Abstract
In a Policy Forum, Alison Holmes and colleagues discuss coordinated approaches to antimicrobial stewardship.
Summary points
Antimicrobial stewardship (AMS) strategies are widely implemented in single healthcare sectors and organisations; however, the extent and impact of integrated AMS initiatives across the whole health economy are unknown.
Assessing degree of integration of AMS across the whole health economy and its impact is essential if we are to achieve a ‘One Health’ approach to addressing antimicrobial resistance (AMR), and therefore we searched systematically for and analysed published examples of integrated AMS initiatives to address this gap.
Application of a system-level framework to analyse integration of AMS initiatives across and within healthcare sectors shows that integration is emerging but needs strengthening.
Findings from a small number of evaluations in high-income countries suggest that antimicrobial prescribing and healthcare-associated infections can be reduced using a multisectoral integrated AMS approach.
More robust research designs to evaluate and understand the impact of multisectoral integrated AMS are needed, particularly with respect to differing health systems in different countries and local organisational contexts.
Our analysis highlights a number of challenges and ways forward for enhancing the delivery of AMS through an integrated approach.
Background
It is estimated that around 700,000 people die annually from drug-resistant infections, with experts predicting an alarming possible increase to 10 million deaths each year by 2050 and major future challenges to the way we practice medicine and surgery [1,2]. It was welcome news that tackling antimicrobial resistance (AMR) and infectious diseases along with health system strengthening were featured at the G20 summit (November, 2018), under the wider aim of improving sustainability, and progress towards more coordinated international efforts will be reviewed at the 73rd session of the UN General Assembly (September 2018) [3]; but how are health professionals, managers, and policymakers assuring coordinated efforts within human healthcare? Globally, there has been much emphasis on a ‘One Health’ approach that involves connecting the health of humans, animals, and the environment to tackle AMR [2]. This is driving much-needed antimicrobial stewardship (AMS) activities in animal production sectors [4]. However, we have yet to achieve and establish joined-up approaches within human health. This paper, therefore, focuses on an analysis of multisectoral AMS in human health. AMS remains a cornerstone for addressing AMR with numerous initiatives implemented with varying degrees of success [5,6]. A critical gap we have identified is that approaches have largely focused efforts separately in primary care or secondary care, and have also heavily targeted medical prescribers. In this paper, we propose that policymakers, clinical leaders, and healthcare managers assess and consolidate AMS activities across the whole health economy, and we use a novel, to our knowledge, approach to demonstrate how such an assessment can be made. We present the extent to which existing AMS initiatives are multisectoral or integrated across a whole health economy within individual countries and their impact on antimicrobial-related outcomes. We then highlight some challenges and key considerations for developing and harnessing potential benefits of integrated AMS approaches.
Need for a whole-health–economy approach
Health systems are required to deliver best outcomes efficiently, facing the challenges of macroeconomic constraints, technology costs, and increasing public need and demand. Consolidating the sometimes disparate programs and initiatives within the health sector is necessary, and integrated models of care across primary, secondary, tertiary, and long-term care can help with coordinated implementation of AMS [7]. Assessment of the degree of integration of AMS across the whole health economy is essential if we are to understand how a ‘One Health’ approach to addressing AMR may be achieved. Much AMS activity has been concentrated in hospital settings, creating a practical but somewhat artificial boundary that neglects bidirectional influences between hospital and community care services. Antimicrobial use in the community is associated with the development of AMR in and outside hospitals [8]. Furthermore, use of accident and emergency departments by ambulatory patients contributes to fragmented care and overuse of antimicrobials [9]. The way people access healthcare has evolved: the availability of blended care and complex patient-care pathways in some countries allows for patient-centred approaches as well as more rational use of services. The availability of antimicrobials without a prescription in some countries and increasing availability of online pharmacies provides an additional challenge for AMS. Fundamentally, AMS is lagging behind the advances made in health service delivery and patient behaviours by remaining sector-based.
What does integration mean and how can we assess it?
The One Health perspective on integration involves multiple sectors communicating and working together to design and implement programs, policies, legislation, and research to achieve better public health outcomes [2]. In practice, in England, new integrated care models are being developed through 50 selected collaborative organisations that will inform potential redesign of the whole health system, and 25 integrated care pioneer sites to test new and different ways of joining up health and social care services [10]. Elsewhere in Europe, the Dutch Ministry of Health, Welfare, and Sport established nine pioneer sites to integrate clinical and community services with the aim of achieving ‘better healthcare at lower cost’ [11]. In the United States, accountable care organisations—which typically involve multiple physician practices and at least one hospital—have been established to improve the quality of care while lowering costs [12]. However, AMS is not explicit in any of these wider health-system–integration models.
To further complicate matters, there is no standard definition of integration, and a number of integrated care models have been proposed in the literature [13–17] (S1 Table). In this analysis, we define and summarise the extent of integration based on the six facets of critical health system function described by Atun and colleagues [16,18] because it provides a practical level of granularity on the concept of intervention integration and is specific to healthcare (Table 1). We appreciate that there may be unpublished initiatives. However, as a novel, to our knowledge, analysis of this issue, the focus was on examining evidence of integrated AMS initiatives from the literature so that some measures of impact and associated context can be synthesised. Our aim was to identify practical considerations to support policymakers seeking to develop integrated AMS across the whole health economy. We carried out a systematic search of the literature published between January, 2006 and December, 2018, selected relevant articles using prespecified inclusion criteria, and reviewed evaluative studies (S1 Appendix). This paper describes an analysis based on 16 AMS initiatives from nine high-income countries and one low-middle–income country (Tables 2 and 3).
Table 1. Critical health system functions and elements of integration adapted from Atun and colleagues [16,18] for AMS initiatives.
| Facets of Critical Health System Function | Elements of Integration Adapted for AMS Initiatives |
|---|---|
| Stewardship and governance | • Regulatory mechanism • Accountability framework |
| Financing | • Pooling of funds • Provider payment methods • Funding source • Cross-program use of funds |
| Planning | • Planning |
| Service delivery | • Human resources for delivery of AMS • Physical infrastructure for laboratory testing |
| Monitoring and evaluation | • Data collection and recording • Data analysis • Reporting systems • Performance management system |
| Demand generation | • Financial incentives • Information, education, and communication |
Definition of full and partial integration: An element was classed as fully or predominantly integrated across the health system if it was exclusively under the management and control of the wider healthcare system. An element was classed as partially integrated if some but not all cases were managed and controlled both by the wider healthcare system and a specific program-related structure. A dimension was not integrated if it was exclusively under the management and control of a specific program-related structure (which is distinct from the wider healthcare system). Abbreviations: AMS, antimicrobial stewardship.
Table 2. Impact of 16 integrated AMS initiatives identified.
| AMS Initiative | Study Design/Type | Reported Impact | Limitations for Future Work |
|---|---|---|---|
| Australia | |||
| Infection control nurse consultant in residential aged care facilities [19] | Uncontrolled before and after study | Reduction in the use of cephalexin, doxycycline, flucloxacillin, clindamycin, and metronidazole. Rates of infection types remained stable, except respiratory tract infection rates increased at one of the two study sites. | No control group |
| National multistrategic AMS program for health professionals and the community [20] | Uncontrolled before and after study | Continued decline in total volume of antibiotics prescribed, GPs and pharmacists perceived the campaign assisted in AMS message promotion to patients, improvement in consumer knowledge and attitudes about self-management of infections | Possible impact of other national level campaigns not known; no control group |
| Canada | |||
| Northern Antibiotic Resistance Partnership [21] | Cohort study | Reduction in MRSA infection rate and an increase in knowledge related to antimicrobial use and hand washing in the community | No data knowledge (adults and children) in nonintervention communities |
| Do Bugs Need Drugs program [22] | Uncontrolled interrupted time series | Program improved clinical knowledge and rate of appropriate antibiotic prescribing for upper respiratory tract infections. Ecological association between program implementation and stabilising of antibiotic prescribing and costs. | No control group |
| Greece | |||
| A multifaceted campaign targeting both physicians and parents of school children on judicious use of antibiotics [23] | Uncontrolled before and after study | Overall antibiotic consumption was unchanged; however, the proportion of amoxicillin and phenoxymethylpenicillin used increased compared with a decrease in macrolides, cephalosporins, and fluoroquinolones | Seasonal and other temporal confounding factors not accounted for |
| Italy | |||
| Toolkit for managing ESBL-E colonisation and infection [24] | Uncontrolled before and after study | Reduction in overall antibiotics prescribed from 60% of patients with asymptomatic ESBL-E to 39% | No control group |
| Sweden | |||
| Strama [25] | Uncontrolled time series and institute publication | Reduction in outpatient antibiotic use, particularly in children aged 5–14 years and for macrolides | No control group |
| United Kingdom | |||
| Enhanced AMS program in hospital and community [26]; Northern Ireland | Interrupted time series | Reduction in fluoroquinolone use and associated reduction in MRSA incidence in the community | No control group |
| Scottish Antimicrobial Prescribing Group [27]; Scotland | Descriptive study | Contributed to the reduction of Clostridium difficile infection rates, improved clinical management of infections | Nonexperimental study design |
| The Cornwall One Health Antimicrobial Resistance Group [28] | Descriptive study | Attributed reductions in antibiotic consumption by 12.8% in total (before and post-group formed) to the implementation of the TARGET toolkit (a national AMS toolkit for general practice) | Nonexperimental study design |
| Mixed persuasive and restrictive antibiotic stewardship intervention [29]; Scotland | Observational and quasiexperimental time-series analysis | Reducing population consumption of fiuoroquinolone, cephalosporins, clindamycin, and macrolides predicted large and sustained declines in C. difficile infection prevalence in both hospitals and the community. Associations with C. difficile infection occurred only where use of these antibiotics exceeded total use thresholds, consistent with the importance of selective pressures favouring epidemic ribotypes. | Further multicentre time-series analyses or cluster-randomised controlled trials would strengthen evidence |
| United States of America | |||
| The Core Elements of Antibiotic Stewardship for Nursing Homes [30] | National guidance | Not evaluated | |
| A household- and office-based patient educational intervention and physician-centred intervention [31] | Controlled trial | Reduction in antibiotic prescription rate post-patient education and minor reduction in antibiotic prescription rate post-physician intervention | Claims data may miss emergency department data |
| Extending hospital-pharmacist–led AMS team services to hospital-affiliated nursing home [32] | Uncontrolled before and after study | Reduction in inappropriate antibiotic prescribing | |
| Introduction of an LID consult team (hospital infectious disease physician and nurse practitioner) to a long-term care facility [33] | Interrupted time-series study and cohort study | Reduced antibiotic use, particularly with tetracyclines, clindamycin sulfamethoxazole/trimethoprim, fluoroquinolones, and beta-lactam/beta-lactamase inhibitor combinations. Reduced positive C. difficile test rate. | Total days of therapy measured (not number of antimicrobial courses initiated) |
| Zambia | |||
| BeatRHDZambia initiative[34] | Uncontrolled before and after study | Substantial changes in the pattern of benzathine penicillin G usage as a result of the intervention was reported but no data were presented | No control group |
Abbreviations: AMS, antimicrobial stewardship; ESBL-E, extended-spectrum beta-lactamase producing Enterobacteriaceae; GP, general practitioner; LID, long-term care facility infectious disease; MRSA, methicillin-resistant Staphylococcus aureus; TARGET, Treat Antibiotics Responsibly, Guidance, Education, Tools.
Table 3. Stakeholders in the integrated AMS initiatives identified.
| Study–AMS initiative | AMS Initiative Developed and Implemented by | Target Recipients for the AMS Initiative |
|---|---|---|
| Australia | ||
| Infection control nurse consultant in residential aged care facilities [19] | GPs, infection control clinical nurse consultant, AMS team in residential aged care facility, and off-site hospital infectious disease physician | GPs in residential aged care facility |
| National multistrategic AMS program for health professionals and the community [20] | National Prescribing Service | GPs, community pharmacists, general public |
| Canada | ||
| Northern Antibiotic Resistance Partnership [21] | University of Saskatchewan, Health Canada Research Ethics Boards | Primary healthcare providers, general public, school staff, and children |
| Do Bugs Need Drugs program [22] | Alberta Health Services (spanning primary and secondary care), Alberta Medical Association, University of Alberta, Alberta Lung Association, British Columbia Ministry of Health and British Columbia Centre for Disease Control. Healthcare providers and healthcare and early childhood education students were trained to deliver the public education sessions. | Children aged 2–5 and 7 years, their parents, older adults in assisted-living facilities, general public, community-based physicians and pharmacists |
| Greece | ||
| A multifaceted campaign targeting both physicians and parents of school children on judicious use of antibiotics [23] | Medical school of the University of Athens, Prefecture of Corinth, Medical Association of Corinth, physician who specialised in infectious diseases | Primary care physicians, paediatricians, parents of children in nursing care and primary school, general public, dentists |
| Italy | ||
| Toolkit for managing ESBL-E colonisation and infection [24] | An initiative led by a network of infectious diseases specialists in Southeastern France developed a warning system combined with a toolkit for managing ESBL-E colonisation or infection in collaboration with microbiologists from private laboratories and community-based GPs. The toolkit promoting French recommendations was implemented in Liguria, Italy (because there were no national recommendations at the time). This comprised a framework for establishing the warning system based on the availability of infectious diseases expert advice and the ESBL-E toolkit. | Prescribers in hospitals, elderly nursing homes, long-term care facilities, GPs |
| Sweden | ||
| Strama [25] | Strama groups were established through the County Medical Officers for Communicable Diseases Control in every county. Groups had representatives from general practice and hospital (including general medicine, infectious diseases, paediatrics, otolaryngology, clinical microbiology, and infection control) and community pharmacies. | Broad audience including policy makers, physicians, and general public |
| United Kingdom | ||
| Enhanced AMS program in hospital and community [26], Northern Ireland | General practice staff and hospital clinical staff | Hospital clinical staff, GPs |
| Scottish Antimicrobial Prescribing Group [27], Scotland | Hospital-based antimicrobial pharmacists, microbiologists, infectious disease specialists, hospital medical and nonmedical leadership, infection prevention specialists, information/antimicrobial surveillance scientists, GPs, dentistry, veterinary medicine, quality improvement, pharmaceutical industry, other expert advisors | Broad audience including policy makers, physicians, and general public |
| The Cornwall One Health Antimicrobial Resistance Group [28] | Developed by a subgroup of the Health & Wellbeing Board’s Health Protection Committee. The Chief Hospital Pharmacist and Medical Director initiated wide stakeholder engagement including members from wider hospital staff, clinical commissioning group, community hospital, out-of-hours GP service, dentistry, veterinary, and farming. | Broad audience including policy makers, physicians, and general public across sectors |
| Mixed persuasive and restrictive antibiotic stewardship intervention [29]; Scotland | Nationally developed but implemented by regional antimicrobial management teams. | Healthcare professionals in primary care, tertiary hospitals, district-general hospitals, and geriatric hospitals |
| United States of America | ||
| The Core Elements of Antibiotic Stewardship for Nursing Homes [30] | Consultant pharmacist (community and/or hospital) and clinical and nursing staff | Nursing home staff |
| A household and office-based patient educational intervention and physician-centred intervention [31] | Colorado medical society and commercial and managed care organisation | Primary care physicians |
| Extending hospital-pharmacist–led AMS team services to hospital-affiliated nursing home [32] | Hospital internal medicine physician, pharmacists, infection control coordinator, and staff from nursing home | Prescribers in nursing home |
| LID consult team in a long-term care facility [33] | Hospital infectious disease physician and nurse practitioner and long-term care facility staff | Long-term care facility staff |
| Zambia | ||
| BeatRHDZambia initiative [34] | Hospital microbiologists, infectious disease consultants, pharmacists, nurses, pharmaceutical advisors, GPs, academics, pharmaceutical advisors, representation from veterinary and farm services, representation from community pharmacy, Public Health England, representation from dental practice, public health educators, and public representation | General public, healthcare workers and vets, GPs, community pharmacies, urgent care centre staff, staff, and patients at the study hospital and government clinics in Lusaka |
AMS initiatives, models, programs, and interventions are terms that are used interchangeably in the literature. Here, we use ‘AMS model’ to refer to a proposed simplified framework that outlines the structure, processes and intended outcomes associated with the goal of AMS [35]. Examples are the internationally recognised AMS model for hospitals from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America [35,36] and British Society for Antimicrobial Chemotherapy [37]. An AMS intervention is any action taken with the aim of improving antimicrobial use, e.g., use of delayed/back-up antibiotic prescriptions or implementation of infection specialist approval for restricted antimicrobials. Accordingly, an AMS program describes a coordinated effort to improve antimicrobial use that involves two or more AMS interventions. Abbreviations: AMS, antimicrobial stewardship; ESBL-E, extended-spectrum beta-lactamase producing Enterobacteriaceae; GP, general practitioner; LID, long-term care facility infectious disease.
Extent of AMS integration across the whole health economy
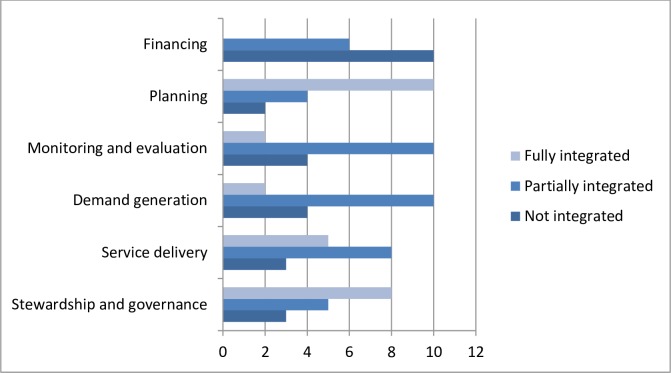
Integration mapping of the 16 initiatives based on Table 1 suggests that a range of approaches have been used to achieve multisectoral AMS (Fig 1). Full integration in Planning was often considered a key factor for establishing many initiatives coupled with an integrated Stewardship and Governance approach. Integration in these two facets was mainly achieved through expansion of the AMS program, by which the primary governance responsibilities remained with the host institution [19,20,30,32,33], rather than through establishment of new structures [25]. AMS initiatives that had a shared governance structure across healthcare organisations (i.e., partially integrated) were either national programs [27] or state-wide programs [26,31]. While these provide examples of an integrated AMS governance approach, effective governance is likely to require much more than a multistakeholder approach to plan and deliver services; a mixed regulatory and persuasive strategy including effective public engagement is needed [38]. In our analysis, nine initiatives were partially integrated for Demand Generation, showing a potential missed opportunity for this critical facet that includes raising awareness and increasing engagement with the public, practitioners, health service managers, and policymakers. Monitoring and Evaluation relate to the functions around data collection, analysis, reporting, and performance-management systems. Full integration was identified in one initiative in which the health system oversaw these functions regionally or was responsible for these functions directly [33]. More often, data collection and analyses were managed by the wider health system; however, performance management roles were not [19,20,22,25,26,32]. Financing relates to the pooling of funds/funding source, cross-program use of funds, and provider payment methods involved in the AMS initiative. The majority of initiatives did not report on how they were or should be financed or how the funds were or should be used [19,20,22,23,26,30,32]. While fund pooling was partially integrated in three initiatives [25,32,33], decisions for provider payment methods were not. Overall, 11 studies evaluated the AMS initiative using mainly quasiexperimental study designs [19–23,25,27,30,31,39] (S2 Table). These reported on a range of positive impacts including reductions in antibiotic prescribing, reductions in the proportion of broad-spectrum antibiotic prescribed, reduction in C. difficile infection rates, and perceived improvement in citizens’ knowledge and attitudes about self-management of minor infections. However, potential for bias should be borne in mind because of study limitations associated with uncontrolled research designs, insufficient data time points, and risk of self-selection by participants who are interested in AMS.
Fig 1. An overview of the extent of multisectoral AMS integration for each of the 16 AMS initiatives identified.
The integration framework is based on all six facets of critical health system function defined by Atun and colleagues [16,18] (Table 1). AMS, antimicrobial stewardship.
Opportunities and implications for policy
Especially when planning new initiatives, a health system function framework as employed here can be critical to minimise duplication of effort and achieve efficiencies from the viewpoint of healthcare professionals and service users. Our assessment has highlighted strengths of initiatives associated with beneficial outcomes, and we present these as three interconnected practical recommendations for policymakers to consider.
A successful integrated AMS approach can be developed through expansion of an existing AMS program
When compared to hospital-based AMS, strategies within primary care and long-term care have generally been slow to develop. Outside of hospitals, structural constraints sometimes include undefined AMS leadership at the organisational level and therefore unclear responsibilities around local AMS objectives and lack of timely pathways to specialist support. An integrated AMS model, particularly one involving secondary care, can overcome some of the community-based issues by either extending existing secondary-care AMS programs [19,26,32,33], adapting from established frameworks for secondary-care AMS [30], or creating a joint platform for multisectoral AMS strategies to be presented, developed, monitored, and/or shared [25,27]. It therefore follows that an integrated AMS program may also be able to address process issues such as fragmented and timely follow-up of patients, their symptom progression, and medical management. However, further research is required to investigate this. Critically, there is a need for establishing sustainable funding for AMS teams working beyond hospital settings that is not solely derived from cost savings through reduced drug expenditure. Instead, funding for developing and supporting AMS teams should be considered within the patient safety and healthcare-quality–related spending [40]. Irrespective of these issues, adoption and uptake of AMS strategies are likely to be influenced by the underlying health system and culture in a country.
Opportunities for success establishing consistent communication channels with responsibilities and common goals clearly defined
Few health systems appear to have effective mechanisms for sharing and disseminating learning about AMS, leading to small-scale local initiatives. Strengthening communication between commissioners, providers, and consumers by having more structured and clear communication pathways, such as in the Strama model developed in Sweden and the similarly structured Scottish Antimicrobial Prescribing Group, can be an effective way to develop, disseminate, and monitor ways to improve AMS [25,27].
Capitalise on existing resources and processes
Patients and the public have a pivotal role in infection prevention and management, yet failure to involve and engage with them in decision-making or achieve sustained behaviour change remains a problem in all health sector settings [41,42]. We found few examples of patient or public involvement in the design and delivery of integrated AMS initiatives (Table 3). However, we know from other studies that patient misconceptions about AMR and what constitutes appropriate antibiotic use is a major driver for inappropriate behaviours around antibiotic use [43]. Furthermore, our stakeholder analysis suggests that there are potentially more opportunities for integration, particularly involving primary care service providers. For instance, in the United Kingdom, it is well recognised that nurses and pharmacists in the community are generally more accessible to the public than general practitioners (GPs). The continuing expansion of their roles in the community, which not only provides support to patients but also reduces the burden on primary care physicians, is testament to this [44,45]. However, there are few AMS initiatives that capitalise on these valuable resources to deliver integrated AMS—by this, we mean appropriate antibiotic access and preservation and knowledge mobilisation for promoting AMS that is aligned with primary, secondary, tertiary, and long-term institutional care sectors. We found little involvement of dental practitioners in most multisectoral AMS initiatives, which is another missed opportunity. Further work is required to investigate such AMS roles in the community and embed these more widely as applicable in the respective country. A more robust evidence base is needed to establish the effectiveness of integrated AMS initiatives and specifically consider contextual antecedents to better inform future sustained improvements.
Overall, we urge policymakers, clinical leaders, and healthcare managers to assess and consider consolidating AMS activities across the whole health economy. Each of these stakeholders have an important role to drive and support clinicians, researchers, and research-active patients to carry out quality research that will inform the development of more robust evidence-based policies and guidelines. The analytic framework presented here can be used to assess the extent of integration of existing or planned multisectoral AMS initiatives, and we have outlined three areas with practical considerations towards how future integration of AMS initiatives across the whole health economy may be achieved. Ultimately, integrated AMS must prove itself as an essential element of efficient redesign if it is to deliver sustained patient benefits.
Supporting information
(PDF)
(PDF)
AMS, antimicrobial stewardship.
(PDF)
Acknowledgments
We thank the following for their support in this work and for giving up their time to assist in article collation: Michiyo Iwami and Elle Clegg. We would also like to acknowledge the National Institute for Health Research Imperial Biomedical Research Centre.
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute for Health Research (NIHR), Public Health England (PHE), or the UK Department of Health and Social Care.
Abbreviations
- AMR
antimicrobial resistance
- AMS
antimicrobial stewardship
- ESBL-E
extended-spectrum beta-lactamase producing Enterobacteriaceae
- GP
general practitioner
- LID
long-term care facility infectious disease
- MRSA
methicillin-resistant Staphylococcus aureus
- NHS
National Health Service
- NIHR
National Institute for Health Research
- PHE
Public Health England
- TARGET
Treat Antibiotics Responsibly, Guidance, Education, Tools.
Funding Statement
This article represents independent research that was partially funded by the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London, in partnership with Public Health England (PHE) in collaboration with The Sanger Institute, the University of Cambridge Veterinary School, and Imperial College Health Partners. MM is supported by the NIHR Imperial Patient Safety Translational Research Centre. RA is supported by an NIHR Fellowship in Knowledge Mobilisation. AH is an NIHR Senior Investigator. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Provenance: Not commissioned; externally peer reviewed.
References
- 1.O’Neill J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. The review on antimicrobial resistance. [Internet]. London; 2014. Available at: https://amr-review.org/sites/default/files/AMRReview Paper—Tackling a crisis for the health and wealth of nations_1.pdf [cited 31 Aug 2017].
- 2.World Health Organization. One Health. In: WHO; [Internet]. World Health Organization; 2017. Available at: http://www.who.int/features/qa/one-health/en/. [cited 1 Nov 2017]. [Google Scholar]
- 3.United Nations. Annotated preliminary list of items to be included in the provisional agenda of the seventy-third regular session of the General Assembly [Internet]. 2018. Available at: https://undocs.org/A/73/100 [cited 9 Aug 2018]. [Google Scholar]
- 4.Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018;15: 1–9. 10.1371/journal.pmed.1002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charani E, Edwards R, Sevdalis N, Alexandrou B, Sibley E, Mullett D, et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis. 2011;53: 651–662. 10.1093/cid/cir445 [DOI] [PubMed] [Google Scholar]
- 6.Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients (Review). Cochrane Database Syst Rev. 2017;2: CD003543 10.1002/14651858.CD003543.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Health Foundation. Infection prevention and control: lessons from acute care in England. Towards a whole health economy approach; London; 2015. [Google Scholar]
- 8.Aldeyab M, Harbarth S, Vernaz N, Kearney MP, Scott MG, Darwish Elhajji FW, et al. The impact of antibiotic use on the incidence and resistance pattern of extended-spectrum beta-lactamase-producing bacteria in primary and secondary healthcare settings. Br J Clin Pharmacol. 2011;74: 171–179. 10.1111/j.1365-2125.2011.04161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May L, Cosgrove S, L’Archeveque M, Talan A, Payne P, Jordan J, et al. A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med. 2013;62: 69–77. 10.1016/j.annemergmed.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NHS England; New care models [Internet]. Available at: https://www.england.nhs.uk/new-care-models/. [cited 21 Mar 2018].
- 11.Drewes HW, Struijs JN, Baan CA. How the Netherlands is Integrating Health and Community Services. In: N Engl J Med Catalyst [Internet]. 2017. Available at: http://catalyst.nejm.org/netherlands-integrating-health-community-services/. [cited 1 Jun 2017]. [Google Scholar]
- 12.Ham C, Alderwick H. Place-based systems of care A way forward for the NHS in England. London: The King's Fund; 2015. [Google Scholar]
- 13.Curry N, Ham C. Clinical and service integration: the route to improved outcomes [Internet]. London; 2010. Available at: www.kingsfund.org.uk/publications [cited 14 May 2017].
- 14.The NHS Confederation. Building integrated care Lessons from the UK and elsewhere. London: The NHS Confederation; 2005. [Google Scholar]
- 15.Contandriopoulos A-P, Denis J-L, Touati N, Rodríguez C. The integration of health care: dimensions and implementation. Montreal: Groupe de recherche interdisciplinaire en sante; 2003. [Google Scholar]
- 16.Atun R, De Jongh T, Secci F, Ohiri K, Adeyi O. Integration of targeted health interventions into health systems: A conceptual framework for analysis. Health Policy Plan. 2010;25: 104–111. 10.1093/heapol/czp055 [DOI] [PubMed] [Google Scholar]
- 17.Leutz WN. Five laws for integrating medical and social services: lessons from the United States and the United Kingdom. Milbank Q. 1999;77: 77–110. 10.1111/1468-0009.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atun R, De Jongh T, Secci F, Ohiri K, Adeyi O. A systematic review of the evidence on integration of targeted health interventions into health systems. Health Policy Plan. 2010;25: 1–14. 10.1093/heapol/czp053 [DOI] [PubMed] [Google Scholar]
- 19.Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthc Infect. 2015;20: 4–6. 10.1071/HI14016 [DOI] [Google Scholar]
- 20.Wutzke SE, Artist MA, Kehoe LA, Fletcher M, Mackson JM, Weekes LM. Evaluation of a national programme to reduce inappropriate use of antibiotics for upper respiratory tract infections: effects on consumer awareness, beliefs, attitudes and behaviour in Australia. Health Promot Int. 2007;22: 53–64. 10.1093/heapro/dal034 [DOI] [PubMed] [Google Scholar]
- 21.Golding GR, Quinn B, Bergstrom K, Stockdale D, Woods S, Nsungu M, et al. Community-based educational intervention to limit the dissemination of community-associated methicillin-resistant Staphylococcus aureus in Northern Saskatchewan, Canada. BMC Public Health. 2012;12: 15 10.1186/1471-2458-12-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay RM, Vrbova L, Fuertes E, Chong M, David S, Dreher K, et al. Evaluation of the do bugs need drugs? Program in British Columbia: Can we curb antibiotic prescribing? Can J Infect Dis Med Microbiol. 2011;22: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plachouras D, Antoniadou A, Giannitsioti E, Galani L, Katsarolis I, Kavatha D, et al. Promoting prudent use of antibiotics: the experience from a multifaceted regional campaign in Greece. BMC Public Health. 2014;14: 866 10.1186/1471-2458-14-866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondain V, Secondo G, Guttmann R, Ferrea G, Dusi A, Giacomini M, et al. A toolkit for the management of infection or colonization by extended-spectrum beta-lactamase producing Enterobacteriaceae in Italy: implementation and outcome of a European project. Eur J Clin Microbiol Infect Dis. Springer Berlin Heidelberg; 2018;37: 987–992. 10.1007/s10096-018-3202-1 [DOI] [PubMed] [Google Scholar]
- 25.Mölstad S, Erntell M, Hanberger H, Melander E, Norman C, Skoog G, et al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis. 2008;8: 125–132. 10.1016/S1473-3099(08)70017-3 [DOI] [PubMed] [Google Scholar]
- 26.Aldeyab MA, Scott MG, Kearney MP, Alahmadi YM, Magee FA, Conlon G, et al. Impact of an enhanced antibiotic stewardship on reducing methicillin-resistant Staphylococcus aureus in primary and secondary healthcare settings. Epidemiol Infect. 2014;142: 494–500. 10.1017/S0950268813001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathwani D, Sneddon J, Malcolm W, Wiuff C, Patton A, Hurding S, et al. Scottish Antimicrobial Prescribing Group (SAPG): Development and impact of the Scottish National Antimicrobial Stewardship Programme. Int J Antimicrob Agents. 2011;38: 16–26. 10.1016/j.ijantimicag.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 28.Powell N, Davidson I, Yelling P, Collinson A, Pollard A, Johnson L, et al. Developing a local antimicrobial resistance action plan: the Cornwall One Health Antimicrobial Resistance Group. J Antimicrob Chemother. 2017;72: 2661–2665. 10.1093/jac/dkx164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawes T, Lopez-Lozano J-M, Nebot CA, Macartney G, Subbarao-Sharma R, Wares KD, et al. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: a non-linear time-series analysis. Lancet Infect Dis. 2017;17: 194–206. 10.1016/S1473-3099(16)30397-8 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. The Core Elements of Antibiotic Stewardship for Nursing Homes [Internet]. Atlanta: US Department of Health and Human Services; 2015. Available at: http://www.cdc.gov/longtermcare/prevention/antibiotic-stewardship.html [cited 22 May 2017]. [Google Scholar]
- 31.Gonzales R, Corbett KK, Leeman-Castillo BA, Glazner J, Erbacher K, Darr CA, et al. The “Minimizing Antibiotic Resistance in Colorado” Project: Impact of Patient Education in Improving Antibiotic Use in Private Office Practices. Health Serv Res. 2005;40: 101–116. 10.1111/j.1475-6773.2005.00344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gugkaeva Z, Franson M. Pharmacist-led model of antibiotic stewardship in a long-term care facility. Ann Long-Term Care. 2012;20: 22–26. [Google Scholar]
- 33.Jump RLP, Olds DM, Seifi N, Kypriotakis G, Jury LA, Peron EP, et al. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol. 2012;33: 1185–1192. 10.1086/668429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long A, Lungu JC, Machila E, Schwaninger S, Spector J, Tadmor B, et al. A programme to increase appropriate usage of benzathine penicillin for management of streptococcal pharyngitis and rheumatic heart disease in Zambia. Cardiovasc J Afr. 2017;28: 242–247. 10.5830/CVJA-2017-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44: 159–77. 10.1086/510393 [DOI] [PubMed] [Google Scholar]
- 36.Barlam TF, Cosgrove SE, Abbo LM, Macdougall C, Schuetz AN, Septimus EJ, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62: e51–e77. 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.British Society for Antimicrobial Chemotherapy. Practical guide to antimicrobial stewardship in hospitals [Internet]. Available at: http://bsac.org.uk/wp-content/uploads/2013/07/Stewardship-Booklet-Practical-Guide-to-Antimicrobial-Stewardship-in-Hospitals.pdf [cited 22 May 2016].
- 38.Birgand G, Castro-Sanchez E, Hansen S, Gastmeier P, Lucet J-C, Ferlie E, et al. Comparison of governance approaches for the control of antimicrobial resistance: Analysis of three European countries. Antimicrob Resist Infect Control. 2018;7: 28 10.1186/s13756-018-0321-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aldeyab MA, Kearney MP, Scott MG, Aldiab MA, Alahmadi YM, Darwish Elhajji FW, et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. 2012;67: 2988–2996. 10.1093/jac/dks330 [DOI] [PubMed] [Google Scholar]
- 40.Pulcini C, Morel CM, Tacconelli E, Beovic B, De With K, Goossens H, et al. Human resources estimates and funding for antibiotic stewardship teams are urgently needed. Clin Microbiol Infect. 2017;23: 785–787. 10.1016/j.cmi.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 41.Rawson TM, Moore LSP, Gilchrist MJ, Holmes AH. Antimicrobial stewardship: Are we failing in cross-specialty clinical engagement? J Antimicrob Chemother. 2016;71: 554–559. 10.1093/jac/dkv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Patient Engagement. In: Technical Series on Safer Primary Care [Internet]. 2016. Available at: http://apps.who.int/iris/bitstream/10665/252269/1/9789241511629-eng.pdf [cited 20 Jun 2017]. [Google Scholar]
- 43.McCullough AR, Parekh S, Rathbone J, Del Mar CB, Hoffmann TC. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother. 2016;71: 27–33. 10.1093/jac/dkv310 [DOI] [PubMed] [Google Scholar]
- 44.England NHS. General Practice Forward View. London: NHS England; 2016. [Google Scholar]
- 45.Klepser ME, Adams AJ, Klepser DG. Antimicrobial stewardship in outpatient settings: leveraging innovative physician-pharmacist collaborations to reduce antibiotic resistance. Health Secur. 2015;13: 166–173. 10.1089/hs.2014.0083 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
AMS, antimicrobial stewardship.
(PDF)