Abstract
Background
We aimed to describe the frequency, risk factors, and costs attributable to drug–drug interactions (DDIs) among an aging French HIV population.
Methods
We conducted a retrospective cohort study using French nationwide health care e-records: the SNIIRAM database. People living with HIV (PLWH) aged >65 years and receiving combined antiretroviral treatment (cART) during 2016 were included. A DDI was defined as “These drugs should not be co-administered,” represented by a red symbol on the University of Liverpool website. Attributable DDIs’ cost was defined as the difference between individuals with and without DDIs regarding all reimbursed health care acts.
Results
Overall, 9076 PLWH met the study criteria. Their baseline characteristics were: mean age, 71.3 ± 4.9 years; 25% female; median HIV duration (interquartile range [IQR]), 16.2 (9.5–20.3) years; median comorbidities (IQR), 2 (1–3). During 2016, they received a median (IQR) of 14 (9–21) comedications (non-cART), and 1529 individuals had at least 1 DDI (16.8%; 95% confidence interval [CI], 16.1–17.6). In multivariate analysis, raltegravir or dolutegravir plus 2 nucleoside reverse-transcriptase inhibitors (NRTIs) significantly and independently reduced the risk of DDIs (adjusted odds ratio [aOR], 0.02; 95% CI, 0.005–0.050; P < .0001) compared with non-nucleoside reverse-transcriptase inhibitor plus 2 NRTIs, whereas cART with boosted agents (protease inhibitors or elvitegravir) significantly increased the risk (aOR, 4.12; 95% CI, 3.34–5.10; P < .0001). Compared with propensity score–matched PLWH without DDIs, the presence of DDIs was associated with a $2693 additional cost per year (P < .0001).
Conclusions
The presence of DDIs is frequent and significantly increases health care costs in the aging population of PLWH.
Keywords: aging HIV population, antiretroviral therapy, costs, drug–drug interaction, nationwide database
Therapeutic progress in the area of combined antiretroviral therapy (cART) during the past 2 decades has dramatically decreased HIV morbidity and mortality [1, 2]. In the Dat’AIDS French cohort of people living with HIV (PLWH), 45.3% and 1.5% were older than age 50 and 75 years, respectively, on December 2015 [3]. Currently, HIV patients aged >65 years account for 6% of PLWH in the United States [4]. Age-related diseases such as cardiovascular disease, hypertension, diabetes mellitus, and renal failure are more prevalent among PLWH, as compared with the general population [5, 6].
Both HIV virus and cART can impair kidney and/or liver function, affecting drug clearance and increasing the risk of drug toxicity [7]. Decreased renal function in the aging population is due to the reduction of nephrons, vascular changes that decrease the blood flow yielding a reduction of glomerular filtration, and decreases of drugs’ elimination. The same mechanism has been described for the alteration of liver function: a progressive reduction in liver volume and liver blood flow that leads to a reduction of the hepatic metabolism of drugs with potential accumulation [8]. Because few clinical trials have enrolled aging PLWH, the magnitude of drug–drug interactions in this population may be underestimated. Multimorbidity and subsequent polypharmacy are associated with a high risk of drug–drug interactions [5, 9]. In addition, many antiretroviral drugs (ARVs) interact with xenobiotic metabolism, mainly via the cytochrome P450 (CYP450) pathway. This may lead to subtherapeutic drug concentrations favoring the emergence of drug resistance. In contrast, this may also induce supra-therapeutic drug concentrations favoring the risk of drug toxicities, such as iatrogenic Cushing’s syndrome [10, 11]. Drug–drug interactions (DDIs) may have clinical consequences such as increased morbidity and mortality [12]. Bastida et al. [13] studied this issue among 265 PLWH aged >65 years in a small, single-center cross-sectional study: 65% had at least 1 potential drug–drug interaction, and 6.6% had a severe potential drug–drug interaction. Therefore, deprescribing medications that are unnecessary (commonly called the Beer list) has the potential to improve the risk–benefit ratio of medication regimens in aging PLWH.
We aimed to estimate the 1-year incidence of DDIs in order to identify independent risk factors associated with DDIs and their associated costs among older PLWH from a large nationwide health care database in France.
METHODS
Study Design and Population
We conducted a retrospective, noninterventional cohort study (POPVIH65, NCT03416881) using the Système National d´Information Inter-Régimes de l’Assurance Maladie (SNIIRAM) pharmacy refill e-records. People affiliated with the Caisse Nationale de l’Assurance Maladie des Travailleurs Salaries (CNAMTS; 98% of the French population [14]) in the SNIIRAM who had HIV illness ALD30 code No. 7 (corresponding to the 10th revision of the International Classification Diseases [ICD-10]; codes B20 to B24 and Z21), aged >65 years and receiving at least 1 ARV drug between January 1, 2016, and December 31, 2016, were included. This academic study was approved by competent authorities: an independent ethics committee (CPP Nord-Ouest III), the French Health Data Institute, and the National Commission Data Protection (CNIL).
Data Collection
Available data for the POPVIH65 cohort study were demographics, presence of a chronic disease with its first registration date, all health care acts reimbursed by health insurance (medical appointments, medications, lab tests, medical transportations, hospitalizations), and their associated costs. These details were anonymous, and individuals were identified with a unique identification number. Pharmacy refill data were listed in different tables: 1 for community pharmacy and several for hospital pharmacy (drugs for home delivered upon discharge and expensive drugs of inpatient not covered by the hospital fees). A row corresponded to a drug with its unique national registration code (CIP) and its entrance date into the SNIIRAM (approximately its delivery date). For simplicity, we defined different groups for cART: 2 nucleoside reverse-transcriptase inhibitors (NRTIs) plus 1 non-NRTI; 2 NTRIs + raltegravir or dolutegravir; 2 NRTIs + a third boosted agent; alternative ARV therapy for cART not corresponding to any of the above definitions; and inconsistent therapy for cART that did not remain the same for more than 6 months.
End Points
The end point of this study was the occurrence of a DDI between 2 drugs (ARV/ARV or ARV/non-ARV) in 2016. Any potential interaction was identified when an ARV drug was delivered between the first and the last delivery date of another drug (ARV or non-ARV). Pro re nata (PRN) drugs were included as comedications, as the SNIIRAM database does not distinguish PRN and non-PRN prescriptions. We assumed PRN drugs were taken following the prescription. Next, this interaction was considered a DDI if it yielded a “Do Not Co-administer” statement (including an “empty” red symbol), according to the University of Liverpool website (www.hiv-druginteractions.org/checker). For fixed-combination products, each active substance was screened separately. For DDIs with lidocaine, dexamethasone, or ketoconazole, only the systemic use of these drugs was included. For more details about the SAS code used to identify DDIs, please see the Appendix. We also estimated the annual cost attributable to DDIs, defined as all reimbursed health care acts recorded in the SNIIRAM during 2016. All costs estimated in euros were converted to US dollars (1€ = $1.2064 USD at February 1, 2018). In a sensitivity analysis, we deducted the annual costs of ARVs. The rationale was to test our hypothesis that increased health care costs associated with DDI were more likely to be attributable to DDI-associated morbidity (ie, lab, consultations, and hospitalization) than to be attributable to DDI-associated higher cost of ARVs.
Statistical Analysis Plan
The characteristics of the studied population were described as number (percentage) for qualitative variables and mean (±SD) or median (interquartile range) for quantitative variables, as appropriate. To compare the groups with or without DDI, we used the Fisher exact or chi-square test for qualitative variables and the Student t or Wilcoxon test for quantitative variables, as appropriate.
The cumulative 1-year incidence of at least 1 DDI was computed with the patient as the statistical unit, with its corresponding 95% confidence interval. The incidence of DDIs per each ARV drug and its 95% confidence interval were estimated by Poisson regression. We used a stepwise multivariate logistic regression model with a P value <.05 to enter and stay in the model to identify independent risk factors for cART prescription and for experiencing at least 1 DDI.
Because DDIs were more likely to occur among individuals with a higher burden of comorbidities, direct comparisons of health care costs could be biased. Therefore, we built a propensity score representing the probability of having a DDI conditional to baseline characteristics by nonparsimonious multivariate logistic regression. Individuals with and without DDIs were subsequently matched based on their propensity score. The baseline characteristics of the propensity score–matched subcohort were compared with the standardized difference as appropriate, with a value 10% indicating a small difference. Comparison of costs and mortality was performed by a generalized estimating equation, taking into account the paired design. All statistical analyses were conducted with SAS software V9.4 (SAS institute, Cary, NC), and a P value <.05 was considered to denote statistical significance.
RESULTS
Baseline Characteristics
During the study period, an estimated 153 710 PLWH were identified in 2016 in France. The flowchart of patients from the initial extraction of SNIIRAM to the final POPVIH65 cohort is described in Supplementary Figure 1. The initial database contained 14 471 subjects, of whom 1246 died before January 1, 2016. Among the 13 225 remaining subjects, 1175 had a condition other than HIV-related immune deficiency and were excluded. Of the 11 450 PLWH aged >65 years old, 2374 (20.7%) did not receive ART during the study period. Among them, 232 (10%) received reimbursed heath care other than drugs, 384 (16%) were not linked to care (no reimbursed care), and 1737 were not linked to HIV care (received reimbursed health care including drugs other than cART). There was a higher likelihood of no prescribed cART (P < .001) among women (odds ratio [OR], 4.0; 95% confidence interval [CI], 3.7–4.5) vs men, among those with a more recent HIV diagnosis (diagnosed in 2016: OR, 9.9; 95% CI, 7.3–13.4; 1 month–10 years: OR, 3.1; 95% CI, 2.6–3.6; 11–20 years: OR, 2.1; 95% CI, 1.8–2.5; vs >20 years: reference), and among PLWH with a diagnosis of dementia (OR, 2.5; 95% CI, 1.6–4.0), adjusting for geographic region, number of comedications, and number of comorbidities.
Among the remaining HIV population, 9076 individuals (5.9%) were aged >65 years while receiving cART in 2016, with a mean age of 71.3 (±4.9) years. Table 1 shows the characteristics of the POPVIH65 population by DDI status during 2016.
Table 1.
Characteristics of POPVIH65 Patients Who Received Antiretroviral Therapy During 2016 (n = 9076)
| Baseline Characteristics | POPVIH65 Cohort (n = 9076) | Patients Without DDI (n = 7547) | Patients With ≥1 DDI (n = 1529) | P Value |
|---|---|---|---|---|
| Male, No. (%) | 6834 (75) | 5704 (76) | 1130 (74) | .172 |
| Age, mean ± SD, y | 71.3 ± 4.9 | 71.3 ± 4.9 | 71.1 ± 4.9 | .295a |
| HIV duration,b median [IQR], y | 16.2 [9.5–20.3] | 16.1 [9.5–20.2] | 16.6 [9.3–20.9] | .158c |
| Diagnosis in 2016, No. (%) | 135 (1) | 117 (1) | 18 (1) | <.001 |
| 1 mo–10 y, No. (%) | 2272 (25) | 1880 (25) | 392 (26) | |
| 11–20 y, No. (%) | 4246 (47) | 3605 (48) | 641 (42) | |
| >20 y, No. (%) | 2423 (27) | 1945 (26) | 478 (31) | |
| Geographic repartition, No. (%) | .085 | |||
| Ile-de-France | 3070 (34) | 2531 (34) | 539 (35) | |
| Occitanie and PACA | 1777 (20) | 1508 (20) | 269 (18) | |
| Others regions | 4229 (47) | 3508 (46) | 721 (47) | |
| Co-medications,d median [IQR] | 14 (9–21) | 13 [8–19] | 19 [13–26] | <.001c |
| Concomitant diseases,e median [IQR] | 2 [1–3] | 2 [1–3] | 3 [1–4] | <.001c |
| Main concomitant diseases, No. (%) | ||||
| Cardiovascular disease | 5838 (64) | 4795 (64) | 1043 (68) | <.001 |
| Dyslipidemia | 3994 (44) | 3292 (44) | 702 (46) | .100 |
| Anxiety, sleep disorders | 1935 (21) | 1519 (20) | 416 (27) | <.001 |
| Diabetes | 1728 (19) | 1391 (18) | 337 (22) | .001 |
| Cancer | 1144 (13) | 950 (13) | 194 (13) | .914 |
| Hospitalizations, median [IQR] | 3 [0–7] | 3 [0–7] | 3 [1–9] | <.001c |
| ARV drugs, median [IQR] | 3 [3–4] | 3 [3–4] | 4 [3–5] | <.001c |
| ARV classes, No. (%) | ||||
| NRTI | 7868 (87) | 6678 (89) | 1190 (78) | <.001 |
| NNRTI | 4443 (49) | 3819 (51) | 624 (41) | <.001 |
| Protease inhibitorsf | 3125 (34) | 2030 (27) | 1095 (72) | <.001 |
| Boosted protease inhibitors | 2874 (32) | 1923 (25) | 951 (62) | <.001 |
| Integrase inhibitors | 4316 (48) | 3661 (49) | 655 (43) | <.001 |
| Entry inhibitors | 213 (2) | 177 (2) | 36 (2) | .983 |
All tests are chi-square tests unless indicated and compare patients with and without DDI.
Abbreviations: ARV, antiretroviral; DDI, drug–drug interaction; IQR, interquartile range; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitors; PACA, Provence-Alpes-Côte-d’Azur.
aStudent t test.
bHIV duration estimated from the date of the first long-term illness exemption related to HIV.
cWilcoxon rank test.
dTotal number of different concomitant medications (non-ARV) delivered during 2016.
eTotal number of different diseases (HIV excluded). For more details about the determination of concomitant diseases, see the Appendix.
fBoosted and unboosted.
Risks of DDI
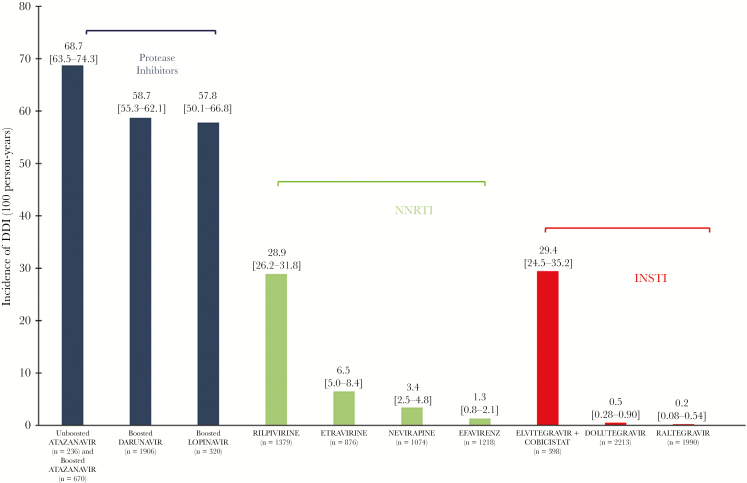
The 1-year cumulative incidence was 1529/9076 patients with at least 1 DDI (16.8%; 95% CI, 16.1–17.6), corresponding to a total of 2772 DDIs, with 161 distinct DDIs identified. On average, aging PLWH had 0.3 (±0.8) DDIs during 2016. The incidence rates of DDIs per 100 person-years for NRTI drugs were as follows: tenofovir DF (n = 4070), 0.0; emtricitabine (n = 3935), 2.2; lamivudine (n = 4058), 2.1; abacavir (n = 3588), 0.3. Figure 1 illustrates the incidence per 100 patient-years of DDIs per non-nucleosidic reverse transcriptase inhibitor agent. Most DDIs revealed interactions between ARV and comedications (2534, 91%). The risk factors for at least 1 DDI are shown in Table 2. Compared with non-NNRTI plus 2 NRTIs, the use of raltegravir or dolutegravir plus 2 NRTIs significantly and independently reduced the rate of DDIs (0.02; 95% CI, 0.005–0.05; P < .0001). Other cART, including ARV regimens utilizing boosted agents, and inconsistent therapy increased the rate of DDIs. The main DDIs identified are detailed in Table 3, with their frequency, mechanism, and risks.
Figure 1.
Incidence per 100 person-years of drug–drug interactions among the POPVIH65 population related to the most common agent received, >3% of prescriptions. Abbreviations: INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Table 2.
Multivariate Analysis to Determine Factors Associated With the Occurrence of ≥1 DDI Among the POPVIH65 Cohort (n = 9076)
| Characteristics | Odds Ratio [95% CI] | P Value |
|---|---|---|
| Age, for 10-y increase | 0.87 [0.76–0.98] | .02 |
| No. of ARV drugs | 1.35 [1.24–1.47]a | <.0001 |
| No. of comedications (non-ARV) | 1.07 [1.06–1.07]a | <.0001 |
| Chronic obstructive pulmonary disease | 1.67 [1.36–2.05] | <.0001 |
| Antiretroviral therapy | ||
| 2 NRTIs + 1 NNRTI (n = 2624) | 1.00 | — |
| 2 NRTIs + raltegravir or dolutegravir (n = 1512) | 0.02 [0.005–0.05] | <.0001 |
| 2 NRTIs + 1 boosted third agentb (n = 1271) | 4.12 [3.34–5.10] | <.0001 |
| Alternative ARV therapy (n = 1485) | 3.58 [2.94–4.37] | <.0001 |
| Inconsistent therapy (n = 2184) | 2.41 [1.88–3.09] | <.0001 |
Abbreviations: ARV, antiretroviral; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor.
aOdds ratio corresponds to 1-unit increase.
bProtease inhibitors or elvitegravir.
Table 3.
Mechanism and Risks of the 10 Most Frequent DDIs Among the POPVIH65 Cohort (n = 9076) in 2016
| DNCIs, No. (%) | Mechanism of Interaction | Potential Risksa | |
|---|---|---|---|
| PI or boost/inhaled glucocorticoidsb | 739 (29) | Inhibition of CYP3A4 yielding to a rise of plasma concentration of inhaled glucocorticoids | Cushing syndrome, adrenal suppression, and other glucocorticoids toxicities |
| Atazanavir or rilpivirine/proton pump inhibitorsc | 676 (27) | Decrease of intestinal absorption of ARV yielding to a subtherapeutic concentration | Ineffective ARV therapy |
| PI or boost/lercanidipine | 285 (11) | Inhibition of CYP3A4 yielding to a rise of plasma concentration of comedications | Not documented (theoretically: hypotension and cardiac rhythm disorders) |
| PI or boost/alfuzosin | 233 (9) | Severe hypotension | |
| PI or boost/domperidone | 136 (5) | Cardiac arythmia like QT interval prolongation | |
| PI or boost/amiodarone | 82 (3) | Cardiac arythmia like QT interval prolongation | |
| PI or boost/simvastatin | 79 (3) | Rhabdomyolysis | |
| PI or boost/apixaban or rivaroxaban | 67 (3) | Bleeding | |
| PI or boost/piroxicam | 51 (2) | Serious respiratory depression and hematologic abnormalities | |
| Darunavir/injectable lidocaine | 38 (2) | Cardiac arythmia like QT interval prolongation | |
| Other combinations | 126 (6) | — | — |
Abbreviations: ARV, antiretroviral; boost, ritonavir or cobicistat; DDIs, drug-drug interactions; PI, protease inhibitor (boosted or not); QT, .
aPotential risks are defined from the Liverpool HIV drug interactions website.
bInhaled glucocorticoids include aerosols of fluticasone or budesonide and nasal sprays of mometasone or triamcinolone.
cProton pump inhibitors include rabeprazole, omeprazole, lansoprazole, pantoprazole, and esomeprazole.
Costs Estimation and Mortality
The mean (SD) cost for the entire population was $16 820 ($13 683) for 2016. This figure was $19 784 ($18 717) for PLWH with DDIs and $16 219 ($12 332) for those without DDIs (unadjusted difference, $3564; P < .0001). The similar baseline characteristics of the 2 groups (n = 1529) after propensity score matching are presented in Table 4. In the propensity score–matched subcohort, the mortality rates were similar: 33/1529 (2.2%) in the group without DDIs and 30/1529 (2.0%) in the group with DDIs died (OR, 1.1; 95% CI, 0.7–1.8; P = .70) in 2016.
Table 4.
Characteristics of the POPVIH65 Population After Matching Based on the Propensity Score Evaluating the Cost of DDIs (n = 3058)
| Baseline Characteristics | Patients Without DDI (n = 1529) | Patients With ≥1 DDI (n = 1529) | Standardized Difference, % |
|---|---|---|---|
| Male, No. (%) | 1142 (75) | 1130 (74) | 1.8 |
| Age, mean ± SD, y | 71.5 ± 5.1 | 71.1 ± 4.9 | 8.0 |
| HIV duration,a median [IQR], y | 16.2 [9.6–20.3] | 16.3 [9.9–20.9] | 2.8 |
| Diagnosis in 2016, No. (%) | 29 (2) | 18 (1) | 5.9 |
| 1 mo–10 y, No. (%) | 369 (24) | 392 (26) | 3.5 |
| 11–20 y, No. (%) | 714 (47) | 641 (42) | 9.6 |
| >20 y, No. (%) | 417 (27) | 478 (31) | 8.8 |
| Geographic repartition, No. (%) | |||
| Ile-de-France | 536 (35) | 539 (35) | 0.4 |
| Occitanie and Provence-Alpes-Côte D’Azur | 274 (18) | 269 (18) | 0.9 |
| Others regions | 719 (47) | 721 (47) | 0.3 |
| Concomitant diseases (non-HIV),b median [IQR] | 3 [1–4] | 3 [1–4] | 1.8 |
| Main concomitant diseases, No. (%) | |||
| Cardiovascular disease | 1068 (70) | 1043 (68) | 3.5 |
| Dyslipidemia | 708 (46) | 702 (46) | 0.8 |
| Anxiety, sleep disorders | 435 (28) | 416 (27) | 2.8 |
| Diabetes | 334 (22) | 337 (22) | 0.5 |
| Cancer | 198 (12) | 194 (13) | 1.0 |
| Hospitalizations during 2015, median [IQR] | 3 [0–8] | 3 [1–9] | 8.8 |
Abbreviations: ARV, antiretroviral; DDI, drug–drug interaction; IQR, interquartile range.
aHIV duration estimated from the date of the first long-term illness exemption related to HIV.
bTotal number of different diseases (HIV excluded). For more details about the determination of concomitant diseases, see the Appendix.
Compared with individuals without DDIs, the associated costs of DDIs from the propensity score–matched subcohort were $2693 for 1-year follow-up (P < .0001). This result was consistent with the sensitivity analysis excluding ARV costs ($1106 attributable to DDIs; P = .029).
DISCUSSION
In a high-income country with free access to cART, 1 out of 5 PLWH aged >65 years did not receive cART, in particular women and late presenters. Most PLWH not receiving ART were linked to care but not to HIV care. Polypharmacy, risk of toxicity, low perceived benefit, and stigmatization may account for this gap between diagnosis and treatment. The 1-year incidence of at least 1 DDI was high, with 16.8% of PLWH >65 years old involved.
Contraindicated interactions range in the literature from 1% to 15% for all ages [15–21] and from 5.0% to 6.6% in the 2 studies that focused on aging PLWH [11, 13]. These cross-sectional studies reported 1-day prevalence, and thus intercurrence of prescriptions was lower. Besides, these studies were mostly conducted at a single site, and they were dependent on prescribing habits. It was also expected that incidence would be higher for an older population due to polypharmacy. Two other studies reported a higher rate of DDI, 27% and 41%, but they had a broader definition of DDI than ours [22, 23].
In previous studies, the most frequent drug interactions reported with ARV therapy involved inhaled glucocorticoids, proton pump inhibitors (PPIs), statins, benzodiazepines, antidepressants, antipsychotics, and drugs for erectile dysfunction [13, 22]. Our study found similar results, except for benzodiazepines and erectile dysfunction drugs. In France, drugs for erectile dysfunction are not reimbursed, so they were not collected in the SNIIRAM database. In addition, most of the combinations between cART and benzodiazepines, antidepressants, or antipsychotics were not identified as a “Do Not Co-administer” DDIs on the Liverpool website.
The most frequent DDIs were combinations of protease inhibitors (PIs) or boosters (ritonavir or cobicistat) with inhaled glucocorticoids. For example, co-administration of fluticasone nasal spray and ritonavir increases fluticasone area under the curve (AUC) by ~350-fold [24]. A switch to a different glucocorticoid, which is not a substrate for CYP3A4 (eg, beclomethasone), should be considered. The second most frequent DDIs were combinations of rilpivirine or atazanavir with PPIs. For example, lansoprazole decreases unboosted atazanavir AUC by 94% [25]. Of note, atazanavir had the highest incidence of DDIs in our study (Figure 1). Removing the booster is only safe for atazanavir and can be attractive among aging PLWH with cardiovascular comorbidities [26]. However, it also increases the risk of subtherapeutic levels due to DDIs. We must acknowledge that atazanavir dosing was not accounted for in the determination of DDIs. Inappropriate PPI therapy is a problem in France and more widely in Europe [27]. In 2015, the American Geriatrics Society updated the Beers criteria for potentially inappropriate medication use in older adults, and PPIs have been added to the list of medications to avoid [28]. Amiodarone and piroxicam were ranked within the most frequently observed DDIs. Amiodarone is part of the Beers list, which recommends that amiodarone be avoided as firstline therapy unless the patient has heart failure or substantial left ventricular hypertrophy. In addition, the American Geriatrics Society recommends avoidance of chronic use of piroxicam. Older PLWH are more impacted by frailty than the older HIV-negative population; thus, careful application of the Beers criteria is even more important in PLWH. The American Geriatrics Society will re-update the Beers criteria shortly.
Polypharmacy and multimorbidity have long been identified as causing drug interactions [29]. Moreover, some ARV classes are more likely to generate drug interactions due to their pharmacokinetic profile, such as B-PIs, which are well known for their inhibitor enzymatic status [30]. However, the results related to the implication of age, with lower risk of DDIs with advancing age, is surprising. We hypothesize that prescribing physicians anticipated and paid more attention to the risk of DDIs in very advanced age individuals (ie, >85 years old) [31].
To our knowledge, this study was one of the first to estimate the associated costs of DDIs in an aging HIV population. Hellinger et al. [32] previously investigated the cost of using atazanavir and tenofovir without ritonavir (referred to as “boosting errors”) and found $4223 higher annual unadjusted cost compared with PLWH receiving the same combination with ritonavir. The cost of an individual living with HIV in our French study ($16 820) is consistent with that reported in the United States (about $18 600 per year) [33]. Avoidance of DDIs may decrease the annual cost of an individual living with HIV by 13.6% in France.
In France, cobicistat was only available coformulated with elvitegravir. Therefore, we were not able to compare the incidence of interactions between ritonavir and cobicistat. Both pharmacokinetic enhancers are strong inhibitors of cytochrome P450 (CYP) 3A4, but cobicistat is a more selective CYP inhibitor than ritonavir [34]. Ritonavir altered exposure to drugs primarily metabolized by CYP1A2, CYP2B6, CYP2C8, CYP2C9, and CYP2C19 or drugs undergoing glucuronidation. Therefore, switching from ritonavir to cobicistat may eventually require a dose adjustment of comedications. Nevertheless, the risk of interaction with both pharmacokinetic enhancers remains very high in our studied population with several comorbidities.
The risks and costs of DDIs are probably underestimated because we had no data about nonreimbursed drugs that can be responsible for several DDIs (eg, “over-the-counter” drugs, herbal products, drugs for erectile dysfunction, including PPIs, Hypericum extract, and sildenafil). Second, our analysis was based on drug deliveries, and we cannot know if and when drugs were actually taken by patients. In addition, we cannot know if physicians had adjusted the dose of a drug to limit the risk of interaction, especially for the combination of atazanavir and PPIs. So the DDIs identified are potential, and we cannot confirm that they were established in our population. Apart from the economic impact, we hypothesized that the increased cost associated with DDIs is the consequence of their associated morbidity. The proportion of patients receiving B-PI was relatively high in France during the study period. This may have contributed to the increase in incidence of DDIs in this population, as compared with other countries. However, the distribution of the prescribing habits did not influence our estimation of the 1-year incidence (Figure 1).
Integrase inhibitors (raltegravir or dolutegravir) were independent protective factors against DDI risk. Therefore, the safety of their use among PLWH aged 65 years and older needs to be discussed. Recent concerns over dolutegravir-related neuropsychiatric toxicity have emerged, particularly among older women living with HIV [35], possibly owning to an increased dolutegravir peak concentration among aging PLWH [36].
This study only investigated the ARV therapies available in 2016. Bictegravir, a new unboosted integrase inhibitor, is a substrate of hepatic isoenzyme CYP3A4 and uridine diphosphate glucuronosyltransferase (UGT) 1A1. Doravirine, a non-nucleosidic reverse transcriptase inhibitor, is also a substrate of CYP3A4. A list of comedications that are inducers or inhibitors of the different families of CYPs can help to predict expected DDIs [37]. For example, comedications that induce CYP3A4 (such as rifampin) are expected to reduce plasma concentrations of bictegravir or doravirine, whereas drugs that inhibit CYP3A4 (such as ketoconazole) are expected to increase the plasma concentrations of these ARV drugs.
In conclusion, the choice of antiretroviral therapy should be made following a discussion between providers (HIV specialists and other specialists), pharmacists, and patients, taking into account various patient factors including comorbidities, pill burden, and the risk of DDIs. Every effort to reduce polypharmacy, boosted antiretroviral agents, and thus DDIs, has the potential to improve the safety of aging HIV-infected patients, a vulnerable population that is likely to grow.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors wish to thank Fabien Chaillot and Jean-Jacques Dutheil from the Caen University Hospital for administrative assistance. The authors also aknowledge Medhi Gabbas and Fabien Belloc from the Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés for technical assistance with the database.
Financial support. This was an academic study funded by Centre Hospitalo-Universitaire de Caen and Caen Normandy University. The funder had no role in the design, conduct, analysis, or reporting of the study.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
SAS Code to Identify DDI
https://docs.google.com/document/d/16__df1B_b0lZwcZA8qnCQnBlTvdqoWebviwiP2rDIp0/edit?usp=sharing.
Determination of Concomitant Diseases
Concomitant diseases determined from both the long-term illness exemption list and the chronic use of drugs during 2016 (determined as all drugs delivered during at least 4 months). Drugs were identified thanks to the Anatomical Therapeutic Chemical (ATC) classification system of the World Health Organization. Only these chronic diseases have been collected:
- Alzheimer’s disease and other types of dementia include patients receiving at least 1 drug with ATC code N06D (“Anti-dementia drugs”) and patients with long-term illness ALD30 code No. 15.
- Anxiety and sleep disorders include patients receiving at least 1 drug with ATC code N05B (“Anxiolytics”) or N05C (“Hypnotics and sedatives”) and patients with long-term illness ALD30 code No. 23 with a CIM10 code F4.
- Behavioral addiction includes patients receiving at least 1 drug with ATC code N07B (“Drugs used in addictive disorders”) and patients with long-term illness ALD30 code No. 23 with a CIM10 code F1.
- Benign prostatic hypertrophy includes patients receiving at least 1 drug with ATC code G04C (“Drugs used in benign prostatic hypertrophy”).
- Cancer includes patients with long-term illness ALD30 code No. 30.
- Cardiovascular disease includes patients receiving at least 1 drug for cardiac disorders: ATC codes C01 (“Cardiac therapy”), C02 (“Antihypertensives”), C03 (“Diuretics”), C07 (“Beta blocking agents”), C08 (“Calcium channel blockers”), C09 (“Agents acting on the renin-angiotensin system”), and B01A (“Antithrombotic agents”) and patients with long-term illness ALD30 code No. 5 or 12 or 13.
- Chronic kidney disease includes patients with long-term illness ALD30 code No. 19.
- Chronic obstructive pulmonary disease includes patients receiving at least 1 drug with ATC code R03 (“Drugs for obstructive airway diseases”) and patients with long-term illness ALD30 code No. 14 with a CIM10 code J180, J41, J42, J45, or J96.
- Chronic viral hepatitis includes patients with long-term illness ALD30 code No. 6 with CIM10 code B18.
- Depression includes patients receiving at least 1 drug with ATC code N06A (“Antidepressants”) and patients with long-term illness ALD30 code No. 23 with a CIM10 code F33 or F32.
- Diabetes includes patients receiving at least 1 drug with ATC code A10 (“Drugs used in diabetes”) and patients with long-term illness ALD30 code No. 8.
- Dyslipidemia includes patients receiving at least 1 drug with ATC code C10 (“Lipid modifying agents”) and patients with long-term illness ALD30 code No. 17 with a CIM10 code E780.
- Dysthyroidia includes patients receiving at least 1 drug with ATC code H03 (“Thyroid therapy”) and patients with long-term illness ALD30 code No. 5 with a CIM10 code E0.
- Epilepsy includes patients receiving at least 1 drug with ATC code No. 3 (“Antiepileptics”) and patients with long-term illness ALD30 code No. 9 with a CIM10 code G40 to G419.
- Fibrosis and cirrhosis include patients with long-term illness ALD30 code No. 6 with CIM10 code K703 or K74.
- Gout includes patients receiving at least 1 drug with ATC code M04 (“Antigout preparations”) and patients with long-term illness ALD30 code No. 99 with a CIM10 code M1.
- Inflammatory bowel disease includes patients with long-term illness ALD30 code No. 24.
- Mood and personality disorders include patients receiving at least 1 drug with ATC code N05A (“Antipsychotics”) and patients with long-term illness ALD30 code No. 23 with a CIM10 code F2 or F6.
- Multiple sclerosis includes patients with long-term illness ALD30 code No. 25 with a CIM10 code G35, G36, or G37.
- Paraplegia includes patients with long-term illness ALD30 code No. 20.
- Parkinson disease, includes patients receiving at least 1 drug with ATC code N04 (“Anti-Parkinson drugs”) and patients with long-term illness ALD30 code No. 16.
- Rheumatoid arthritis and spondylarthritis include patients with long-term illness ALD30 code No. 22 or 27.
- Stroke includes patients with long-term illness ALD30 code No. 1.
- Tuberculosis includes patients with long-term illness ALD30 code No. 29.
References
- 1. Samji H, Cescon A, Hogg RS, et al. . North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet Lond Engl 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allavena C, Hanf M, Rey D, et al. . Dat’AIDS Study Group Antiretroviral exposure and comorbidities in an aging HIV-infected population: the challenge of geriatric patients. PLoS One 2018; 13:e0203895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC. HIV among people aged 50 and over | age | HIV by Group | HIV/AIDS 2017. https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed 5 January 2018.
- 5. Guaraldi G, Orlando G, Zona S, et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 6. Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep 2016; 13:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Effros RB, Fletcher CV, Gebo K, et al. . Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008; 47:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004; 57:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nozza S, Malagoli A, Maia L, et al. . GEPPO Study Group Antiretroviral therapy in geriatric HIV patients: the GEPPO cohort study. J Antimicrob Chemother 2017; 72:2879–86. [DOI] [PubMed] [Google Scholar]
- 10. Clevenbergh P, Corcostegui M, Gérard D, et al. . Iatrogenic Cushing’s syndrome in an HIV-infected patient treated with inhaled corticosteroids (fluticasone propionate) and low dose ritonavir enhanced PI containing regimen. J Infect 2002; 44:194–5. [DOI] [PubMed] [Google Scholar]
- 11. Molas E, Luque S, Retamero A, et al. . Frequency and severity of potential drug interactions in a cohort of HIV-infected patients identified through a multidisciplinary team. HIV Clin Trials 2018; 19:1–7. [DOI] [PubMed] [Google Scholar]
- 12. Hare CB, Vu MP, Grunfeld C, Lampiris HW. Simvastatin-nelfinavir interaction implicated in rhabdomyolysis and death. Clin Infect Dis 2002; 35:e111–2. [DOI] [PubMed] [Google Scholar]
- 13. Bastida C, Grau A, Márquez M, et al. . Polypharmacy and potential drug-drug interactions in an HIV-infected elderly population. Farm Hosp 2017; 41:618–24. [DOI] [PubMed] [Google Scholar]
- 14. ameli.fr - Rapport d’activité 2016. 2017. https://www.ameli.fr/l-assurance-maladie/connaitre-l-assurance-maladie/rapport-d-activite-2016.php. Accessed 28 December 2017.
- 15. Siefried KJ, Mao L, Cysique LA, et al. . PAART study investigators Concomitant medication polypharmacy, interactions and imperfect adherence are common in Australian adults on suppressive antiretroviral therapy. AIDS 2018; 32:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baecke C, Gyssens IC, Decoutere L, et al. . Prevalence of drug-drug interactions in the era of HIV integrase inhibitors: a retrospective clinical study. Neth J Med 2017; 75:235–40. [PubMed] [Google Scholar]
- 17. Jakeman B, Nasiri M, Ruth L, et al. . Comparing the frequencies of contraindicated drug-drug interactions between differing antiretroviral regimens in HIV-infected patients. Ann Pharmacother 2017; 51:365–72. [DOI] [PubMed] [Google Scholar]
- 18. Iniesta-Navalón C, Franco-Miguel JJ, Gascón-Cánovas JJ, Rentero-Redondo L. Identification of potential clinically significant drug interactions in HIV-infected patients: a comprehensive therapeutic approach. HIV Med 2015; 16:273–9. [DOI] [PubMed] [Google Scholar]
- 19. Holtzman C, Armon C, Tedaldi E, et al. . and the HOPS Investigators Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013; 28:1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tseng A, Szadkowski L, Walmsley S, et al. . Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother 2013; 47:1429–39. [DOI] [PubMed] [Google Scholar]
- 21. Rastegar DA, Knight AM, Monolakis JS. Antiretroviral medication errors among hospitalized patients with HIV infection. Clin Infect Dis 2006; 43:933–8. [DOI] [PubMed] [Google Scholar]
- 22. Miller CD, El-Kholi R, Faragon JJ, Lodise TP. Prevalence and risk factors for clinically significant drug interactions with antiretroviral therapy. Pharmacotherapy 2007; 27:1379–86. [DOI] [PubMed] [Google Scholar]
- 23. Evans-Jones JG, Cottle LE, Back DJ, et al. . Recognition of risk for clinically significant drug interactions among HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2010; 50:1419–21. [DOI] [PubMed] [Google Scholar]
- 24. Liverpool HIV interactions - ritonavir + fluticasone. 2018. https://www.hiv-druginteractions.org/interactions/69555. Accessed 4 January 2018.
- 25. Liverpool HIV interactions - atazanavir + lansoprazole. 2018. https://www.hiv-druginteractions.org/interactions/68972. Accessed 4 January 2018.
- 26. Hocqueloux L, Choisy P, Moal GL, et al. . Pharmacologic Boosting of Atazanavir in Maintenance HIV-1 Therapy: The COREYA Propensity-Score Adjusted Study. PLOS ONE 2012; 7:e49289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lanas A. We are using too many PPIs, and we need to stop: a European perspective. Am J Gastroenterol 2016; 111:1085–6. [DOI] [PubMed] [Google Scholar]
- 28. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–46. [DOI] [PubMed] [Google Scholar]
- 29.ICH experts working group. ICH E7 Guideline - Studies in support of special populations: Geriatrics. 1993. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E7/Step4/E7_Guideline.pdf. Accessed 3 January 2018.
- 30. Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother 2004; 53:4–9. [DOI] [PubMed] [Google Scholar]
- 31. Günthard HF, Saag MS, Benson CA, et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hellinger FJ, Encinosa WE. The cost and incidence of prescribing errors among privately insured HIV patients. PharmacoEconomics 2010; 28:23–34. [DOI] [PubMed] [Google Scholar]
- 33. Chen RY, Accortt NA, Westfall AO, et al. . Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis 2006; 42:1003–10. [DOI] [PubMed] [Google Scholar]
- 34. Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug–drug interaction profiles with co-medications. J Antimicrob Chemother 2016; 71:1755–1758. [DOI] [PubMed] [Google Scholar]
- 35. Hoffmann C, Welz T, Sabranski M, et al. . Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 2017; 18:56–63. [DOI] [PubMed] [Google Scholar]
- 36. Elliot ER, Wang X, Singh S, et al. . Increased dolutegravir peak concentrations in people living with human immunodeficiency virus aged 60 and over, and analysis of sleep quality and cognition. Clin Infect Dis 2019; 68:87–95. [DOI] [PubMed] [Google Scholar]
- 37. Flockhart DA. Drug Interactions: Cytochrome P450 drug interaction table Indiana University School of Medicine; 2007. Available at: https://drug-interactions.medicine.iu.edu. Accessed 20 December 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.