Abstract
Blood stasis in left atrium (LA) or LA appendage (LAA) is thought to be the main cause of thrombus formation and systemic embolism in atrial fibrillation (AF) patients. Paroxysmal and non-paroxysmal AF differ significantly in various aspects. Impact of cardiac hemodynamics on systemic embolism might also differ between the 2 distinct AF entities. This study was performed to evaluate the influence of cardiac hemodynamics on systemic embolism in both paroxysmal and non-paroxysmal AF. Consecutive AF patients undergoing radiofrequency catheter ablation (RFCA) in Korea University Medical Center Anam Hospital between June 1998 and February 2018 were analyzed. Among 2,801 patients who underwent first-time RFCA, a total of 231 patients had either previous ischemic stroke, transient ischemic attack, or arterial embolism. In paroxysmal AF, LA diameter, LA volume (measured with magnetic resonance imaging), left ventricular (LV) ejection fraction, E/e’, LAA flow velocity, and prevalence of spontaneous echocontrast (SEC) and dense SEC were significantly different between patients with and without thromboembolic events. However, only E/e’ was different between patients with and without thromboembolic events in non-paroxysmal AF. The influence of LA diameter, LA volume, LV EF, LAA flow velocity, and dense SEC on thromboembolic events was significantly moderated by the type of AF. In conclusion, paroxysmal and non-paroxysmal AF might have a different mechanism responsible for thrombus formation and consequent embolic events. Relative contribution of hemodynamic parameters and other factors such as atrial myopathy to thromboembolic events in paroxysmal versus non-paroxysmal AF needs further evaluation.
Introduction
Thromboembolic events are major complications of atrial fibrillation (AF) [1, 2]. Blood stasis in left atrium (LA) and LA appendage (LAA) due to rapid and disorganized contraction is considered to be a main mechanism of thrombus formation and consequent embolization [3, 4]. The observation that decreased flow velocity in LAA and resulting spontaneous echocontrast (SEC) are associated with increased risk of thrombus formation and clinical events, such as ischemic stroke, supports this rhythm theory [3–5]. Recent studies suggest that radiofrequency catheter ablation (RFCA) in AF patients, which can cure AF or at least decrease the burden, is associated with significant reduction in the cumulative incidence of ischemic stroke [6, 7]. However, there are emerging evidences indicating atrial myopathy is the culprit pathology for thromboembolic complications in AF patients [8–10]. In the TREND study, more than 75% of AF patients who experienced ischemic stroke during thorough rhythm monitoring by implanted pacemakers, had no AF in the preceding 30 days [9]. The ASSERT trial also reported that 51% of patients who had ischemic stroke but without clinical AF experienced device-detected AF later during the follow up period and only 8% of patients with ischemic stroke had device-detected AF in the 30 days before the stroke [10]. Therefore, rhythm status cannot explain the underlying cause of thromboembolic complications in certain circumstances and according to the substrate theory, atrial fibrosis also known as atrial myopathy is the main cause of thrombus formation and subsequent adverse clinical events.
Paroxysmal and non-paroxysmal AF differs in various clinical and cardiac hemodynamic aspects. Notably, atrial fibrosis is the hallmark of non-paroxysmal AF and previous studies indicate increased burden of atrial fibrosis is clearly associated with increased risk of ischemic stroke [11, 12]. Therefore, the underlying mechanism of thromboembolic events in paroxysmal and non-paroxysmal AF might be different. We performed this analysis to compare the influence of various cardiac hemodynamic parameters on thromboembolic events in paroxysmal versus non-paroxysmal AF.
Materials and methods
Patients
RFCA registry of Korea University Medical Center Anam Hospital were utilized [13]. Patients who underwent first-time RFCA for AF between June 1998 and February 2018 in our institution were included and there was no specific exclusion criteria. Institutional Review Board of Korea University Medical Center Anam Hospital specifically approved this study. Written informed consent was waived because the current study was a retrospective analysis. The protocol of the current study was consistent with the ethical guidelines of the 2008 Helsinki Declaration. The aim of this study was to examine the underlying mechanism of thrombus formation and subsequent thromboembolic events in the two distinct type of AF: paroxysmal versus non-paroxysmal AF.
Imaging evaluation
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were performed prior to RFCA procedure and various echocardiographic parameters were measured. Diameter of LA, left ventricular (LV) ejection fraction, and E/e’ were measured by TTE. Presence of spontaneous echocontrast (SEC) or thrombus in LA or LAA was thoroughly examined. Emptying, filling, and average flow velocity of LAA were measured during TEE evaluation. In the main analysis, average flow velocity was used. In patients who performed cardiac magnetic resonance imaging (MRI) studies, LA volume was measured.
Definitions
The current study evaluated the impact of various cardiac hemodynamic parameters measured with either echocardiography or cardiac MRI on previous thromboembolic events. Previous thromboembolic events were defined as confirmed prior diagnosis of ischemic stroke, transient ischemic attack, or arterial embolism. Ischemic stroke was defined as any neurologic symptoms lasting for more than 24 hours which cannot be explained by other medical conditions. Transient ischemic attack was defined as any neurologic symptoms which are not attributable to any other medical conditions which resolved completely within 24 hours. Arterial embolism was defined as occlusion of any artery that needed medical or surgical treatment. Modified CHA2DS2-VASc score (mCHA2DS2-VASc) was calculated as same method with CHA2DS2-VASc score except for excluding previous thromboembolic events for score summation. Paroxysmal AF was defined as AF episodes not lasting for more than 7 days. Non-paroxysmal AF was defined as AF episodes lasting for more than 7 days or requiring direct current cardioversion to terminate.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables are presented as percentile value. Unpaired t-test was used to compare continuous variables. Categorical variables were compared with either chi-square test or Fisher’s exact test as appropriate. Receiver operating characteristic (ROC) curve analysis was performed to calculate area under curve (AUC). Binary logistic regression analysis was performed to calculate odds ratio (OR) of thromboembolic events for each risk factor. OR was calculated separately for paroxysmal and non-paroxysmal AF and interaction term analysis was performed to evaluate whether the association between each risk factor and thromboembolic events was moderated by AF type. All significance tests were two-tailed and p values of less than 0.05 were considered statistically significant. All statistical analyses were performed with SPSS version 24.0 (SPSS Inc., Armonk, NY, USA).
Data availability statement
All relevant data are within the paper and its Supporting Information files. The authors confirm that these data constitute the minimal data set required to support all the conclusions of the present study. However, the underlying individual-level patient data are restricted because they contain sensitive information such as age, sex, body weight, and medical comorbidities. The authors of the present study confirm that the de-identified individual patient data are not required to validate or confirm our study’s conclusions. However, these de-identified patient-level data will nevertheless be made available to interested researchers who meet the criteria for access to confidential information. These data can be requested from the Institutional Review Board of Korea University Medical Center Anam Hospital (+82-02-920-6566 or eirbadmin@kumc.or.kr).
Results
Patient characteristics
A total of 2,801 patients undergoing first-time RFCA were analyzed. Baseline characteristics of the study population are summarized in Table 1. Mean age was 55.58 ± 10.97 and 79.2% were male. Mean LA diameter was 41.16 ± 6.06 mm and 40.9% of patients had non-paroxysmal AF. CHA2DS2-VASc and mCHA2DS2-VASc scores were 1.25 ± 1.26 and 1.08 ± 1.10 respectively. Before undergoing RFCA, TTE, TEE, and cardiac MRI was performed in 2,742 (97.9%), 2,580 (92.1%), and 932 (33.3%) patients, respectively. A total of 1,064 patients underwent follow-up TTE (38.0%).
Table 1. Baseline clinical and echocardiographic characteristics.
| Total patients (N = 2,801) |
Thromboembolic events (-) (n = 2,570) |
Thromboembolic events (+) (n = 231) |
p value | |
|---|---|---|---|---|
| Clinical findings | ||||
| Age | 55.58 ± 10.97 | 55.21 ± 11.10 | 59.68 ± 8.46 | < 0.001 |
| Male sex | 2,217 (79.2%) | 2,038 (79.3%) | 179 (77.5%) | 0.516 |
| Body weight (kg) | 70.80 ± 11.21 | 71.00 ± 11.29 | 68.62 ± 10.06 | 0.002 |
| Height (cm) | 168.18 ± 8.26 | 168.30 ± 8.29 | 166.85 ± 7.82 | 0.011 |
| Body mass index (kg/m2) | 24.97 ± 3.07 | 25.00 ± 3.08 | 24.61 ± 2.90 | 0.065 |
| Heart failure | 139 (5.0%) | 124 (4.8%) | 15 (6.5%) | 0.263 |
| Hypertension | 1054 (37.6%) | 957 (37.2%) | 97 (42.0%) | 0.153 |
| Diabetes mellitus | 266 (9.5%) | 240 (9.3%) | 26 (11.3%) | 0.341 |
| Vascular disease | 226 (8.1%) | 194 (7.5%) | 32 (13.9%) | 0.001 |
| CHA2DS2-VASc | 1.25 ± 1.26 | 1.06 ± 1.09 | 3.33 ± 1.14 | < 0.001 |
| mCHA2DS2-VASc | 1.08 ± 1.10 | 1.06 ± 1.09 | 1.33 ± 1.14 | < 0.001 |
| Non-paroxysmal AF | 1,145 (40.9%) | 1,022 (39.8%) | 123 (53.2%) | < 0.001 |
| AF duration (years) | 4.72 ± 4.62 | 4.70 ± 4.50 | 4.94 ± 5.77 | 0.524 |
| Echocardiographic findings | ||||
| LA diameter (mm) | 41.16 ± 6.06 | 41.06 ± 6.11 | 42.28 ± 5.37 | 0.001 |
| LV ejection fraction (%) | 54.77 ± 6.11 | 54.86 ± 6.05 | 53.80 ± 6.64 | 0.020 |
| E/e’ | 8.79 ± 3.38 | 8.68 ± 3.27 | 10.02 ± 4.17 | < 0.001 |
| Pulmonary artery pressure (mmHg) | 30.63 ± 5.25 | 30.62 ± 5.17 | 30.78 ± 6.01 | 0.684 |
| LAA emptying velocity (cm/sec) | 47.81 ± 21.81 | 48.28 ± 21.94 | 42.74 ± 19.73 | < 0.001 |
| LAA filling velocity (cm/sec) | 49.28 ± 22.89 | 49.77 ± 23.00 | 43.95 ± 20.92 | < 0.001 |
| LAA average velocity (cm/sec) | 48.55 ± 21.24 | 49.03 ± 21.36 | 43.34 ± 19.28 | < 0.001 |
| SEC | 530 (20.6%) | 471 (20.0%) | 59 (27.1%) | 0.013 |
| Dense SEC | 88 (3.4%) | 75 (3.2%) | 13 (6.0%) | 0.031 |
AF: atrial fibrillation; LA: left atrium; LAA: left atrial appendage; LV: left ventricle; SEC: spontaneous echocontrast.
History of previous thromboembolic events
Among 2,801 patients, 231 (8.3%) patients had a history of thromboembolic events. Clinical and echocardiographic characteristics of patients with and without previous thromboembolic events are summarized in Table 1. Patients with thromboembolic events showed significantly older age (55.21 ± 11.10 vs. 59.68 ± 8.46, p < 0.001), lower body weight (71.00 ± 11.29 vs. 68.62 ± 10.06, p = 0.002), small height (168.30 ± 8.29 vs. 166.85 ± 7.82, p = 0.011), and higher mCHA2DS2-VASc score (1.06 ± 1.09 vs. 1.33 ± 1.14, p < 0.001). Worse cardiac hemodynamics were observed in patients who experienced thromboembolic events: large LA diameter (41.06 ± 6.11 vs. 42.28 ± 5.37; p = 0.001), low LV ejection fraction (54.86 ± 6.05 vs. 53.80 ± 6.64, p = 0.020), high E/e’ (8.68 ± 3.27 vs. 10.02 ± 4.17; p < 0.001), low LAA flow velocity (49.03 ± 21.36 vs. 43.34 ± 19.28; p < 0.001), and higher prevalence of SEC (20.0% vs. 27.1%; p = 0.013) and dense SEC (3.2% vs. 6.0%; p = 0.031) (Table 1).
Type of AF
Baseline characteristics of patients with paroxysmal AF and non-paroxysmal AF are presented in Table 2. Patients with non-paroxysmal AF showed significantly higher rate of previous thromboembolic events (6.5% vs. 10.7%; p < 0.001) despite similar mCHA2DS2-VASc score. Clinical heart failure was more prevalent in non-paroxysmal AF (3.1% vs. 7.6%; p < 0.001). The CHA2DS2-VASc score was higher and AF duration was longer in patients with non-paroxysmal AF. Cardiac hemodynamics including LA diameter, LA volume, LV ejection fraction, LAA flow velocity, SEC, and dense SEC were worse in patients with non-paroxysmal AF.
Table 2. Baseline characteristics between paroxysmal and non-paroxysmal AF.
| Paroxysmal AF (n = 1,656) |
Non-paroxysmal AF (n = 1,145) |
p value | |
|---|---|---|---|
| Clinical findings | |||
| Age | 55.04 ± 11.42 | 56.37 ± 10.26 | 0.001 |
| Male sex | 1,269 (76.6%) | 948 (82.8%) | < 0.001 |
| Body weight (kg) | 69.68 ± 11.07 | 72.40 ± 11.23 | < 0.001 |
| Height (cm) | 167.86 ± 8.38 | 168.62 ± 8.06 | 0.018 |
| Body mass index (kg/m2) | 24.66 ± 2.98 | 25.41 ± 3.13 | < 0.001 |
| Heart failure | 52 (3.1%) | 87 (7.6%) | < 0.001 |
| Hypertension | 623 (37.6%) | 431 (37.6%) | > 0.999 |
| Diabetes mellitus | 157 (9.5%) | 109 (9.5%) | > 0.999 |
| Thromboembolic events | 108 (6.5%) | 123 (10.7%) | < 0.001 |
| Vascular disease | 127 (7.7%) | 99 (8.6%) | 0.351 |
| CHA2DS2-VASc | 1.21 ± 1.21 | 1.31 ± 1.33 | 0.045 |
| mCHA2DS2-VASc | 1.08 ± 1.08 | 1.09 ± 1.12 | 0.733 |
| AF duration (years) | 4.30 ± 4.42 | 5.32 ± 4.83 | < 0.001 |
| Echocardiographic findings | |||
| LA diameter (mm) | 39.00 ± 5.26 | 44.20 ± 5.79 | < 0.001 |
| LV ejection fraction (%) | 56.05 ± 4.99 | 52.97 ± 7.02 | < 0.001 |
| E/e’ | 8.68 ± 3.21 | 8.95 ± 3.60 | 0.051 |
| Pulmonary artery pressure (mmHg) | 30.71 ± 5.52 | 30.53 ± 4.89 | 0.423 |
| LAA emptying velocity (cm/sec) | 56.57 ± 20.50 | 36.45 ± 17.86 | < 0.001 |
| LAA filling velocity (cm/sec) | 58.00 ± 21.67 | 37.97 ± 19.18 | < 0.001 |
| LAA average velocity (cm/sec) | 57.28 ± 19.57 | 37.21 ± 17.68 | < 0.001 |
| SEC | 138 (9.5%) | 392 (35.0%) | < 0.001 |
| Dense SEC | 16 (1.1%) | 72 (6.5%) | < 0.001 |
| MRI findings | |||
| LA volume (ml) | 81.73 ± 26.29 | 107.96 ± 37.21 | < 0.001 |
| VENC (ml/sec) | 61.95 ± 33.01 | 37.14 ± 27.98 | < 0.001 |
AF: atrial fibrillation; LA: left atrium; LAA: left atrial appendage; LGE: late gadolinium enhancement; LV: left ventricle; MRI: magnetic resonance imaging; SEC: spontaneous echocontrast; VENC: velocity encoded cardiac MRI.
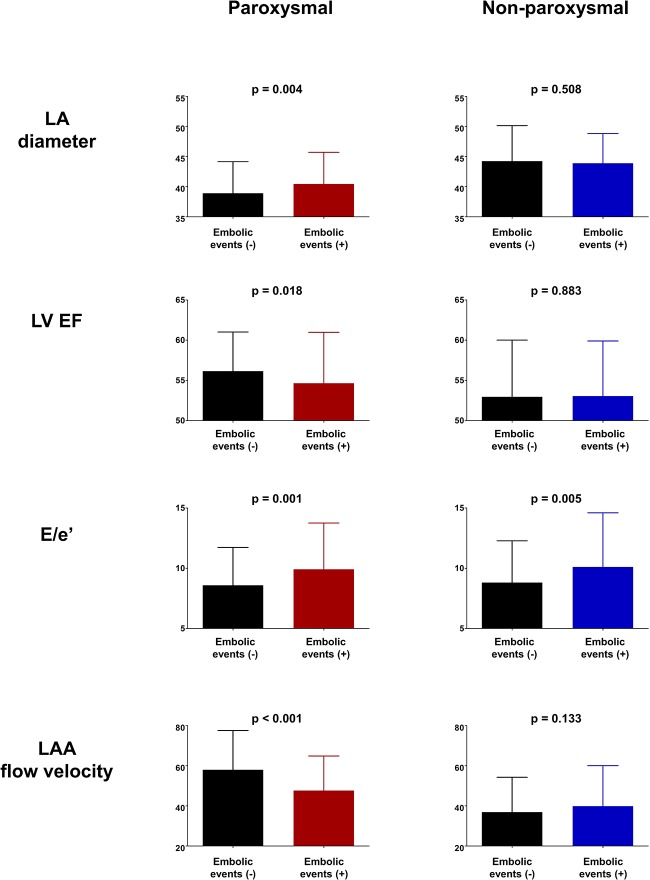
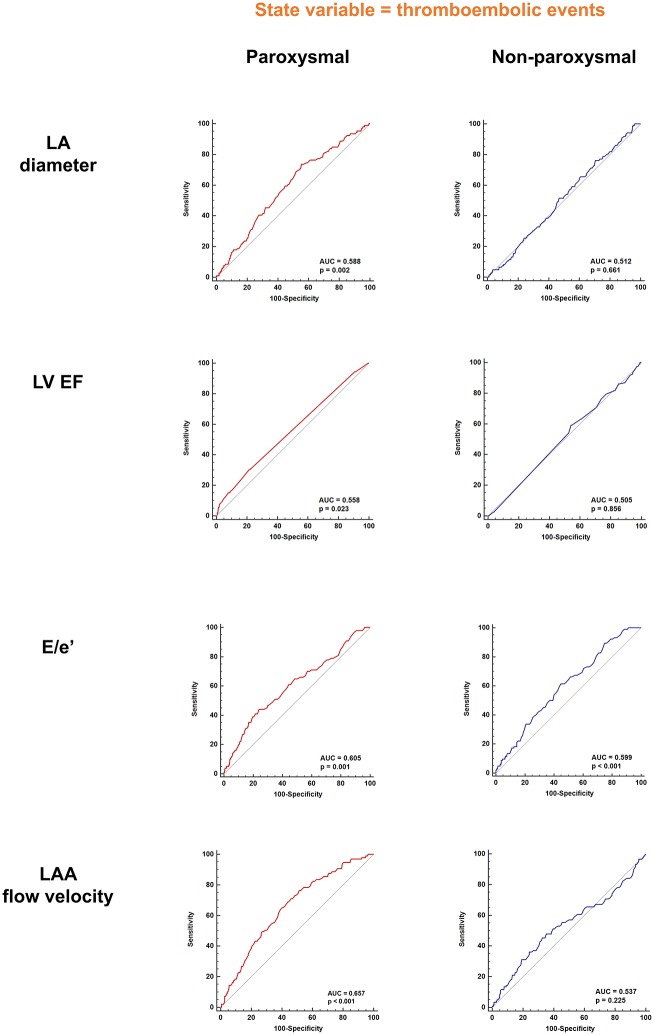
In paroxysmal AF, patients who had previous thromboembolic events showed significantly large LA diameter (38.90 ± 5.25 vs. 40.44 ± 5.27, p = 0.004; Fig 1), lower LV ejection fraction (56.15 ± 4.87 vs. 54.65 ± 6.32, p = 0.018; Fig 1), higher E/e’ (8.59 ± 3.14 vs. 9.91 ± 3.83, p = 0.001; Fig 1), and lower LAA flow velocity (57.98 ± 19.55 vs. 47.67 ± 17.17, p < 0.001; Fig 1). In patients with non-paroxysmal AF, however, there were no significant differences in LA diameter (44.24 ± 5.89 vs. 43.88 ± 4.94, p = 0.508; Fig 1), LV ejection fraction (52.86 ± 7.05 vs. 53.06 ± 6.84, p = 0.883; Fig 1), and LAA flow velocity (36.89 ± 17.34 vs. 39.82 ± 20.23, p = 0.133; Fig 1) between patients with and without previous history of thromboembolic events. However, E/e’ was significantly higher in patients with previous thromboembolic events (8.81 ± 3.46 vs. 10.11 ± 4.49, p = 0.005; Fig 1). In ROC curve analysis, LA diameter, LV ejection fraction, and LAA flow velocity had statistically significant AUC only in paroxysmal AF patients (Fig 2). However, E/e’ showed significant predictive value for previous thromboembolic events in both paroxysmal and non-paroxysmal AF (Fig 2). The volume of LA measured with cardiac MRI also showed similar pattern as compared with LA diameter measured with TTE. The volume of LA had prognostic value for previous thromboembolic events only in paroxysmal AF (S1 and S2 Figs). In paroxysmal AF, patients with history of thromboembolic events showed significantly higher prevalence of both SEC (9.0% vs. 15.3%, p = 0.041; Table 3) and dense SEC (0.9% vs. 4.1%, p = 0.019; Table 3). In non-paroxysmal AF, however, no significant difference was observed in the prevalence of SEC (34.8% vs. 36.7%, p = 0.691; Table 3) and dense SEC (6.3% vs. 7.5%, p = 0.623; Table 3) between patients with and without thromboembolic events. Logistic regression analysis also revealed that SEC and dense SEC had increased odds ratio for previous thromboembolic events only in paroxysmal AF patients (Table 3). Odds ratios of each risk factor for thromboembolic events for both paroxysmal and non-paroxysmal AF patients are presented in Table 4. Type of AF demonstrated a significant moderator effect on individual risk factors for thromboembolic events (Table 4). However, the impact of E/e’ on thromboembolic events was not influenced by the type of AF and high E/e’ was a significant risk factor for thromboembolic events in both paroxysmal and non-paroxysmal AF patients. Change in LA diameter and LV ejection fraction before and after RFCA is summarized in S3 Fig.
Fig 1. Thromboembolic events, various hemodynamic parameters, and AF type.
In patients with paroxysmal AF, those with previous thromboembolic events had large LA, low LV EF, high E/e’, and decreased LAA flow velocity. However, no difference in LA diameter, LV EF, and LAA flow velocity was observed between between patients with and without history of thromboembolic events in non-paroxysmal type of AF. AF: atrial fibrillation; LA: left atrium; LAA: left atrial appendage; LV EF: left ventricular ejection fraction.
Fig 2. ROC curve analysis for cardiac hemodynamic parameters.
LA diameter, LV EF, and LAA flow velocity was able to predict previous thromboembolic events only in paroxysmal type of AF. However, E/e’ was able to predict thromboembolic events in both paroxysmal and non-paroxysmal AF. AF: atrial fibrillation; LA: left atrium; LAA: left atrial appendage; LV EF: left ventricular ejection fraction; ROC: receiver operating characteristic.
Table 3. Relationship among SEC, type of AF, and thromboembolic events.
| Paroxysmal | Non-paroxysmal | |||||
|---|---|---|---|---|---|---|
| Thromboembolic events (-) | Thromboembolic events (+) | p value | Thromboembolic events (-) | Thromboembolic events (+) | p value | |
| SEC | 123 (9.0%) | 15 (15.3%) | 0.041 | 348 (34.8%) | 44 (36.7%) | 0.691 |
| Dense SEC | 12 (0.9%) | 4 (4.1%) | 0.019 | 63 (6.3%) | 9 (7.5%) | 0.623 |
| OR | 95% CI | p value | OR | 95% CI | p value | |
| SEC | 1.818 | 1.017–3.247 | 0.044 | 1.083 | 0.731–1.605 | 0.691 |
| Dense SEC | 4.773 | 1.510–15.085 | 0.008 | 1.199 | 0.581–2.478 | 0.623 |
AF: atrial fibrillation; CI: confidence interval; OR: odds ratio; SEC: spontaneous echocontrast.
Table 4. Influence of AF type on the effect of each risk factors for thromboembolic events.
| Paroxysmal | Non-paroxysmal | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | p value | |
| LA diameter | 1.056 | 1.018–1.096 | 0.004 | 0.989 | 0.957–1.022 | 0.507 | 0.936 | 0.009 |
| LA volume | 1.012 | 1.002–1.022 | 0.016 | 0.996 | 0.987–1.006 | 0.440 | 0.985 | 0.024 |
| LAA flow velocity | 0.972 | 0.961–0.983 | < 0.001 | 1.009 | 0.999–1.020 | 0.089 | 1.039 | < 0.001 |
| E/e’ | 1.105 | 1.050–1.162 | < 0.001 | 1.082 | 1.034–1.133 | 0.001 | 0.979 | 0.549 |
| LV EF | 0.954 | 0.924–0.984 | 0.003 | 1.002 | 0.975–1.029 | 0.883 | 1.051 | 0.019 |
| Dense SEC | 4.773 | 1.510–15.085 | 0.008 | 1.199 | 0.581–2.478 | 0.623 | 0.251 | 0.047 |
AF: atrial fibrillation; CI: confidence interval; LA: left atrium; LAA: left atrial appendage, LV EF: left ventricular ejection fraction; OR: odds ratio; SEC: spontaneous echocontrast.
Discussion
The main findings of the current study can be summarized as follows: (i) increased LA diameter, decreased LV ejection fraction, decreased LAA flow velocity, and presence of SEC or dense SEC are risk factors for thromboembolic events only in patients with paroxysmal AF; (ii) increased E/e’ is a risk factor for thromboembolic events regardless of AF type; (ii) impact of aforementioned risk factors on thromboembolic events, except for E/e’, is significantly moderated by the type of AF. This is the first study to analyze the impact of multiple TTE and TEE parameters, which reflects various aspects of cardiac hemodynamics, on thromboembolic risk in patients with paroxysmal and non-paroxysmal AF.
Paroxysmal vs. non-paroxysmal
The major difference between paroxysmal AF and non-paroxysmal AF is whether AF is sustained [14–16]. In order to sustain AF, atrial remodeling which is often called as ‘substrate’ is required [14–16]. Therefore, non-paroxysmal AF, by its definition, has more substrate than paroxysmal AF which is demonstrated by significant lower LA voltage in non-paroxysmal AF even after LA volume adjustment [17]. In cardiac MRI evaluation, increased late gadolinium enhancement which is a reliable marker for atrial fibrosis, is observed with higher degree in patients with non-paroxysmal AF as compared with paroxysmal AF [11]. Increased amount of atrial fibrosis is also shown to be associated with increased risk of thromboembolic events [11]. In the current study, patients with non-paroxysmal AF had significantly higher prevalence of previous thromboembolic events. Our study also revealed that non-paroxysmal AF has worse cardiac hemodynamics such as enlarged LA, low LV ejection fraction, decreased LAA flow velocity, and higher prevalence of SEC and dense SEC as compared with paroxysmal AF. These parameters, however, was associated with increased risk of thromboembolic events only in paroxysmal AF and not in non-paroxysmal AF suggesting that another underlying mechanism for thrombus formation and embolization is present for non-paroxysmal AF patients.
Influence of AF type
In the current analysis, most of echocardiographic risk factors for thromboembolic events, such as LA size, LV ejection fraction, LAA flow velocity, or SEC, were only valid in paroxysmal AF and impact of these risk factors on thromboembolic events was clearly moderated by the type of AF. In contrast, increased E/e’ was a clear risk factor for thromboembolic events regardless of AF type. Mechanism of thrombus formation in LA or LAA is usually explained by 2 theories: rhythm or substrate. In the rhythm theory, rapid and disorganized contraction of LA and LAA result in blood stasis which in turn, provide a nice nidus for SEC and thrombus formation [3, 4]. The substrate theory, however, proposes that atrial fibrosis increases the risk of thromboembolic events independently of atrial rhythm status [8, 18, 19]. Increased atrial fibrosis might reduce contractility of LA and LAA which will in turn, results in increased LA size, decreased LAA blood flow, and formation of SEC. Furthermore, replacement of normal myocyte lining in the endocardium with fibrotic tissue might provoke coagulation cascade and initiate thrombosis formation irrespective of cardiac hemodynamics. Our data support this concept. In patients with non-paroxysmal AF, markers of cardiac hemodynamics, except for E/e’, were not useful to identify patients at higher risk of thromboembolic events. Therefore, it might be the atrial fibrosis itself rather than cardiac hemodynamics which is the main driver of thrombus formation and consequent thromboembolic events in patients with non-paroxysmal AF.
Atrial fibrosis observed in AF patients is characterized by excessive accumulation of collagenous material in the extracellular space [18, 20, 21]. In the endovascular system, collagen, which is highly thrombogenic, is exposed to blood after endothelial disruption, starting the formation of a thrombus [22]. Collagen material can have similar effect in cardiac chambers. Replacement of normal atrial myocyte with collagen material might increase thrombogenecity by not only decreasing LA function but also by thrombogenic effect of collagen itself. The impact of guiding anticoagulation treatment based on the degree of atrial fibrosis should be examined in future clinical trials.
In addition to rhythm and substrate, abnormality of blood constituents is another important factor associated with thromboembolic events in AF patients [23]. Elevation patterns of Von Willebrand factor, fibrinogen, and P-selectin differed among paroxysmal, persistent, and permanent AF according to the previous study [24]. Furthermore, the duration of AF was independently associated with abnormal measured factors [24]. Therefore, paroxysmal and non-paroxysmal AF might have different degree of abnormality of blood constituents which might explain different impact of hemodynamic parameters on thromboembolic events in paroxysmal versus non-paroxysmal AF in this study. Inflammatory markers such as C-reactive protein or interleukin-6 are also associated with prothrombotic state in patients with AF [23, 25]. Whether these inflammatory markers have different role in paroxysmal versus non-paroxysmal AF is an area of future research.
Limitations
The current study has several limitations. First, TTE and TEE evaluations were done after the occurrence of previous thromboembolic events. Second, previous thromboembolic events were diagnosed based on patient history and medical records rather than imaging modalities. Third, although total patient number was quite large, the sample size of patients with previous history of thromboembolic events was of moderate number. Fourth, this study included only patients undergoing RFCA for AF and therefore, do not reflect the whole AF patient. Our study population was consisted of East Asian patients and caution is needed when applying our results to different ethnicity. Fifth, AUC of individual TTE and TEE risk factors was not high and therefore, the risk of thromboembolic events cannot be fully explained by individual hemodynamic parameters. It will be helpful to take into account multiple cardiac hemodynamic parameters comprehensively in addition to clinical factors and markers of atrial myopathy when estimating future risk of thromboembolic events in a given AF patient.
Conclusions
Hemodynamic parameters of LA and LAA had different impact on thromboembolic events between patients with paroxysmal and non-paroxysmal AF. This study suggests that mechanisms of thrombus formation and subsequent thromboembolic events in the two distinct type of AF might be different.
Supporting information
In paroxysmal AF, patients with previous thromboembolic events showed significantly large LA volume which was measured with cardiac MRI. However, LA volume was not different between patients with and without thromboembolic events in non-paroxysmal AF. AF: atrial fibrillation; LA: left atrium; MRI: magnetic resonance imaging.
(TIF)
LA volume was able to predict previous thromboembolic events only in paroxysmal AF patients. AF: atrial fibrillation; LA: left atrium; ROC: receiver operating characteristic.
(TIF)
LA diameter was decreased after ablation. LV EF was increased after ablation but the degree of improvement was negligible. AF: atrial fibrillation; LA: left atrium; LV EF: left ventricular ejection fraction; RFCA: radiofrequency catheter ablation.
(TIF)
Abbreviations
- AF
atrial fibrillation
- AUC
area under curve
- CT
computed tomography
- HR
hazard ratio
- LA
left atrium
- LAA
left atrial appendage
- mCHA2DS2-VASc
modified CHA2DS2-VASc
- MRI
magnetic resonance imaging
- OR
odds ratio
- RFCA
radiofrequency catheter ablation
- ROC
receiver operating characteristic
- SEC
spontaneous echocontrast
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
Data Availability
All relevant data are within the paper and its Supporting Information files. The authors confirm that these data constitute the minimal data set required to support all the conclusions of the present study. However, the underlying individual-level patient data are restricted because they contain sensitive information such as age, sex, body weight, and medical comorbidities. The authors of the present study confirm that the de-identified individual patient data are not required to validate or confirm our study’s conclusions. However, these de-identified patient-level data will nevertheless be made available to interested researchers who meet the criteria for access to confidential information. These data can be requested from the Institutional Review Board of Korea University Medical Center Anam Hospital (+82-02-920-6566 or eirbadmin@kumc.or.kr).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Petersen P. Thromboembolic complications in atrial fibrillation. Stroke. 1990;21(1):4–13. Epub 1990/01/01. . [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379(9816):648–61. Epub 2011/12/15. 10.1016/S0140-6736(11)61514-6 . [DOI] [PubMed] [Google Scholar]
- 3.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31(7):1622–6. Epub 1998/06/17. . [DOI] [PubMed] [Google Scholar]
- 4.Black IW. Spontaneous echo contrast: where there’s smoke there’s fire. Echocardiography. 2000;17(4):373–82. Epub 2000/09/09. . [DOI] [PubMed] [Google Scholar]
- 5.Black IW, Chesterman CN, Hopkins AP, Lee LC, Chong BH, Walsh WF. Hematologic correlates of left atrial spontaneous echo contrast and thromboembolism in nonvalvular atrial fibrillation. Journal of the American College of Cardiology. 1993;21(2):451–7. Epub 1993/02/01. . [DOI] [PubMed] [Google Scholar]
- 6.Karasoy D, Gislason GH, Hansen J, Johannessen A, Kober L, Hvidtfeldt M, et al. Oral anticoagulation therapy after radiofrequency ablation of atrial fibrillation and the risk of thromboembolism and serious bleeding: long-term follow-up in nationwide cohort of Denmark. Eur Heart J. 2015;36(5):307–14a. Epub 2014/11/05. 10.1093/eurheartj/ehu421 . [DOI] [PubMed] [Google Scholar]
- 7.Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. European heart journal. 2016;37(31):2478–87. Epub 2016/03/18. 10.1093/eurheartj/ehw087 . [DOI] [PubMed] [Google Scholar]
- 8.Calenda BW, Fuster V, Halperin JL, Granger CB. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13(9):549–59. Epub 2016/07/08. 10.1038/nrcardio.2016.106 . [DOI] [PubMed] [Google Scholar]
- 9.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circulation Arrhythmia and electrophysiology. 2009;2(5):474–80. Epub 2009/10/22. 10.1161/CIRCEP.109.849638 . [DOI] [PubMed] [Google Scholar]
- 10.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. The New England journal of medicine. 2012;366(2):120–9. Epub 2012/01/13. 10.1056/NEJMoa1105575 . [DOI] [PubMed] [Google Scholar]
- 11.Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57(7):831–8. Epub 2011/02/12. 10.1016/j.jacc.2010.09.049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaita F, Blandino A. Atrial fibrillation. Left atrial fibrosis—a promising stroke risk factor? Nature reviews Cardiology. 2011;8(6):307–8. Epub 2011/04/20. 10.1038/nrcardio.2011.63 . [DOI] [PubMed] [Google Scholar]
- 13.Kim YG, Shim J, Oh SK, Park HS, Lee KN, Hwang SH, et al. Different Responses of Left Atrium and Left Atrial Appendage to Radiofrequency Catheter Ablation of Atrial Fibrillation: a Follow Up MRI study. Sci Rep. 2018;8(1):7871 Epub 2018/05/20. 10.1038/s41598-018-26212-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Heart Lung Circ. 2017;26(9):887–93. Epub 2017/06/15. 10.1016/j.hlc.2017.05.119 . [DOI] [PubMed] [Google Scholar]
- 15.Lau DH, Schotten U, Mahajan R, Antic NA, Hatem SN, Pathak RK, et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J. 2016;37(20):1573–81. Epub 2015/11/19. 10.1093/eurheartj/ehv375 [DOI] [PubMed] [Google Scholar]
- 16.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–68. Epub 2014/04/26. 10.1161/CIRCRESAHA.114.303211 . [DOI] [PubMed] [Google Scholar]
- 17.Fiala M, Wichterle D, Chovancik J, Bulkova V, Wojnarova D, Nevralova R, et al. Left atrial voltage during atrial fibrillation in paroxysmal and persistent atrial fibrillation patients. Pacing Clin Electrophysiol. 2010;33(5):541–8. Epub 2009/12/23. . [DOI] [PubMed] [Google Scholar]
- 18.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34(35):2731–8. Epub 2013/06/14. 10.1093/eurheartj/eht194 . [DOI] [PubMed] [Google Scholar]
- 19.Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol. 2012;23(7):797–9. Epub 2012/05/05. . [DOI] [PubMed] [Google Scholar]
- 20.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54(2):361–79. Epub 2002/06/14. . [DOI] [PubMed] [Google Scholar]
- 21.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90(4):400–5. Epub 2004/03/17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez-Garza E, Jerjes-Sanchez C, Navarrete A, Joya-Harrison J, Rodriguez D. Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. J Thromb Thrombolysis. 2017;44(3):377–85. Epub 2017/07/22. 10.1007/s11239-017-1528-7 . [DOI] [PubMed] [Google Scholar]
- 23.Khan AA, Lip GYH. The prothrombotic state in atrial fibrillation: pathophysiological and management implications. Cardiovascular research. 2019;115(1):31–45. Epub 2018/11/06. 10.1093/cvr/cvy272 . [DOI] [PubMed] [Google Scholar]
- 24.Hatzinikolaou-Kotsakou E, Kartasis Z, Tziakas D, Hotidis A, Stakos D, Tsatalas K, et al. Atrial fibrillation and hypercoagulability: dependent on clinical factors or/and on genetic alterations? Journal of thrombosis and thrombolysis. 2003;16(3):155–61. Epub 2004/04/17. 10.1023/B:THRO.0000024053.45693.fc . [DOI] [PubMed] [Google Scholar]
- 25.Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. Journal of the American College of Cardiology. 2004;43(11):2075–82. Epub 2004/06/03. 10.1016/j.jacc.2003.11.062 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In paroxysmal AF, patients with previous thromboembolic events showed significantly large LA volume which was measured with cardiac MRI. However, LA volume was not different between patients with and without thromboembolic events in non-paroxysmal AF. AF: atrial fibrillation; LA: left atrium; MRI: magnetic resonance imaging.
(TIF)
LA volume was able to predict previous thromboembolic events only in paroxysmal AF patients. AF: atrial fibrillation; LA: left atrium; ROC: receiver operating characteristic.
(TIF)
LA diameter was decreased after ablation. LV EF was increased after ablation but the degree of improvement was negligible. AF: atrial fibrillation; LA: left atrium; LV EF: left ventricular ejection fraction; RFCA: radiofrequency catheter ablation.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The authors confirm that these data constitute the minimal data set required to support all the conclusions of the present study. However, the underlying individual-level patient data are restricted because they contain sensitive information such as age, sex, body weight, and medical comorbidities. The authors of the present study confirm that the de-identified individual patient data are not required to validate or confirm our study’s conclusions. However, these de-identified patient-level data will nevertheless be made available to interested researchers who meet the criteria for access to confidential information. These data can be requested from the Institutional Review Board of Korea University Medical Center Anam Hospital (+82-02-920-6566 or eirbadmin@kumc.or.kr).
All relevant data are within the paper and its Supporting Information files. The authors confirm that these data constitute the minimal data set required to support all the conclusions of the present study. However, the underlying individual-level patient data are restricted because they contain sensitive information such as age, sex, body weight, and medical comorbidities. The authors of the present study confirm that the de-identified individual patient data are not required to validate or confirm our study’s conclusions. However, these de-identified patient-level data will nevertheless be made available to interested researchers who meet the criteria for access to confidential information. These data can be requested from the Institutional Review Board of Korea University Medical Center Anam Hospital (+82-02-920-6566 or eirbadmin@kumc.or.kr).