Abstract
Circadian (~24h) rhythms depend on intra-cellular transcription-translation negative feedback loops (TTFLs). How these self-sustained cellular clocks achieve multi-cellular integration and thereby direct daily rhythms of behavior in animals is largely obscure. The suprachiasmatic nucleus (SCN) is the fulcrum of this gene-to-cell-to-circuit-to-behavior pathway in mammals. We describe cell-type-specific, functionally distinct, TTFLs in neurons and astrocytes of the SCN and show that- in the absence of other cellular clocks- the cell autonomous astrocytic TTFL alone can drive molecular oscillations in the SCN and circadian behavior in mice. Astrocytic clocks achieve this by reinstating clock gene expression and circadian function of SCN neurons via glutamatergic signals. These results provide a first demonstration that astrocytes can autonomously initiate and sustain complex mammalian behavior.
The transcription-translation negative feedback loop mechanisms (TTFL) responsible for intra-cellular circadian (~24h) time-keeping in animals are understood in molecular detail (1). The TTFL of mammals involves transcriptional activation by Clock/Bmal1 hetero-dimers, which drive daytime expression of Period (Per) and Cryptochrome (Cry) genes through E-box regulatory sequences. Following dimerization and transport to the nucleus, Per-Cry complexes repress Clock-Bmal1 activity during circadian night, until progressive degradation of Per-Cry allows initiation of a new cycle. This self-sustaining cell-autonomous TTFL is universally active across mammalian tissues, so how cellular clocks interact to achieve multi-cellular integration and ultimately direct daily rhythms of behavior is a matter of considerable interest. The suprachiasmatic nucleus of the hypothalamus (SCN) is the fulcrum of this gene-to-cell-to-circuit-to-behavior pathway. Its tightly co-ordinated multi-cellular oscillations can continue indefinitely to direct internal synchronization of cellular clocks across the body. The conventional view is that robust pacemaking relies on the intrinsic inter-neuronal connectivity of the SCN, albeit with principles still largely unknown (2). However, circadian time-keeping in the SCN is also influenced by a sophisticated interplay between its neurons and astrocytes (3). In common with other cell types, astrocytes have a TTFL that is assumed to be maintained by input from SCN neurons (4, 5). In light of the intimacy of the astrocytic-neuronal interaction in the SCN, however, we wondered whether SCN astrocytes really are “slaves” to their neuronal partners, or whether the SCN pacemaker might instead be considered a bi-partite cellular system in which astrocytes can also direct neuronal time-keeping and behavior.
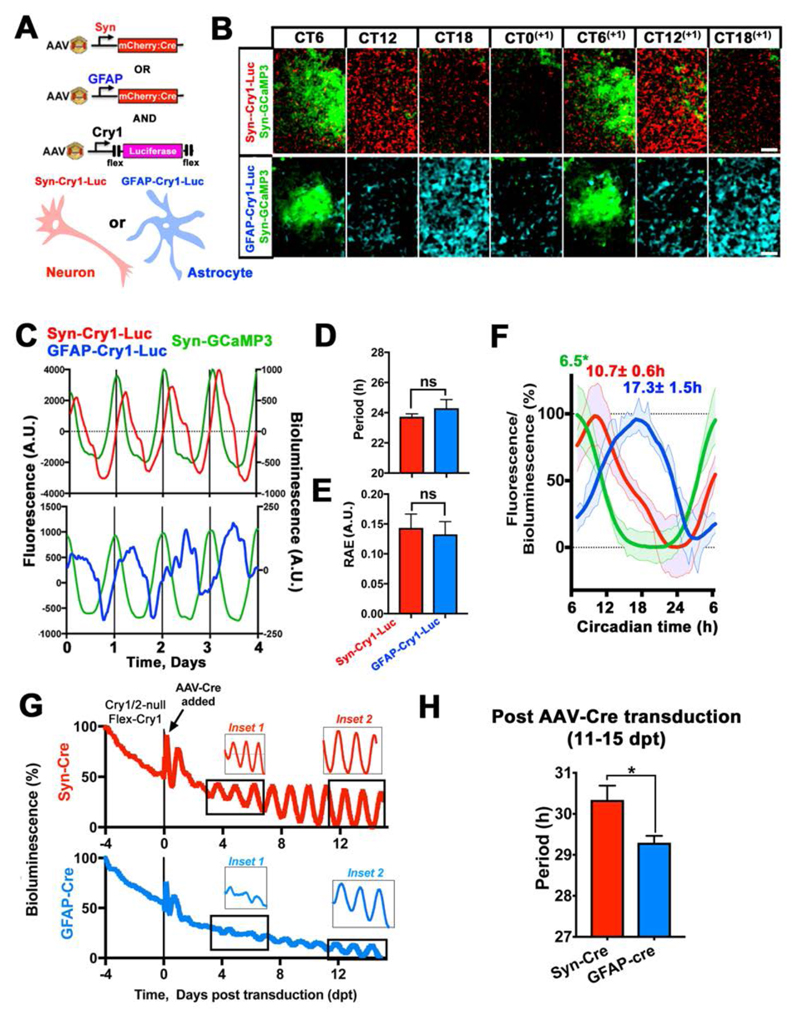
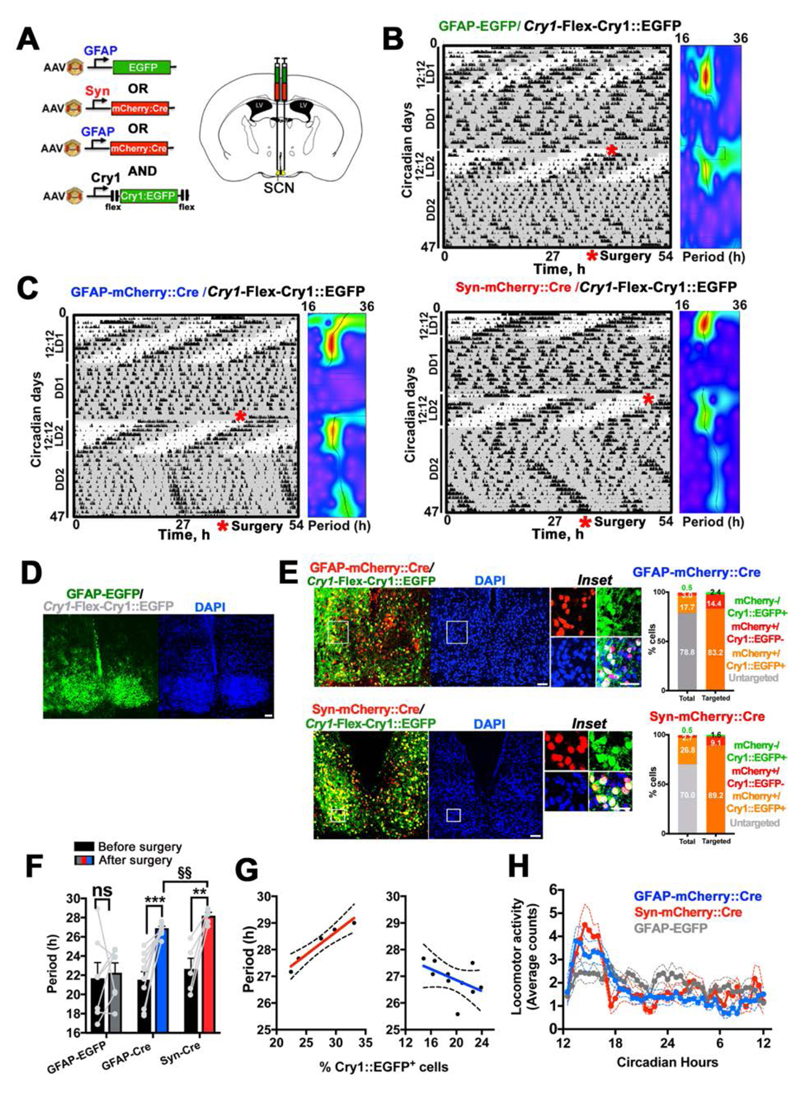
To address this, we used adeno-associated viral vectors (AAVs) and genetics to characterise the distinctive properties of the TTFLs of SCN astrocytes and neurons. We assessed cell-type-specific TTFL function with an AAV encoding a Cre recombinase-dependent (6) reporter in which firefly luciferase is driven by a minimal mouse Cry1 promoter (Cry1) containing E-boxes (Flex-Cry1-Luc) (Flex= Cre-dependent flip-excision) (7, 8). Co-transduction of SCN slices with AAVs driving Cre by the Glial Fibrillary Acidic Protein (GFAP) or human Synapsin 1 (Syn) promoters restricted Flex-Cry1-Luc expression to astrocytes or neurons, respectively (3) (Fig. 1A to F). Bioluminescent recording revealed sustained circadian oscillations of Cry1-Luc in both SCN neurons and astrocytes. Although these oscillations had the same period and robustness as measured by the relative amplitude error (RAE), their waveforms differed (Fig. 1F), reminiscent of the distinctive waveforms of intracellular calcium rhythms observed in astrocytes and neurons (3). These SCN slices were also co-transduced with AAVs encoding the calcium reporter GCaMP3 driven by the Syn promoter (Syn-GCaMP3) to track circadian concentrations of neuronal intracellular calcium ([Ca2+]i). We used this reporter, which peaks during the mid circadian day (circadian time 6.5 hours- CT6.5) (3, 9), to internally register the circadian phase of the detected Cry1-Luc expression in SCN astrocytes and neurons. This showed that the peak of expression of astrocytically restricted Cry1-Luc was phase-delayed by ~6.5 hours (~CT17), when compared to that of the neurons, which peaked at ~CT11 (Fig. 1B, C and F). Thus neurons and astrocytes exhibit cell-type-specific functionally distinct Cry1-reported TTFLs in the SCN, characterized by different phases and waveforms. To test the potential contribution of the astrocytic TTFL to SCN time-keeping, we used cell-type-specific genetic complementation in SCN of mice lacking both Cry genes (Cry 1/2-null mice) (10). In the absence of the Cry repressors the endogenous TTFL does not function, so molecular circadian oscillations, as monitored by the Per2::Luc reporter, are compromised (11) (Fig. 1G). Generalised (pan-cellular) expression of Cry1 can initiate circadian molecular rhythms in Cry-deficient SCN slices (8). Using Cre-dependent AAVs encoding a Cry1::EGFP fusion protein driven by the Cry1 promoter (Cry1-Flex-Cry1::EGFP), we expressed Cry1 specifically in either neurons or astrocytes of Cry1/2-null SCN, restricted by Syn-Cre or GFAP-Cre. As anticipated, expressing Cry1 in neurons was sufficient to initiate self-sustained circadian oscillations of Per2::Luc in the SCN. Expression of Cry1::EGFP solely in astrocytes was also effective, however, highlighting astrocytes as pacemakers within the SCN circuit (Fig. S1, Fig. 1G and H). There were, nevertheless, appreciable differences both in the early and the late phases of Cry1 expression between the two cell-type-specific manipulations. The effects on Per2::Luc oscillations of neuronally restricted Cry1::EGFP became apparent within ~2 days post-transduction (dpt), whereas astrocytically restricted Cry1 took appreciably longer (>7 dpt) to initiate rhythms. In the later stages (11-15 dpt), Cry1 maintained stable, longer-than-24-hours oscillations (as appropriate to a Cry2-null background) (10), when expressed in either neurons or astrocytes, although astrocytically dependent rhythms had a significantly shorter period than neuronally driven rhythms (Fig. 1H). Thus, not only SCN neurons, but also astrocytes can autonomously initiate and sustain stable oscillations of clock gene expression in the SCN, and their instructive, rather than simply permissive role is evidenced by the observed period differences. As shown by SCN transplantation between animals with contrasting genetically specified circadian periods (12, 13), the defining property of the SCN as the master circadian pacemaker is its ability to initiate circadian patterns of behavior, imposing its intrinsic periodicity to the rest of the body. We therefore tested whether the cell-autonomous astrocytic TTFL could drive circadian locomotor activity rhythms in otherwise “clockless” adult mice and compared it to similarly restricted manipulations of the neuronal TTFL (Fig. 2). The SCN of Cry1/2-null mice were stereotaxically injected with Cre-conditional AAV-Cry1-Flex-Cry1::EGFP together with: 1) AAV-GFAP-mCherry::Cre, or 2) AAV-Syn-mCherry::Cre, or 3) AAV-GFAP-EGFP as a Cre-negative control group (Fig. 2A to C, Fig. S2A). We confirmed high specificity and efficiency of Cre-dependent expression of Cry1::EGFP by evaluating post hoc the histological co-localisation of the Cry1::EGFP signal with GFAP-mCherry::Cre, or SynmCherry::Cre, respectively (Fig. 2D and E). We further confirmed that the GFAP driven Cre recombinase efficiently restricts expression of Cry1 to astrocytes by co-localizing the Cry1::EGFP signal with the astrocytic marker AldH1L1 (3, 4) (Fig. S2B). Locomotor activity of mice was recorded before and after surgery under constant dim red light (DD) to assess their intrinsic free-running circadian rhythmicity. Before surgery, Cry1/2-null mice did not show any consistent circadian rhythmicity in DD (DD1). However, after surgery (DD2), and in contrast to Cre-negative control mice, both AAV-GFAP-mCherry::Cre and AAV-SynmCherry::Cre treated mice showed sustained circadian patterns of locomotor behavior (Fig. 2F, Fig. S2A). The periods of the induced rhythms were consistent with the molecular cycles of Per2::Luc observed in SCN explants, being >26h and with the astrocytically driven rhythm being ca. 1 hour shorter than that of mice with neuronally expressed Cry1 (Fig. 2F and Fig. 1H). Furthermore, across animals the number of Cry1::EGFP+ neurons correlated positively with the behavioral period, whereas when Cry1 was expressed in SCN astrocytes there was a negative relationship (Fig. 2G), supporting the view that increasing numbers of targeted neurons and astrocytes can drive the locomotor rhythm to a period progressively closer to that of their respective cell-autonomous TTFLs. Nevertheless, the daily profiles of circadian behavior were equivalent whether neuronally or astrocytically controlled (Fig. 2H and Fig. S2C). Thus, SCN astrocytes can specifically instruct new circadian behavior in an otherwise arrhythmic mouse.
Fig. 1. An astrocytic clockwork can autonomously drive circadian clock gene expression in the SCN.
(A) Experimental design to restrict expression of Cry1-Flex-Luc to neurons or astrocytes by co-transduced Syn-mCherry::Cre or GFAP-mCherry::Cre AAVs. (B) Stills from live-imaging recordings of SCN slices co-transduced with Cry1-Flex-Luc, alongside Syn-mCherry::Cre or GFAP-mCherry::Cre, showing circadian variation of the bioluminescent Cry1-Luc signal, phase aligned to Syn-GCaMP3. Signals are false LUT colors. (C) Representative de-trended traces of neuronally or astrocytically restricted Cry1-Flex-Luc circadian oscillations, phase-aligned to Syn-GCaMP3. (D, E) Period and RAE values of Cry1-Luc oscillations, restricted to neurons or astrocytes. Mean±SEM n=5 per group. (F) Waveform traces of neuronal and astrocytic Cry1-Flex-Luc expression phase-aligned to Syn-GCaMP3. Mean±SEM n=5 for each experimental group. *Circadian phase based on previous data (3, 9). (G) Representative Per2::Luc traces from SCN slices of Cry1/2-null pups sequentially transduced with Cry1-Flex-Cry1::EGFP and then either Syn-mCherry::Cre or GFAP-mCherry::Cre AAVs to restore Cry1 expression in neurons or astrocytes, respectively. Insets show amplitudes of Per2::Luc in the early (inset 1) and late (inset 2) stages of neuronally and astrocytically restricted Cry1 expression. (H) Period values following neuronally or astrocytically restricted Cry1 expression in the late phases of the treatment. Mean±SEM, n=4. Statistical test is unpaired two-tailed t-test. *=p<0.05. Scale Bars= 50 μm.
Fig. 2. Genetic complementation of Cry1 in SCN astrocytes initiates and sustains robust circadian patterns of locomotor activity in circadian incompetent Cry1/2-null mice.
(A) Experimental design of in vivo expression of Flex-Cry1::EGFP restricted to SCN astrocytes or neurons by Syn- or GFAP-driven Cre, respectively. (B, C) Representative actograms and wavelet analyses of Cry1/2-null mice targeted with Cry1-Flex-Cry1::EGFP together with GFAP-EGFP (control) (B), or Cre expressing AAVs (C). Rhythmicity in LD1 and 2 is due to masking effect of the light-dark cycle. (D, E) Representative confocal tiled microphotographs of SCN sections from control and Cre-treated mice evaluated post-hoc to assess effective targeting of the SCN. Histograms represent co-localisation of fluorescence signals from mCherry::Cre and Cry1::EGFP in Cre-treated mice (insets). Total number of cells counted: GFAP-Cre: N(DAPI+)= 5491, n=5 targeted mice; Syn-Cre: N(DAPI+)= 6037, n=5 targetted mice. (F) Periods of circadian activity rhythms of control and Cre-treated mice before (DD1) and after (DD2) stereotaxic surgery. (G) Correlation analysis of number of Cry1::EGFP+ astrocytes or neurons and behavioral period (Syn-mCherry-Cre r=1 n=5, p= 0.02; GFAP-mCherry-Cre r=-0.70, n=10, p= 0.03, 2-way Spearman test). (H) Locomotor activity plotted across the circadian day (Mean±SEM). Group size is n(GFAP-EGFP)=7; n(GFAP-Cre)=10; nSyn-Cre)=5. Statistical test is 2-way RM-ANOVA, with a Bonferroni correction. **=p<0.01; ***=p<0.001. §§=p<0.01 Ad-hoc unpaired 2-tailed t-test with a Sidak-Bonferroni correction, Scale bars= 50 μm.
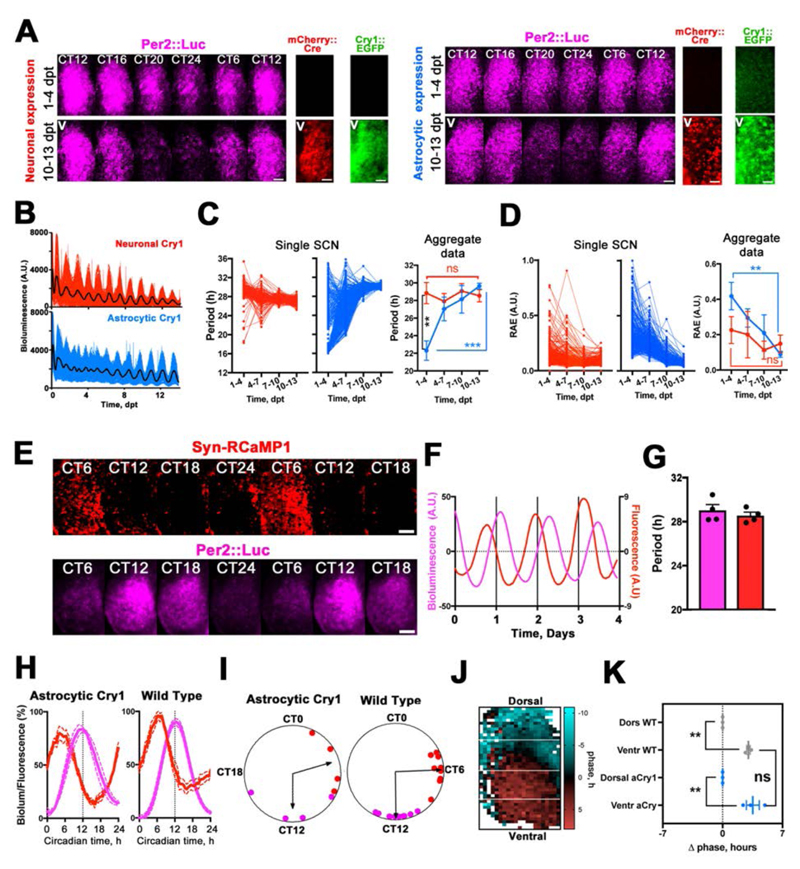
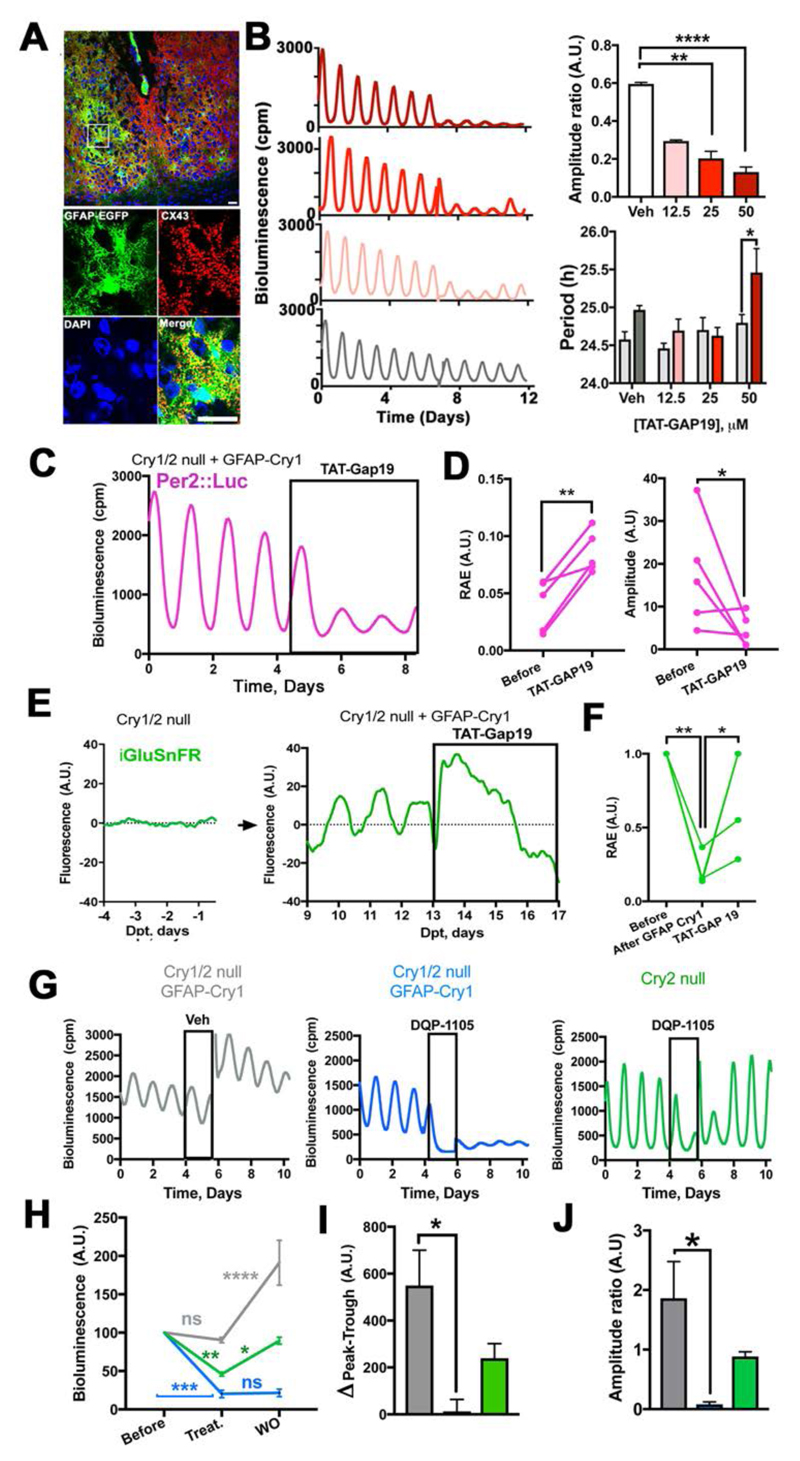
Given that astrocytes are not directly connected to motor centres, we hypothesised that they may rely on recruiting the (TTFL-incompetent) SCN neuronal circuitry in order to engage the behavioral output. To test for such indirect mechanisms, we imaged single cell- and circuit-level TTFL dynamics in Cry1/2-null SCN slices during the early phases of neuronal or astrocytic Cry1 expression (Fig. 3). Neuronal expression of Cry1 immediately generated robust cellular Per2::Luc oscillations, consistent with a direct effect on the neuronal TTFL, and similar to that observed after non-restricted expression of Cry1 (8) (Fig. S3). In contrast, although expression of Cry1 in astrocytes also initiated stable long-period oscillations, it took >7 days to do so (Movie S1, Fig. 3A, B). Analysis of individual Per2::Luc+ cells in the SCN revealed that Cry1 expression in astrocytes produced a progressive strengthening of Per2::Luc cellular rhythms with periods initially differing by >16 hours and slowly converging to a single ~28.5 hours period (Fig. 3C and D). This progressive effectiveness of astrocytes is consistent with an indirect engagement of the wider neuronal circuit. To monitor neuronal activity directly, SCN expressing Cry1 only in astrocytes were super-transduced with AAVs encoding the synapsin-driven red genetically encoded calcium indicator RCaMP1h (Syn-RCaMP1h) (3). This revealed astrocytically driven circadian oscillations of neuronal [Ca2+]i that were phase-advanced to Per2::Luc by ~6 circadian hours, as observed in wild-type SCN (Fig. 3E to I; Movie S2). Circadian peaks of [Ca2+]i and clock gene expression travel across the SCN in a stereotypical spatiotemporal wave, with neurons in the dorsal SCN phase-leading the ventral ones in a pattern strictly dependent on the SCN circuit properties (15, 16). To confirm that astrocytically restricted Cry1 expression also established appropriate spatio-temporal patterns of neuronal [Ca2+]i across the SCN, we compared the calcium signal in wild-type SCN and SCN with astrocytically restricted Cry1 expression and found comparable dorsal-to-ventral organization of neuronal [Ca2+]i (16) (Fig. 3J and K, Movie S2). Given that Per gene promoters harbour calcium-responsive elements that phase-lock Per expression to neuronal [Ca2+]i, astrocytes may engage the E-box-based TTFL of neurons by driving neuronal [Ca2+]i (9), sustaining intracellular oscillations of clock gene expression across SCN space and circadian time. Critically, this happens in the absence of Cry genes in neurons, thus revealing that the neuronal E-box-based TTFL may be dispensable for circuit-level circadian time-keeping. Thus, genetic complementation of Cry1 in SCN astrocytes can initiate and sustain mammalian circadian function by recruiting the latent SCN neuronal circuit. To investigate the relevant mechanisms, we tested the role of Connexin 43 (Cx43) a major component of gap junctions and hemichannels specifically expressed in astrocytes, which coordinates astrocytic networks and recently implicated in hypothalamic regulation of sleep-wake cycles (17, 18). Cx43 is highly expressed in the SCN, extensively decorating astrocytic processes, as shown by co-localization with the GFAP-EGFP tag from control surgery mice (Fig. 2D and Fig. 4A). We then assessed the effects of Cx43 inhibition on circadian oscillations of clock gene expression in SCN slices by using the mimetic peptide TAT-Gap19 (19, 20). TAT-Gap19 elicited a dose-dependent and reversible reduction in the amplitude and period-lengthening of Per2::Luc oscillations (Fig. 4B, Fig. S4), confirming the role of astrocytes in circadian function of wild type SCN. We then showed that Cx43 inhibition by TAT-Gap19 significantly compromised Per2::Luc oscillations driven by astrocytically restricted expression of Cry1 in Cry1/2-null slices (Fig. 4C and D). TAT-Gap19 specifically inhibits the hemichannel form of Cx43, involved in paracrine astrocytic release of gliotransmitters, including ATP and glutamate (19, 21). Astrocytically released glutamate is a major gliotransmitter in the SCN (3) and therefore we tested its key role in driving circadian rhythmicity in Cry1/2-null mice where Cry1 was expressed in astrocytes. Extracellular glutamate levels of Cry1/2-null SCN slices, measured using the AAV-encoded glutamate indicator iGluSnFR driven by Syn (3, 22), exhibited no detectable circadian oscillations, but GFAP-Cre restricted expression of Cry1 initiated robust circadian glutamate oscillations. Moreover, these were strongly impaired by Cx43 inhibitor (TAT-Gap19) (Fig. 4E and F). These data support the role of astrocytically derived circadian oscillations of glutamate in mediating astrocytic control of circadian oscillations in Cry1/2-null SCN. To determine whether glutamate is specifically responsible for astrocytically dependent circadian time-keeping in Cry1/2-null SCN, slices received DQP-1105, an antagonist for N-methyl-D-aspartate glutamate receptor assemblies containing the NR2C/D subunit (NMDAR2C) (23). NMDAR2C inhibition by DQP-1105 damps circadian rhythms of membrane potential and clock gene expression in WT SCN neurons over several days (3). The effect of the drug was much more marked in Cry1/2-null SCN with rhythms driven by astrocytically expressed Cry1, as shown by the immediate drop in the baseline of the Per2::Luc rhythms and abolition of the peak-to-trough difference. Moreover, rhythmicity was restored on washout of the drug, but the amplitude was irreversibly reduced. We interpreted this as protracted misalignment of circadian activity of SCN neurons and astrocytes. The dramatic effects of DQP-1105 were not evident in Cry2-null SCN, which retain Cry1 expression in both neurons and astrocytes, thus ruling out any confound of Cry2 deficiency in our astrocytic Cry1 rescue model (Fig. 4G to J). Thus, glutamate is a necessary mediator of astrocytic control of circadian function in the SCN, as shown by two independent pharmacological approaches: interference with glutamate release by astrocytes (via Cx43 inhibition) and with neuronal glutamate sensing (via NMDAR2C antagonism) (2, 3).
Fig. 3. Temporal dynamics of circadian bioluminescence rhythms of single cells initiated in Cry1/2-null SCN explants following neuronally or astrocytically restricted expression of Cry1.
(A) Stills from live-imaging recordings of Per2::Luc expression from Cry1/2-null SCN slices showing circadian variation of the bioluminescent signal in the early (upper rows) and late stages (lower rows) of neuronal or astrocytic Cry1 expression. Co-detected mCherry and EGFP are shown to compare spatial distribution and temporal dynamics of mCherry::Cre and Cry1::EGFP expression. (B) Representative single-cell (colored lines) and mean traces (black lines) of Per2::Luc oscillations, following Cre-mediated expression of Cry1 in either neurons or astrocytes within SCN slices. (C, D) Period and Relative Amplitude Error (RAE) following neuronal or astrocytic expression of Cry1 in an individual SCN and across multiple explants. Traces for aggregate data are Mean±SEM. Group size is n=3 for each group. Statistical test is 2-way RM-ANOVA, with a Bonferroni correction. (E) Stills from live-imaging recordings showing circadian variations of Per2::Luc and Syn-RCaMP1h in Cry1/2-null SCN slices transduced with GFAP-mCherry::Cre and Cry1-Flex-Cry1::EGFP. (F) Representative traces of data presented in (E). (G) Period quantification of Per2::Luc and Syn-RCaMP1h in Cry1/2-null SCN expressing Cry1 only in astrocytes. Mean±SEM, n=4 (H, I) Mean traces±SEM (H) and Rayleigh plots (I) showing waveforms and phase differences of Per2::Luc and Syn-RCaMP1h oscillations in GFAP-mCherry::Cre/ Cry1-Flex-Cry1::EGFP SCN slices and wild type SCN. (J, K) Representative spatial phase map of Syn-RCaMP1h signal (J) and quantification of the dorsal to ventral phase relationship (K) in SCN expressing astrocytic Cry1, compared to wild type. Phase data normalized to dorsal values. Values are Mean±SEM, group size as plotted. **=p<0.01, ***=p<0.001, ****=p<0.0001. Statistical tests: paired two-tailed t-test in (G) and unpaired ANOVA in (K). Scale bars= 50 μm.
Fig. 4. Astrocytically released glutamate mediates astrocytic control of circuit-level circadian time-keeping in Cry1/2-null SCN expressing GFAP-restricted Cry1.
(A) Confocal tiled microphotographs of adult SCN showing co-localization of GFAP-EGFP and Cx43, detected by polyclonal antiserum (representative of 3 independent brains). (B) Representative Per2::Luc PMT traces and group data (Mean+SEM) showing dose-response effects of TAT-Gap19 on amplitude (ratio= with drug/before drug) and period in wild type SCN slices. Statistical test: Amplitude ratio= unpaired ANOVA, with a Bonferroni correction. Period is 2-way RM-ANOVA, with a Bonferroni correction, n=3 for each group, except Veh n=4. (C, D) Representative Per2::Luc PMT trace (C) and paired scatter plot of RAE and amplitude (D) of Cry1/2-null SCN slices transduced with GFAP-mCherry::Cre and Cry1-Flex-Cry1::EGFP and treated with TAT-Gap19 (50 μM). Statistical test: paired one-tailed t-test, n=5. (E, F) Representative iGluSnFR traces (E) and paired scatter plot of RAE (F) of Cry1/2-null SCN slices before and after GFAP-mCherry::Cre/ Cry1-Flex-Cry1::EGFP transduction and with TAT-Gap19 (50 μM). Statistical test: RM-ANOVA, with a Bonferroni correction, n=4. (G) Representative Per2::Luc PMT traces of Cry2-null and Cry1/2-null SCN slices transduced with GFAP-mCherry::Cre and Cry1-Flex-Cry1::EGFP on treatment with DQP-1105 (50 μM) or vehicle, and subsequent washout. (H) Group data (Mean+SEM) of bioluminescence baseline traces represented in (G) before, in the presence of, and after removal of, DQP-1105. Statistical test is 2-ways RM-ANOVA, with a Bonferroni correction. (I) Group data (Mean+SEM) showing peak-trough difference in the presence of DQP-1105 of traces represented in (G). (J) Group data (Mean+SEM) showing amplitude (ratio= after drug/with drug) of data presented in (G) Statistical test in (H) is 2-ways RM-ANOVA, with a Bonferroni correction. Statistical test in (I) and (J) is unpaired ANOVA, with a Bonferroni correction; n=4 for Cry2-null and Veh groups; n=3 for DQP-1105 in Cry1/2-null group *=p<0.05; **=p<0.01; ***=p<0.001; ****=p<0.0001, n=4. Scale bars= 20 μm.
A growing body of evidence has challenged a neurono-centric view of the control of behavior in mammals, by showing that astrocytes can modulate complex neural processes, including cognition, fear, sleep and circadian rhythms (3, 4, 17, 24, 25). However, most studies rely on the presence of a pre-existing neuronally encoded behavior and show that behavioral performances are affected when astrocytic function is modified (24). Thus, regardless of the specificity of the astrocytic-neuronal interactions described (3, 25, 26), these studies only address the ability of astrocytes to modulate neuronally dependent behavior, but do not establish their sufficiency in controlling behavior. Here we show that astrocytes of the SCN can autonomously encode circadian information and instruct their neuronal partners, which lack a competent TTFL clock, to initiate and indefinitely sustain circadian patterns of neuronal activity and behavior.
Supplementary Material
One Sentence Summary.
The cell-autonomous circadian clock of SCN astrocytes can drive mammalian SCN neuronal time-keeping and circadian behavior.
Acknowledgments
We thank LMB Biological Services Group and Ares staff for excellent technical support. Medical Research Council UK (core funding MC_U105170643 to M.H.H.) supported this work. M.B. designed, performed, and analyzed all the experiments. M.H.H. contributed to the experimental design. M.D.E and N.J.S. developed and validated Cry1-Flex-Luc and Cry1-Flex-Cry1::EGFP. A.P.P, E.S.M. and J.E.C. conducted preliminary experiments. All authors contributed to project discussions. M.B. and M.H.H. wrote the manuscript. Authors declare no competing interests. All data are available in the manuscript or supplementary data
References
- 1.A Nobel Pursuit May Not Run like Clockwork. Cell. 2017;171:1246–1251. doi: 10.1016/j.cell.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018;19:453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 3.Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron. 2017;93:1420–1435.e5. doi: 10.1016/j.neuron.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tso CF, et al. Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr Biol. 2017;27:1055–1061. doi: 10.1016/j.cub.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci Off J Soc Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 7.Fustin JM, O’Neill JS, Hastings MH, Hazlerigg DG, Dardente H. Cry1 circadian phase in vitro: wrapped up with an E-box. J Biol Rhythms. 2009;24:16–24. doi: 10.1177/0748730408329267. [DOI] [PubMed] [Google Scholar]
- 8.Edwards MD, Brancaccio M, Chesham JE, Maywood ES, Hastings MH. Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc Natl Acad Sci. 2016;113:2732–2737. doi: 10.1073/pnas.1519044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brancaccio M, Maywood ES, Chesham JE, Loudon ASI, Hastings MH. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron. 2013;78:714–728. doi: 10.1016/j.neuron.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Horst GTJ, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 11.Yoo S-H, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 13.King VM, et al. A hVIPR transgene as a novel tool for the analysis of circadian function in the mouse suprachiasmatic nucleus. Eur J Neurosci. 2003;17:822–832. [PubMed] [Google Scholar]
- 14.Smyllie NJ, Chesham JE, Hamnett R, Maywood ES, Hastings MH. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 2016;113:3657–3662. doi: 10.1073/pnas.1511351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brancaccio M, et al. Network-mediated encoding of circadian time: the suprachiasmatic nucleus (SCN) from genes to neurons to circuits, and back. J Neurosci Off J Soc Neurosci. 2014;34:15192–15199. doi: 10.1523/JNEUROSCI.3233-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enoki R, et al. Synchronous circadian voltage rhythms with asynchronous calcium rhythms in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 2017;114:E2476–E2485. doi: 10.1073/pnas.1616815114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clasadonte J, Scemes E, Wang Z, Boison D, Haydon PG. Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle. Neuron. 2017;95:1365–1380.e5. doi: 10.1016/j.neuron.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayorquin LC, Rodriguez AV, Sutachan J-J, Albarracín SL. Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons. Front Mol Neurosci. 2018;11 doi: 10.3389/fnmol.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abudara V, et al. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8 doi: 10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walrave L, et al. Inhibition of astroglial connexin43 hemichannels with TAT-Gap19 exerts anticonvulsant effects in rodents. Glia. 2018;66:1788–1804. doi: 10.1002/glia.23341. [DOI] [PubMed] [Google Scholar]
- 21.Ye Z-C, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci Off J Soc Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marvin JS, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acker TM, et al. Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol Pharmacol. 2011;80:782–795. doi: 10.1124/mol.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira JF, Sardinha VM, Guerra-Gomes S, Araque A, Sousa N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015;38:535–549. doi: 10.1016/j.tins.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Papouin T, Dunphy JM, Tolman M, Dineley KT, Haydon PG. Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron. 2017;94:840–854.e7. doi: 10.1016/j.neuron.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Fernandez M, et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci. 2017;20:1540–1548. doi: 10.1038/nn.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ. Strengths and Limitations of Period Estimation Methods for Circadian Data. PLoS ONE. 2014;9:e96462. doi: 10.1371/journal.pone.0096462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore A, Zielinski T, Millar AJ. Online period estimation and determination of rhythmicity in circadian data, using the BioDare data infrastructure. Methods Mol Biol Clifton NJ. 2014;1158:13–44. doi: 10.1007/978-1-4939-0700-7_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.