO was a similar design to CRUISE, recruiting 397 patients with BRVO. Over the 12 months of the study, mean BCVA improved by 16.4 and 18.3 letters in the 0.3 and 0.5 mg groups, respectively.17
Extension studies following BRAVO and CRUISE have given insight into outcomes of anti-VEGF therapy for RVO of up to 4 years but with significant loss to follow-up, with only 205 of the initial 397 BRVO and 181 of the initial 392 eyes with CRVO left at the end of the HORIZON study (2 years after starting treatment).20 Mean gains of 17.5 and 15.6 letters from BRAVO baseline were observed for patients initially randomised to 0.5 mg and sham groups, respectively. In contrast, mean change in BCVA of CRUISE patients at 12 months from HORIZON baseline was a loss of 5.2 and 4.1 letters in the 0.3 and 0.5 mg treatment groups, respectively.20 After 2 years of treatment, patients in the HORIZON study were still undergoing treatment for persistent fluid.
The RETAIN study was an extension of the HORIZON study which followed patients for a further 24 months. It included just 26 eyes from the BRAVO trial and 27 eyes from the CRUISE study, with data on 4-year outcomes. RETAIN study demonstrated 56% of patient’s required frequent injections; however, 80% of those with BRVO and 64.3% with CRVO were able to maintain a BCVA of 20/40 or better at 4 years.21 Of note, only 26 of 34 eyes with BRVO and 27 of 32 eyes with CRVO completed the study.21 A clinical study by Rezar et al included just 28 patients with RVO treated with bevacizumab or ranibizumab with a mean of 5 years follow-up. However, because of limitations of the study design, especially in bevacizumab-treated eyes, the mean time to start treatment was delayed 5 months with the major conclusion of the study being that final functional outcomes were significantly superior if treatment was initiated within <3 months of diagnosis.22
There is very limited data available on long-term (5 years +) outcomes of RVO treated with VEGF inhibitors and the number of injections required for these outcomes. The aim of this study was to provide such data from a real-world setting. This is a retrospective study assessing long-term outcomes in patients with macular oedema secondary to RVO at 5 years after initiation of anti-VEGF inhibitor therapy.
Methods
Study design
This was a retrospective, single-centre case series. The study was approved by the University of Sydney institutional review board. The study adhered to the Declaration of Helsinki.
Study participants
We conducted a search of records with patients diagnosed with RVO who commenced anti-VEGF therapy between January 2010 and January 2013. Patients required at least 5 years of follow-up from initiation of anti-VEGF treatment. Any exclusions were recorded using a Consolidated Standards of Reporting Trials-like approach to minimise bias.
Inclusion criteria included macular oedema secondary to RVO diagnosed clinically, confirmed by fundus fluorescein angiography (FFA) and spectral domain optical coherence tomography (SD-OCT); commencement of IVT anti-VEGF therapy between January 2010 and January 2013, central foveal thickness (CFT) ≥300 µm and presence of CMO defined as intraretinal oedema and or subretinal fluid on SD-OCT, with 5 years of follow-up.
Exclusion criteria for the study eye included the following: (1) previous IVT injections of anti-VEGF or corticosteroids; (2) presence of active retinal or ocular disease that may confound the results (including diabetic retinopathy, macular degeneration, macular hole, and uveitis); (3) history of vitreoretinal surgery and (4) patients who had inadequate imaging.
Data collection
For eligible patients, the following data were collected from their medical records: age, gender, history of systemic hypertension (including use of antihypertensive medication), macular laser treatment, VA and CFT at baseline and yearly to 5 years, IVT injections of VEGF inhibitors—type and frequency any additional treatments.
Outcome measures
Main outcome measures were the mean change in VA and CFT from baseline to year 5. Secondary outcomes included perfusion status, ellipsoid zone (EZ) integrity, and area of macular ischaemia and foveal avascular zone (FAZ) from fluorescein angiography, the number and frequency of injections and visits, and the proportion of eyes avoiding severe VA loss (< 15 letters); the proportion of eyes with good VA (≥ 70 letters [20/40]) and poor VA (≤ 35 letters [20/200]), the proportion of eyes that were fluid free at 5 years and the proportion of patients where anti-VEGF therapy was successfully ceased.
Optical coherence tomography analysis
All patients had SD-OCT tomography (Heidelberg Spectralis; Heidelberg Engineering; Heidelberg, Germany) performed at each visit as standard of care. For this analysis, yearly scans were used and CFT recorded. CFT was defined as the distance between the inner limiting membrane and Bruch’s membrane in the central 1 mm diameter area centred on the fovea. Images were independently evaluated by two image graders, and a third grader adjudicated in the event of disagreement. Segmentation lines were manually adjusted in the case of software error. If the fovea could not be identified, the scan was excluded from analysis. Follow-up scans were obtained by use of the progression scanning tool. OCT images were graded for any disruption to the EZ by two independent masked graders and any differences in grading adjudicated by a third grader.
Analysis of fundus fluorescein angiogram
Macular ischaemia and FAZ area were measured using a single FFA image. The quantification of macular nonperfusion in FFA-captured images was calculated by manually outlining using the Heidelberg Eye explorer program (Heidelberg Spectralis V.1.0.15.0). No image manipulation was performed prior to FAZ area measurement. FFA images were graded by two independent masked graders and any differences in grading was adjudicated by a third grader. Macular ischaemia was defined as the absence or presence of retinal capillary loss in ≥1 centre, inner or outer fields of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid.23 Retinal ischaemic subtype was defined as more than 10 disc areas of retinal capillary nonperfusion based on FFA.24
Statistical analyses
Statistical analysis was performed using SPSS software (V.24.0, SPSS, Chicago, IL, USA). Snellen VA was converted to ETDRS letters for statistical analyses.25 Descriptive data were presented as means and SD. Paired t-test was used to compare outcome variables between baseline and follow-up visits. Interobserver agreement was assessed using the interclass correlation coefficient. Baseline VA was stratified into five subgroups based on the criteria set out in the International Classification of Diseases, 10th revision, Australian Modification: legal blindness, baseline VA<35 ETDRS letters (Snellen VA<20/200); low vision, VA≥35 (≥20/200) and <60 letters (<20/60); reduced vision, VA≥60 (≥20/60) and <70 letters (<20/40); mildly reduced vision to the Australian legal driving limit, VA≥70 and <85 letters (≥20/40 and <20/20); and normal vision, VA≥85 letters (≥20/20).
Changes in VA and CFT were evaluated separately in each subgroup according to baseline VA in order to determine the potential confounding ‘ceiling’ and ‘floor’ effects resulting from good or poor baseline vision. One-way analysis of variance was used to compare means of VA and CFT changes, respectively, over the 5 years across the five subgroups of baseline vision.
Multivariate logistic regression analysis was performed to identify prognostic factors for final visual outcomes. Variables evaluated in the multivariate analyses included age, gender, baseline VA and baseline CFT. A p value of <0.05 was considered statistically significant.
Results
Study patients
After evaluation of inclusion criteria, 68 eyes from 66 patients were eligible for inclusion in this study. At RVO diagnosis, mean age was 67±11.1 years (range: 43.0–88). Of these 41 (62%) were males. The population was predominately Caucasian, with one patient (1.5%) being southeast Asian. The mean time from diagnosis of RVO to beginning of anti-VEGF therapy was 8.1±4.2 weeks, with the majority of patients (81%) being diagnosed within 3 months prior to initiation of anti-VEGF therapy. Baseline characteristics are summarised in table 1.
Table 1.
Demographic and clinical characteristics of included patients
| CRVO/HRVO (n=37) |
BRVO (n=31) |
|
| Age, years | 66.1±11.1 | 67.8±11.1 |
| BCVA (ETDRS letters) | 54.1±14.5 | 61.4±17.0 |
| CFT (µm) | 561.5±239.6 | 451.3±129.3 |
| IOP (mm Hg) | 14.1±4.1 | 12.8±3.3 |
| Gender | ||
| Male | 24 (65%) | 17 (55%) |
| Female | 13 (35%) | 12 (39%) |
| Ischaemic type | 12 (32%) | 7 (23%) |
| Hypertension | 28 (76%) | 27 (87%) |
| Hypercholesterolaemia | 21 (57%) | 19 (61%) |
| Diabetes mellitus | 8 (22%) | 3 (10%) |
| Smoker | 10 (27%) | 8 (26%) |
BCVA, best-corrected visual acuity; CFT, central foveal thickness; ETDRS, Early Treatment Diabetic Retinopathy Study; IOP, intraocular pressure.
In all, 31 eyes were classified as BRVO; 35 CRVO and two hemi-RVO. For the purposes of analysis, the eyes with HRVO were included with CRVO eyes as the occlusion was located at the anterior aspect of the central retinal vein. Macular laser treatment had been applied to 13 (19%) eyes prior to initiation of anti-VEGF therapy. In total, 42 eyes (62%) were treated with bevacizumab only, 11 eyes (16%) were switched from bevacizumab to ranibizumab, 4 eyes (6%) were switched from bevacizumab to aflibercept, 2 eyes (3%) were treated with ranibizumab only and 8 (12%) eyes were treated with all three agents during the course of their treatment.
BRVO outcomes
Before treatment, mean VA of eyes with BRVO was 61.4±17.0 ETDRS letters, improving to 75.5±12.7 letters at 5 years (p<0.001) (figure 1). Statistically significant mean VA gains were seen at all time points. At 5 years after initiation of therapy, 39% of eyes with BRVO had gained at least 15 letters, whereas 6% had lost 15 letters or more. Greatest visual gains were seen in those patients with a baseline VA worse than the median of 68 ETDRS letters, CFT of ≥400 µm and preservation of the EZ (table 2). The vision at the end of 1 year was a predictor for the final VA at year 5 (R2=0.7, p<0.001).
Figure 1.

BRVO analysis: (a) mean change in visual acuity and (b) central foveal thickness over 5 years. BRVO, branch retinal vein occlusion; ETDRS, Early Treatment Diabetic Retinopathy Study.
Table 2.
Change in visual acuity from baseline over 5 years following commencement of anti-VEGF therapy
| Baseline visual acuity (ETDRS letters) | BRVO | CRVO | ||||
| N | Mean (95% CI) | P value | N | Mean (95% CI) | P value | |
| <35 | 2 | 36.0 (8.7 to 48.7) | 0.05 | 2 | 27 (15.0 to 39.0) | 0.02 |
| ≥35 and<60 | 8 | 23.9 (13.5 to 31.5) | 0.03 | 20 | 15.2 (2.1 to 31.5) | 0.02 |
| ≥60 and<70 | 7 | 14.9 (6.9 to 21.6) | 0.03 | 9 | 5.75 (8.9 to 20.9) | 0.01 |
| ≥70 and<85 | 14 | 5.1 (4.8 to 12.1) | 0.02 | 6 | 8.3 (3.0 to 14.6) | 0.05 |
| ≥85 | 0 | – | – | 0 | – | – |
BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; ETDRS, Early Treatment Diabetic Retinopathy Study; VEGF, vascular endothelial growth factor.
There was no difference in final VA among patients that switched anti-VEGF (n=12) throughout the study compared with those that remained on a single therapy (n=19) (14.4±19.5 vs 14.0±12.6 letters, p=0.09).
Mean CFT of eyes with BRVO decreased by 152.1±138.0 µm at year 5 (p<0.001) (figure 2). There was no statistical difference seen between patients that remained on a single anti-VEGF and those that were switched during the study (−158.7±140.9 µm vs −141.6±134.5 µm, p=0.06).
Figure 2.

BRVO analysis: mean change in central foveal thickness over 5 years. BRVO, branch retinal vein occlusion.
Eyes (11/31, 35%) which had undergone macular laser prior to initiation of anti-VEGF therapy had a similar baseline VA (57.3±15.5 vs 63.8±17.2 letters, p=0.08) and final VA (72.6±13.8 vs 77.1±12.9, p=0.09) compared with those which were treatment naive. Similarly, there was no difference in reduction of CFT among these two groups at year 5 (−155.5±162.1 vs -150.2±127.5 µm, p=0.08).
At baseline, the central EZ on SD-OCT was preserved or only partially disrupted for most eyes: 24 (77%). Two eyes (6%) were not gradeable due to gross oedema or haemorrhage. After 5 years of treatment, 27 of 31 eyes (87%) had preservation of the EZ with a final mean VA of 78.9±11.5 letters compared with those with disrupted EZ of 68.4±17.5 letters (p=0.03). In all, 11 eyes (36%) had persistent macular oedema. Seven eyes (23%) had successfully ceased anti-VEGF therapy with no recurrence of oedema by year 5. Four eyes (13%) had successfully ceased treatment by year 2. There were no RPE changes observed.
Of the 879 anti-VEGF injections administered in the eyes with BRVO during the study period, 201 (23%) were ranibizumab, 637 (72%) were bevacizumab and 41 (5%) were aflibercept. Most patients (59%) received repeated injections of a single anti-VEGF agent, but in 41% of patients, the type of anti-VEGF therapy used was switched at least once during the study period. The most common switch in anti-VEGF therapy was from bevacizumab to ranibizumab (69%).
The mean number of injections administered during the 5-year period was 28.4±16.6. Three eyes (10%) required only three initial IVT injections without recurrence of CMO. The average number of injections administered during the first year was 6.9±3.3. Subsequent years demonstrated no variance in frequency of IVT administration, with a mean of 5.5±3.8 per year (range: 1–13) (figure 3). The number of injections administered in the second year predicted the number of injections in the fourth year and fifth year (R2=0.5, p<0.001, and R2=0.5, p<0.001, respectively).
Figure 3.
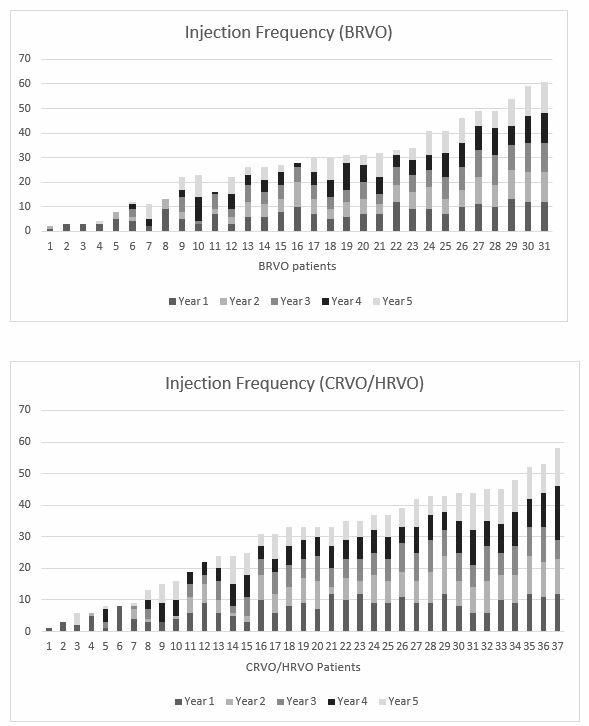
Injection frequency by patient over 5 years of anti-VEGF therapy. BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; HVRO, hemi-retinal vein occlusion; VEGF, vascular endothelial growth factor.
CRVO outcomes
Among the 37 eyes with CRVO, mean baseline VA was 54.1±14.5 letters and on average gained 11.1±20.3 letters by 5 years. There was a statistically significant difference between eyes that switched therapy (n=12) during the course of the study and those that remained on the same anti-VEGF (n=25) (3.75±20.4 vs 14.6±19.8 letters, p=0.02). There was also a significant difference in reduction of final CFT (−227.5±188.1 µm vs −272.6±276.9 µm, p=0.02). The eyes that switched anti-VEGF therapy also required more IVT injections over the duration of the study compared with those which were not switched (37.7±10.9 vs 25.5±16.0 injections, p=0.03).
Mean CFT of eyes with CRVO decreased by 189.6±206.0 µm at year 1 and decreased further at year 5 by 257.6±249.8 µm (p<0.001) (figure 4). Greater baseline CFT was associated with a greater mean reduction in CFT at 5 years (p<0.001). Change in CFT and VA among perfusion status in CRVO is demonstrated in figure 5.
Figure 4.

CRVO subtype analysis: mean change in CFT in microns from baseline to 5 years follow-up. CFT, central foveal thickness; CRVO, central retinal vein occlusion; HRVO, hemi-retinal vein occlusion.
Figure 5.
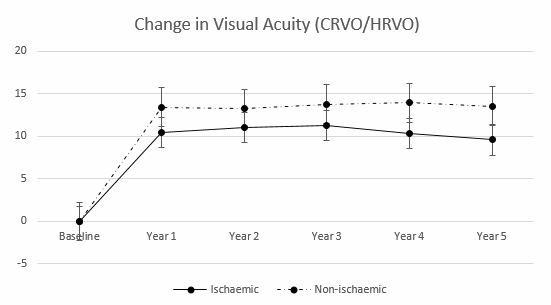
CRVO subgroup analysis: mean change in visual acuity in ETDRS letters from baseline to 5 years. CRVO, central retinal vein occlusion; ETDRS, Early Treatment Diabetic Retinopathy Study; HRVO, hemi-retinal vein occlusion.
At baseline, the central EZ on SD-OCT was preserved or only partially disrupted for most eyes; 24 (65%), 5 eyes were ungradeable due to gross oedema or haemorrhage. After 5 years of treatment, 29 eyes (78%) had preservation of the EZ with a final mean VA of 66.8±18.2 letters compared with those eyes with disrupted EZ of 53.3±16.5 letters (p=0.02). In all, 13 eyes (35%) had persistent macular oedema. The progression of capillary nonperfusion was observed in six (16%) eyes over the 5 years of follow-up. In five eyes (14%), anti-VEGF therapy was discontinued in the first 2 years, with no recurrence of oedema at 5 years. Anti-VEGF was ceased in one further eye after the second year.
FAZ measurements were available for 35/37 eyes. Two eyes from two patients were excluded from the measurement of FAZ due to a high quantity of motion artefacts. Patients with macular ischaemia presented with a mean FAZ of 0.93±0.32 mm2, and patients with non-ischaemia 0.29±0.15 mm (p=0.03). Mean VA was negatively associated with macular ischaemia (R2=−0.6, p=0.05); those eyes with no macular ischaemia had better VA. FAZ after therapy was positively correlated with final VA score (R2=0.6, p=0.03).
Univariate logistic regression demonstrated that baseline vision and age were significant factors contributing to a final VA improvement of ≥15 ETDRS letters (p=0.009). Gender and history of hypertension were not significant. Multivariate analyses revealed no significance among these possible factors. No correlation was identified between baseline CFT and VA changes over 5 years (R2=0.09, p=0.508).
The mean number of injections administered during the 5-year period was 29.5±15.5. Two eyes (5%) required only three initial IVT injections without recurrence of CMO. The average number of injections was administered during the first year was 7.3±3.3. Subsequent years demonstrated no variance in frequency of IVT administration, with a mean of 5.5±3.8 per year (range: 2–12) (figure 3). The number of injections administered in the second year predicted the number of injections in the fourth year and fifth year (R2=0.6, p<0.001, and R2=0.6, p<0.001, respectively).
Of the 1091 anti-VEGF injections administered in the study eyes during the study period, 14% were ranibizumab, 79% were bevacizumab and 7% were aflibercept. Most patients (n=24) received repeated injections of a single anti-VEGF agent, but in 32% of patients (n=13), the type of anti-VEGF therapy used was switched.
Safety outcomes
No cases of endophthalmitis, uveitis or cardiovascular events were reported in patients in this study. In all, 20 eyes with CRVO (54%) were treated for intraocular pressure (IOP) rise with topical medications (increase in IOP of >10 mm Hg from baseline) due to ocular hypertension, a common association of CRVO. One eye developed rubeosis, receiving laser panretinal photocoagulation. Five eyes (7%) underwent phacoemulsification during the course of follow-up. One patient was diagnosed with myelofibrosis.
Discussion
This study reports the long-term outcomes of VEGF inhibitors in RVO in a real-world setting. Although significant shorter-term benefits of VEGF inhibitors in RVO have been reported in numerous clinical trials and studies,26–30 limited data are available on long-term outcomes,21 and even less on real-world outcomes. The present study provides additional information to the limited long-term data currently available.22 31
The gain in vision seen after 1 year was +11 letters among both eyes with BRVO and CRVO, consistent with other real-world studies,32–37 and only a little inferior to the major RVO clinical trials.16–18 38 Similarly, the proportion of eyes achieving a 15-letter improvement after 1 year was 34% of eyes with BRVO and 50% of eyes with CRVO, again a little inferior to clinical trials (VIBRANT: 52.7%; COPERNICUS: 56% and GALILEO: 60%). At 5 years, the majority of patients (83.8%) improved or maintained vision, with 22% gaining≥15 ETDRS letters. Greater visual gains were correlated with a poorer baseline VA which may have been due to the greater potential for improvement and ceiling effect of those eyes with better baseline VA.22
Our population differed from that of previously reported clinical trials. Based on the inclusion and exclusion criteria of the BRAVO and CRUISE studies, 16 patients from the present study would have not met the inclusion criteria: 14 (21%) based on vision requirements and 2 (3%) based on CFT. The inclusion criteria of clinical trials rarely reflect real-world populations. Participants in clinical trials tend to be healthier on average, as they have to commit to intensive treatment schedules and close monitoring. These differences may cause discrepancies between real-world studies and clinical trials.
There are few data on long-term outcomes of eyes with RVO. In the RETAIN study, data were available for only approximately 10.5% (n=66) of the primary BRAVO and CRUISE patients at 4 years,21 which may not be representative of the original cohort. Patients with BRVO had a mean BCVA improvement of 16.4 letters from BRAVO baseline, which is a loss of 2.1 letters from the end of BRAVO.17 Patients with CRVO had a mean gain of 14 letters from CRUISE baseline, similar to the 13.1 letters achieved at the end of CRUISE.21 In our retrospective study, significant visual improvement was seen and more importantly, maintained for 5 years. In Hayreh et al’s 2011 study following the natural history of CRVO, vision at 5 years in those with resolution of macular oedema was found to be 50 ETDRS letters in 83% of non-ischaemic eyes and 12% ischaemic CRVO eyes.39 In our cohort, even in those without complete resolution of macular oedema, vision was ≥50 letters in 84% of non-ischaemic CRVO and 67% of those with ischaemic CRVO. This reflects the hypothesised association between retinal ischaemia and increased production of VEGF,40 with many studies reporting reperfusion of ischaemic retina with anti-VEGF treatment.41–43
Reduction in CFT during the first year was also maintained during the longer follow-up of 5 years. Macular oedema was absent on SD-OCT imaging at 5 years in the majority of patients (65%). In the RETAIN study, while 50% of eyes with BRVO had resolution of macular oedema at 4 years, just 44% of eyes with CRVO had resolution of macular oedema.21 This demonstrates the superiority of anti-VEGF therapy to the natural history, with reported values of macular oedema resolution being much lower (39% of ischaemic CRVO and 51% of non-ischaemic CRVO at 24 months).44
There was a positive association between the number of injections received and improvement in VA at 5 years in our study. This association is consistent with other studies where more frequent injections generate greater visual outcomes.31 However, the mean number of anti-VEGF injections received by patients in this study was 5–6 per year, or 8–10 weekly, which may not always have been the optimal interval as prescribed by the treating physician. Patients in clinical trials tend to be healthier and younger and need to be able to commit to the rigorous schedule of the clinical trial. It is more common for under treatment of conditions to occur outside of clinical trials.45 We did not see the decline in injection frequency from year 2 to year 5 commonly seen in eyes being managed for diabetic macular oedema.
Progression of ischaemia is a significant cause of permanent vision loss in eyes with RVO.46 In this study, progression of capillary nonperfusion was observed in six (8.8%) eyes despite use of anti-VEGF over the 5 years of follow-up. Worsening VA can occur due to damage to photoreceptors caused by macular oedema at the fovea, leading to impairment of bipolar and ganglion cells by ischaemia, as indicated by the presence of nonperfusion areas. The eyes which progressed to ischaemic RVO tended to have more frequent injections over the course of the study, but with less effect on CFT and VA compared with those which remained well perfused. It is unknown whether an early change in VEGF inhibitor may have been of benefit. On the other hand, retinal atrophy and chronic thinning of the neuroretinal were observed in most patients with decreased vision, an indicator of macular ischaemia.
However, those poorly responsive eyes in the first year did present with a subsequent later response to treatment. Interestingly, this subgroup required fewer injections than the entire cohort (21.4 vs 36.6; p=0.03) and had a mean gain of +21 letters over the 5 years, demonstrating that a limited initial response to anti-VEGF therapy does not entirely preclude the possibility of a complete future response. Although eyes with ischaemia had worse vision compared with eyes with non-ischaemia throughout the study, they still achieved significant visual improvements.47
The good long-term functional and anatomical outcomes of VEGF inhibition in this cohort support this treatment in real-world clinical setting. In the present study, only 9% required additional focal/grid laser treatment. The use of laser treatment was less frequent than seen in clinical trials (BRAVO trial 20.1% in 0.3 mg ranibizumab arm and 21.4% in 0.5 mg ranibizumab arm).17 In our study, switching to ranibizumab or aflibercept was associated with a significant improvement in VA in CRVO but not in eyes with BRVO, although our study was not designed to specifically assess this.
There are several limitations of the present study. Primarily, the retrospective study design, lack of control group and small sample size. The choice of drug was dependent on what was available at the time of initiation of treatment. In Australia, ranibizumab became funded by the Pharmaceutical Benefits Scheme (PBS) for RVO in July 2015; and aflibercept in October 2015 for CRVO and December 2016 for BRVO. Bevacizumab was available as an off-label medication when PBS-funded drug was not available. The study design did not allow comparison between the different drugs regarding their effectiveness. Although a small percentage of patients received additional laser therapy, potentially confounding the treatment effect, however, it should be noted that rescue laser is frequently seen in many clinical trials.17 48 49
The major strength of the study is the long follow-up period. It demonstrated that many patients with RVO receiving long-term anti-VEGF therapy are able to maintain the gain in vision seen in the first year for an additional 5 years, although frequent injections are still required. There is still need for additional long-term, prospective, studies to better ascertain the best long-term management strategies for patients with macular oedema secondary to RVO.
bmjophth-2018-000249supp001.docx (24.6KB, docx)
Footnotes
Contributors: KS conceived and planned the study, data collection, statistics and manuscript drafting. TH, SFB and AC gave critical appraisal and final approval of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AAC has acted as a consultant for Novartis, Bayer and Alcon. SF-B has acted as a consultant for Bayer, Novartis and Allergan.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res 2014;41:1–25. 10.1016/j.preteyeres.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang Y, Mieler WF. Update on the use of anti-VEGF intravitreal therapies for retinal vein occlusions. Asia Pac J Ophthalmol 2017;6:546–53. 10.22608/APO.2017459 [DOI] [PubMed] [Google Scholar]
- 3. Li J, Paulus YM, Shuai Y, et al. New developments in the classification, pathogenesis, risk factors, natural history, and treatment of branch retinal vein occlusion. J Ophthalmol 2017;2017:1–18. 10.1155/2017/4936924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appiah AP, Trempe CL. Differences in contributory factors among hemicentral, central, and branch retinal vein occlusions. Ophthalmology 1989;96:364–6. 10.1016/S0161-6420(89)32884-3 [DOI] [PubMed] [Google Scholar]
- 5. Deramo VA, Cox TA, Syed AB, et al. Vision-related quality of life in people with central retinal vein occlusion using the 25-item National Eye Institute visual function questionnaire. Arch Ophthalmol 2003;121:1297–302. 10.1001/archopht.121.9.1297 [DOI] [PubMed] [Google Scholar]
- 6. Awdeh RM, Elsing SH, Deramo VA, et al. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item National Eye Institute visual function questionnaire. Br J Ophthalmol 2010;94:319–23. 10.1136/bjo.2007.135913 [DOI] [PubMed] [Google Scholar]
- 7. Tsukada K, Tsujikawa A, Murakami T, et al. Lamellar macular hole formation in chronic cystoid macular edema associated with retinal vein occlusion. Jpn J Ophthalmol 2011;55:506–13. 10.1007/s10384-011-0056-9 [DOI] [PubMed] [Google Scholar]
- 8. Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clin Ophthalmol 2010;4:809–16. 10.2147/OPTH.S7631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 2005;140:256.e1–256.e7. 10.1016/j.ajo.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 10. Yoshimura T, Sonoda K-hei, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One 2009;4:e8158 10.1371/journal.pone.0008158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2010;117:1113–23. 10.1016/j.ophtha.2010.01.060 [DOI] [PubMed] [Google Scholar]
- 12. Decroos FC, Fekrat S. The natural history of retinal vein occlusion: what do we really know? Am J Ophthalmol 2011;151:739–41. 10.1016/j.ajo.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 13. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;98:1636–41. 10.1136/bjophthalmol-2014-305252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edington M, Connolly J, Chong NV. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin Drug Metab Toxicol 2017;13:1217–24. 10.1080/17425255.2017.1404987 [DOI] [PubMed] [Google Scholar]
- 15. Moisseiev E, Waisbourd M, Ben-Artsi E, et al. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch Clin Exp Ophthalmol 2014;252:331–7. 10.1007/s00417-013-2495-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korobelnik J-F, Holz FG, Roider J, et al. Intravitreal Aflibercept Injection for Macular Edema Resulting from Central Retinal Vein Occlusion: One-Year Results of the Phase 3 GALILEO Study. Ophthalmology 2014;121:202–8. 10.1016/j.ophtha.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 17. Thach AB, Yau L, Hoang C, et al. Time to clinically significant visual acuity gains after ranibizumab treatment for retinal vein occlusion. Ophthalmology 2014;121:1059–66. 10.1016/j.ophtha.2013.11.022 [DOI] [PubMed] [Google Scholar]
- 18. Clark WL, Boyer DS, Heier JS, et al. Intravitreal Aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the Vibrant study. Ophthalmology 2016;123:330–6. 10.1016/j.ophtha.2015.09.035 [DOI] [PubMed] [Google Scholar]
- 19. Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA 2017;317 10.1001/jama.2017.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the horizon trial. Ophthalmology 2012;119:802–9. [DOI] [PubMed] [Google Scholar]
- 21. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab. Ophthalmology 2014;121:209–19. 10.1016/j.ophtha.2013.08.038 [DOI] [PubMed] [Google Scholar]
- 22. Rezar S, Eibenberger K, Bühl W, et al. Anti-VEGF treatment in branch retinal vein occlusion: a real-world experience over 4 years. Acta Ophthalmologica 2015;93:719–25. 10.1111/aos.12772 [DOI] [PubMed] [Google Scholar]
- 23. Larsen M, Waldstein SM, Boscia F, et al. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion: Twelve-Month results of the crystal study. Ophthalmology 2016;123:1101–11. 10.1016/j.ophtha.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 24. Brown DM, Wykoff CC, Wong TP, et al. Ranibizumab in preproliferative (ischemic) central retinal vein occlusion: the rubeosis anti-VEGF (RAVE) trial. Retina 2014;34:1728–35. 10.1097/IAE.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 25. Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina 2010;30:1046–50. 10.1097/IAE.0b013e3181d87e04 [DOI] [PubMed] [Google Scholar]
- 26. Wirth MA, Becker MD, Graf N, et al. Aflibercept in branch retinal vein occlusion as second line therapy: clinical outcome 12 months after changing treatment from bevacizumab/ranibizumab—a pilot study. Int J Retin Vitr 2016;2 10.1186/s40942-016-0045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evoy KE, Abel SR. Aflibercept: newly approved for the treatment of macular edema following central retinal vein occlusion. Ann Pharmacother 2013;47:819–27. 10.1345/aph.1R705 [DOI] [PubMed] [Google Scholar]
- 28. Rishi P, Raka N, Rishi E. Analysis of potential ischemic effect of intravitreal bevacizumab on unaffected retina in treatment-naïve macular edema due to branch retinal vein occlusion: a prospective, interventional Case-Series. PLoS One 2016;11:e0162533 10.1371/journal.pone.0162533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitry D, Bunce C, Charteris D, et al. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database of Systematic Reviews 2013;118 10.1002/14651858.CD009510.pub2 [DOI] [PubMed] [Google Scholar]
- 30. Osaka R, Muraoka Y, Miwa Y, et al. Anti-vascular endothelial growth factor therapy for macular edema following central retinal vein occlusion: 1 initial injection versus 3 monthly injections. Ophthalmologica 2018;239:27–35. 10.1159/000479049 [DOI] [PubMed] [Google Scholar]
- 31. Wecker T, Ehlken C, Bühler A, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol 2017;101:353–9. 10.1136/bjophthalmol-2016-308668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan M, Wai KM, Silva FQ, et al. Comparison of ranibizumab and bevacizumab for macular edema secondary to retinal vein occlusions in routine clinical practice. Ophthalmic Surg Lasers Imaging Retina 2017;48:465–72. 10.3928/23258160-20170601-04 [DOI] [PubMed] [Google Scholar]
- 33. Sakanishi Y, Lee A, Usui-Ouchi A, et al. Twelve-month outcomes in patients with retinal vein occlusion treated with low-frequency intravitreal ranibizumab. Clin Ophthalmol 2016;10:1161–5. 10.2147/OPTH.S107594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brynskov T, Kemp H, Sørensen TL. Intravitreal ranibizumab for retinal vein occlusion through 1 year in clinical practice. Retina 2014;34:1637–43. 10.1097/IAE.0000000000000111 [DOI] [PubMed] [Google Scholar]
- 35. Chatziralli I, Theodossiadis G, Chatzirallis A, et al. Ranibizumab for retinal vein occlusion: predictive factors and long-term outcomes in real-life data. Retina 2018;38:559–68. 10.1097/IAE.0000000000001579 [DOI] [PubMed] [Google Scholar]
- 36. Kornhauser T, Schwartz R, Goldstein M, et al. Bevacizumab treatment of macular edema in CRVO and BRVO: long-term follow-up. (BERVOLT study: bevacizumab for RVO long-term follow-up). Graefes Arch Clin Exp Ophthalmol 2016;254:835–44. 10.1007/s00417-015-3130-z [DOI] [PubMed] [Google Scholar]
- 37. Tan MH, McAllister IL, Gillies ME, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. Am J Ophthalmol 2014;157:237–47. 10.1016/j.ajo.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 38. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 2013;155:429–37. 10.1016/j.ajo.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 39. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology 2011;118:e1-2.:119–33. 10.1016/j.ophtha.2010.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyd SR, Zachary I, Chakravarthy U, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol 2002;120:1644–50. 10.1001/archopht.120.12.1644 [DOI] [PubMed] [Google Scholar]
- 41. Campochiaro PA, Bhisitkul RB, Shapiro H, et al. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013;120:795–802. 10.1016/j.ophtha.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 42. Campochiaro PA, Wykoff CC, Shapiro H, et al. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology 2014;121:1783–9. 10.1016/j.ophtha.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 43. Mir TA, Kherani S, Hafiz G, et al. Changes in retinal Nonperfusion associated with suppression of vascular endothelial growth factor in retinal vein occlusion. Ophthalmology 2016;123:625–34. 10.1016/j.ophtha.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hayreh SS, Zimmerman MB. Fundus changes in branch retinal vein occlusion. Retina 2015;35:1016–27. 10.1097/IAE.0000000000000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. The Lancet 2005;365:82–93. 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
- 46. Winegarner A, Wakabayashi T, Fukushima Y, et al. Changes in retinal microvasculature and visual acuity after antivascular endothelial growth factor therapy in retinal vein occlusion. Invest Ophthalmol Vis Sci 2018;59:2708–16. 10.1167/iovs.17-23437 [DOI] [PubMed] [Google Scholar]
- 47. DeCroos FC, Ehlers JP, Stinnett S, et al. Intravitreal bevacizumab for macular edema due to central retinal vein occlusion: perfused vs. ischemic and early vs. late treatment. Curr Eye Res 2011;36:1164–70. 10.3109/02713683.2011.607537 [DOI] [PubMed] [Google Scholar]
- 48. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102–12. 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 49. Varma R, Bressler NM, Suñer I, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and cruise trials. Ophthalmology 2012;119:2108–18. 10.1016/j.ophtha.2012.05.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2018-000249supp001.docx (24.6KB, docx)


