Abstract
We report on the mineralogy, petrography, and oxygen isotopic compositions of primary olivine and plagioclase/feldspathic mesostases in chondrules and of secondary magnetite and fayalite in chondrules and matrix of an oxidized Bali-like CV3.1 carbonaceous chondrite, Kaba. In this meteorite, compositionally nearly pure fayalite (Fa98–100) associates with hedenbergite (Fs~50Wo~50), magnetite, and Fe,Ni-sulfides. There are several textural occurrences of this mineral paragenesis: (i) coarse-grained intergrowths in interchondrule matrix, (ii) veins starting at the opaque nodules in the peripheries of type I chondrules and crosscutting finegrained rims around them, and (iii) rims overgrowing olivine of type I and type II chondrule fragments. Oxygen isotopic compositions of fayalite and magnetite are in disequilibrium with chondrule olivines. On a three-isotope oxygen diagram, δ17O vs. δ18O, compositions of olivine plot along primitive chondrule minerals (PCM) line having a slope of ~1.0; deviations from the terrestrial fractionation line, Δ17O = δ17O − 0.52 × δ18O, range from ~−8‰ to ~−5‰. In contrast, fayalite and magnetite plot along mass-dependent fractionation line with a slope of ~0.5; their δ18O values range from −1 to ~+9‰; Δ17O is nearly constant (average ± 2SE = −1.5±1‰). Oxygen isotopic compositions of chondrule plagioclase and feldspathic mesostases are in disequilibrium with chondrule olivines: they deviate to the right from the PCM line by ~12‰ and plot close to the mass-dependent fractionation line defined by fayalite and magnetite.
Based on the mineralogy, petrography, oxygen isotopic compositions of fayalite and magnetite, and the previously published thermodynamic analysis of the fayalite-bearing assemblages in ordinary and carbonaceous chondrites, we conclude that Kaba fayalite and magnetite formed during aqueous fluid-rock interaction at low water/rock ratio (0.1–0.2) and elevated temperatures (~200–300°C) on the CV chondrite parent asteroid. The Δ17O values of Kaba fayalite and magnetite (−1.5±1‰) correspond to Δ17O of aqueous fluid that operated on the CV chondrite parent asteroid and resulted in its alteration. Plagioclase and feldspathic mesostases in Kaba chondrules experienced postcrystallization oxygen isotopic exchange with this 16O-depleted fluid; olivine grains retained their original compositions acquired during chondrule melts crystallization. The inferred oxygen isotopic exchange in Kaba chondrules appear to have not affected their Al-Mg isotope systematics.
Keywords: Kaba, chondrules, oxygen isotopes, fayalite, magnetite, oxygen-isotope exchange
Introduction and Previous Studies
Kaba is an oxidized Bali-like CVOxB3.1 (Vigarano type) carbonaceous chondrite that experienced aqueous/hydrothermal alteration, but largely avoided subsequent thermal and/or shock metamorphism (e.g., KROT et al. 1998; BONAL et al. 2006). Nearly pure fayalite was first described in the CVOxB chondrites, Kaba and Mokoia, by HUA & BUSECK (1995). Fayalite grains in these meteorites associate with magnetite, troilite, and pentlandite, reach up to 100 μm in size, and occur in the matrix, chondrules, and finegrained rims around chondrules and Ca,Al-rich inclusions (CAIs). HUA & BUSECK (1995) suggested fayalite in CV chondrites formed through nebular reaction of gaseous Sio, released by decomposition of enstatite, with magnetite and sulfides under highly oxidizing conditions, with H2O/H2 ratio much higher than the canonical solar nebula of ~5×10−4. Subsequently, based on the mineralogical observations, including the presence of fayalite-bearing veins crosscutting fine-grained rims around chondrules, thermodynamic analysis, and 53Mn-53Cr dating, it was concluded that fayalite-bearing assemblages in Kaba and Mokoia formed during aqueous fluid-rock interaction Ma after formation of CV CAIs at ~200–300°C and low water-to-rock ratio (0.1–0.2) on the CV chondrite parent asteroid (e.g., KROT et al. 1998; CHOI et al. 2000; HUA et al. 2005; ZOLOTOV et al. 2006; BREARLEY & KROT 2013; DOYLE et al. 2015).
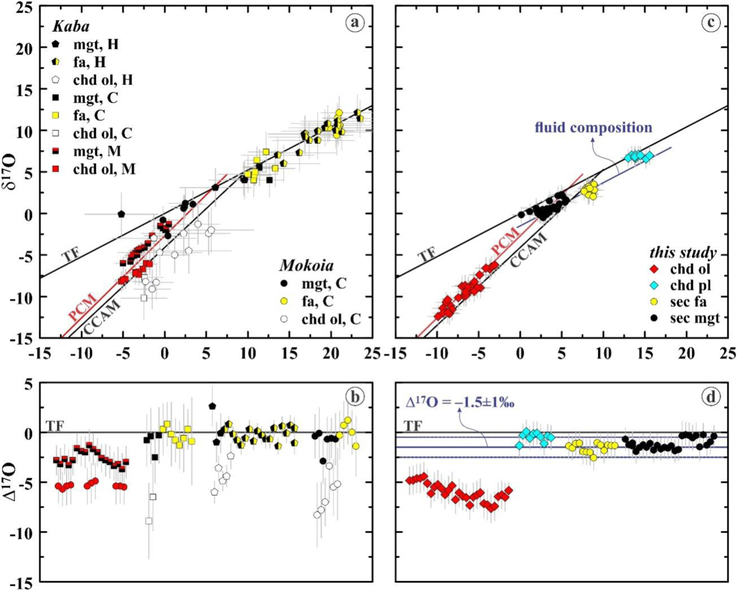
CHOI et al. (2000) and HUA et al. (2005) reported first relatively low-precision oxygen isotopic compositions (2σ for δ17O and δ18O ~3–4‰) of fayalite and magnetite in Kaba and Mokoia (Figs. 1a,b). Both groups found that on a three-isotope oxygen diagram, the compositions of magnetite and fayalite plot along a mass-dependent fractionation line close to the terrestrial fractionation (TF) line (average Δ17O ~−0.2±1.5‰, 2SE). There is, however, an important disagreement between these datasets: compositions of the Kaba fayalite and magnetite reported by HUA et al. (2005) have significantly different δ18O than those reported by CHOI et al. (2000), −5 to +10‰ and +17 to +24‰ vs. +10 to +13‰ and +10 to +13‰, respectively. Assuming contemporaneous formation of magnetite and fayalite in equilibrium with an aqueous solution, these differences may significantly affect the temperature estimates of fayalite and magnetite formation (ZHENG 1991, 1993).
Figure 1:
(a, c) Three-isotope oxygen diagram and (b, d) Δ17O values of the chondrule olivine phenocrysts (chd ol), chondrule plagioclase/feldspathic mesostasis (chd pl), and secondary fayalite (secfa) and magnetite (sec mgt) from the Bali-like oxidized CV3 chondrites, Kaba and Mokoia. (a, b) — Previously published data from (CHOI et al 2000) — (C), (HUA et al 2005) — (H), and (MARROCCHI et al. 2016) — (M). (c, d) — this study. Carbonaceous chondrite anhydrous mineral (CCAM) line (CLAYTON et al. 1977), primitive chondrule minerals (PCM) line (USHIKUBO et al. 2012), and the terrestrìalfractionation (TF) line are shown for reference. Chondrule olivines plot along the PCM line; magnetite andfayalite plot along mass-dependentfractionation line with Δ17O of ~−1.5‰, which corresponds to Δ17O ofan aqueousfluid resulting on formation offayalite and magnetite. Compositions of chondrule olivines are in isotope disequilibrium with chondrule plagioclase/feldspathic mesostases; the latter, however, are in isotopic equilibrium with secondary fayalite and magnetite, suggesting oxygen isotopic exchange with the aqueous fluid
Recently, MARROCCHI et al. (2016) reported high-precision oxygen isotopic compositions (2σ for δ17O and δ17O ~0.5–1‰,) of magnetite and olivine in Kaba chondrnles (Figs. 1a,b). The magnetites show a range of δ18O (−5 to 3.3‰) and Δ17O (−3.7 to 0.1%) and are in isotope disequilibrium with chondrule olivines (Δ17O ~−5‰). On a three-isotope oxygen diagram, the Kaba magnetites plot close to the primitive chondrule minerals (PCM) line and having a slope of ~1.0 (Fig. 1a). The PCM line was defined by high-precision oxygen isotopic measurements of chondrule silicates in Acfer 094 (USHIKUBO et al. 2012), which is one of the most primitive (C3.00) carbonaceous chondrites (GRESHAKE 1997). In contrast to previously reached conclusions (CHOI et al. 2000; HUE et al. 2005), MARROCCHI et al. (2016) suggested that magnetite in Kaba chondrules crystallized from chondrule melt under highly-oxidizing conditions. We note, however, that considering the observed range in δ18O of ~8‰ and the typical uncertainties of Δ17O of magnetite reported by MARROCCHI et al. (2016), ~1.5‰ (2σ), the limited range of Δ17O does not allow us to distinguish the mass-independent and the mass-dependent fractionation lines (Figs. 1a,b). Oxygen isotopic compositions of fayalite, which appear to have formed contemporaneously with magnetite (e.g., KROT et al. 1998; DOYLE et al. 2005), could help to resolve this issue and provide additional constraints on the formation conditions of fayalite-magnetite-bearing assemblages.
In order to test the mechanism of magnetite formation proposed by MARROCCHI et al. (2016), to contrain oxygen-isotope composition of aqueous fluids on the CV parent asteroid, and to investigate a possible role of the fluid-rock interaction in oxygen isotopic exchange of high-temperature primary minerals in chondrules, we studied oxygen isotopic compositions of chondrule olivines and plagioclase/feldspathic mesostases, as well as fayalite and magnetite in chondrules and matrix from Kaba with the Universisty of Hawai’i (UH) Cameca ims-1280 secondary ion mass-spectrometer (SIMS or ion microprobe).
Analytical procedures
The mineralogy and petrography of fayalite, magnetite, and chondrules in Kaba were studied in backscattered electron (BSE) images using the UHJXA-8500F field emission electron microprobe equipped with an energy dispersive spectrometer. Quantitative electron microprobe analyses were performed with JXA-8500F operated at 15 kV accelerating voltage, 15 nA beam current, and fully focused beam using five wavelength-dispersive spectroscopy detectors. For each element, counting times on both peak and background were 30 sec. Minerals with known chemical compositions were used as standards. A ZAF matrix correction method was used (a phi-rho-z correction; ARMSTRONG 1988). The element detection limits with these analytical conditions were (in wt%): Al2O3 and K2O (0.01), SiO2, MgO, CaO, and Na2O (0.02), TiO2, Cr2O3, and MnO (0.03), and FeO (0.04).
Oxygen-isotope compositions of fayalite, magnetite, ferrmagnesian olivines, and plagioclase/feldspathic mesostasis were measured in situ with the UH Cameca ims-1280 SIMS using a 15–20 pA focused Cs+ primary ion beam of 20 keV impact energy and ~2 μm in diameter. The secondary ion mass spectrometer was operated at −10 keV with a 50 eV energy window. 16O− and 18O− were measured on multicollector Faraday Cup (FC) and electron multiplier (EM), respectively. 17O− was measured with the axial EM. The mass resolving power (m/Δm) for 16O− and 18O− was ~2000, and that for 17O− was ~5500, sufficient to separate interfering 16OH−. A normal-incidence electron flood gun was used for charge compensation. To verify the positions of the sputtered regions, the spots analyzed for oxygen isotopes were studied with BSE images using the UH JEOL JXA-8500F electron microprobe before and after SIMS measurements.
Oxygen-isotope compositions are reported as δ17O and δ18O, deviations from Vienna Standard Mean Ocean Water (SMOW; 17O/16OVSMOW = 0.000380; 18O/16OVSMOW = 0.002005; DE LAETER et al. 2003) in parts per thousand: δ17,18OSMOW = [(17,18O/16Osample) / (17,18O/16OVSMOW) − 1] × 1000, and as Δ17O.
Data for plagioclase/feldspathic mesostasis, ferramagnesian olivine, fayalite, and magnetite were corrected using Miyke-jima anorthite, San Carlos olivine, terrestrial fayalite and magnetite standards, respectively. The reported 2σ uncertainties include both the internal measurement precision on an individual analysis and the external reproducibility for standard measurements during a given analytical session. The external reproducibility on the multiple analyses of the standard was ~1.5–2‰ (2σ) for both δ17O and δ18O.
Results
Mineralogy and petrography
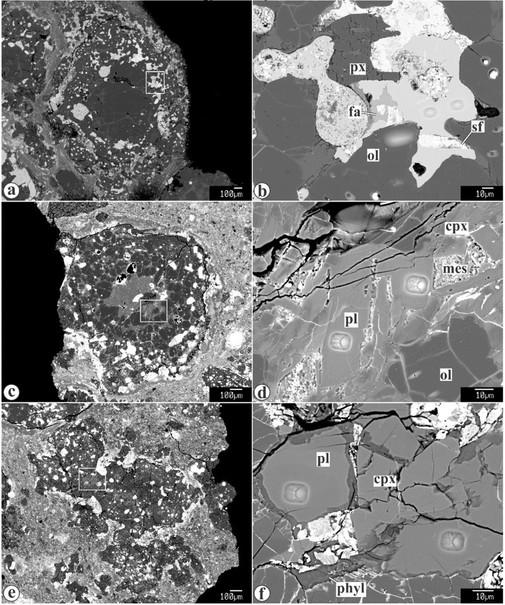
Fayalite-magnetite±hedenbergite assemblages occur in all Kaba components — refractory inclusions, chondrules, and matrix, and have been previously characterized in detail (HUA & BUSECK 1995; KROT et al. 1998; this study). In Figures 2 and 3a,b, we illustrated typical occurrences of Kaba fayalite and magnetite, including (i) coarse fayalite grains inside type I chondrules (Figs. 2a,b), (ii) fayalite-magnetite-sulfide veins crosscutting fine-grained rims around chondrules (Figs. 2c,d), (iii) fayalite±hedenbergite overgrowths on chondrule fragments (Fig. 2e), and (iv) coarse fayalite grains in the matrix (Fig. 2f). All textural occurrences of fayalite grains measured are nearly pure Fe2SiO4 (Fa>98)
Figure 2:

Backscattered electron images of textural occurrences of secondary fayalite in Kaba. chr = chromite;fa = fayalite; hed = hedenbergite; mes = mesostasis; mgt = magnetite; ol = ferromagnesian olivine; sf= sulfide. Fayalite associated with magnetite, hedenbergite, and sulfides (a, b) replaces Fe,Ni-metal nodules in type I chondrules, (c, d) forms veins crosscuttingfine-grained rims around chondrules, (e) overgrowths chondrule olivines, and (f) occurs as euhedral grains in the matrix.
Figure 3:
Backscattered electron images of Kaba chondrules with magnetite, fayalite, plagioclase and olivinephenocrysts measuredfor oxygen-isotope compositions. Regions outlined in (a, c, and d) are shown in detail in (b, d, and f, respectively). cpx = high-Capyroxene;fa = fayalite; mes = mesostasis; mgt = magnetite; ol = olivine;pl = plagioclase/feldspathic mesostasis;phyl = phyllosilicates;px = low-Ca pyroxene; sf= Fe,Ni-sulfides.
There are two major textural occurrences of magnetite in Kaba: magnetite-sulfide nodules replacing Fe,Ni-metal in type I chondrules, and massive subhedral magnetite grains, often with elongated inclusions of Fe,Ni-sulfides, overgrowing magnetite-sulfide nodules (Figs. 3a,b). Nodular magnetite contains high chromium contents (up to 1 wt%), whereas massive magnetite is compositionally pure FeFe2O4.
Plagioclase and glassy mesostasis in magnetite±fayalite-bearing chondrules are replaced to various degrees by phyllosilicates and unidentified FeO-rich secondary minerals (Figs. 3b–f).
Oxygen isotopic compositions
Oxygen isotopic compositions of chondrule olivines, chondrule plagioclase/feldspathic mesostasis, fayalite, and magnetite are plotted in Figures 1c and d. Chondrule olivines show a range of Δ17O (−7.6 to −4.4‰) and on a three-isotope oxygen diagram plot along the PCM line. The similar results for chondrule olivine and low-Ca pyroxene phenocrysts in Kaba were reported by HERTWIG et al. (2016). In contrast to isotopically uniform chondrules from Acfer 094 (USHIKUBO et al. 2012), the plagioclase/feldspathic mesostasis in Kaba chondrules are in oxygen-isotope disequilibrium with olivine phenocrysts; the former plot significantly to the right from the PCM line and have Δ17O of −0.5±0.9‰.
All three major textural occurrences of fayalite analyzed (matrix, chondrule overgrowths and coarse grains inside chondrules) have similar oxygen isotopic compositions: δ18O range from ~7 to ~9‰; the average Δ17O is ~−1.5±1.3‰ (2SE). Massive magnetite grains in type I chondrules have average Δ17O ~−1.2±1.1‰, and a relatively small range of δ18O, from ~−1 to ~+6‰. On a three-isotope oxygen diagram (Fig. 1c), the compositions of fayalite and magnetite plot along a single mass-dependent fractionation line with Δ17O of ~−1.5‰. Within uncertainty of our measurements, compositions of chondrule plagioclase/mesostasis plot along this line as well (Figs. 1c,d).
Discussion
Oxygen isotopic exchange during aqueous fluid-rock interaction
Mineralogical observations and thermodynamic analysis suggest that fayalite and magnetite in Kaba resulted from fluid-assisted metasomatic alteration at ~200–300°C on the CV parent asteroid (KROT et al. 1998; ZOLOTOV et al. 2006; BREARLEY & KROT 2013; DOYLE et al. 2015). Since these minerals formed by oxidation of Fe,Ni-metal and/or by direct precipitation from an aqueous fluid (KROT et al. 1998; BREARLEY & KROT 2013), their Δ17O of−1.5±1‰ must correspond to Δ17O of the fluid (KROT et al. 2015).
In most chondrules from the least metamorphosed chondrites [petrologic type <3.0, e.g., Acfer 094 (C3.0 ungrouped), Yamato-81020 (CO3.0), and CR2–3s], olivine and low-Ca pyroxene phenocrysts, plagioclase, and glassy mesostases are in oxygen isotopic equilibrium, i.e., within an individual chondrule, these minerals have the same Δ17O (within uncertainty of SIMS measurements), suggesting crystallization from a melt having nearly constant Δ17O (e.g., USHIKUBO et al. 2012; TENNER et al. 2013, 2015). In contrast, olivine phenocrysts and plagioclase/feldspathic mesostasis in Kaba chondrules are in oxygen isotopic disequilibrium (Figs. 1c,d), indicating postcrystallization isotope exchange that affected only the plagioclase/feldspathic mesostasis. On a three-isotope oxygen diagram (Fig. 1c), the olivine phenocrysts plot along the PCM line, whereas the plagioclase grains deviate to the right from the PCM line and plot along mass-dependent fractionation line with Δ17O of ~−1.5‰ defined by the Kaba magnetite and fayalite, suggesting the exchange resulted from interaction with the fluid. Plagioclase/feldspathic mesostasis in Kaba chondrules have nearly the same (within uncertainty of our SIMS measurements) Δ17O as magnetite and fayalite, indicating that the exchange was nearly complete.
It has been previously shown that the presence of water leads to significantly enhanced oxygen-diffusion rates in many silicates compared to those under dry conditions (e.g., FARVER 2010 and references therein). In plagioclase, oxygen-diffusion coefficient under wet (hydrothermal) conditions (GILETTI et al. 1978) is considerably higher than that under dry conditions (YURIMOTO et al. 1989; RYERSON & MCKEEGAN 1994) (Fig. 4a). Under the inferred temperature of metasomatic alteration experienced by Kaba, 200–300°C (ZOLOTOV et al. 2006), a 10 μm-sized crystal of anorthite (typical grain sizes of plagioclase in Kaba chondrules) would completely exchange its oxygen isotopic composition with an aqueous fluid within ~0.1–0.001 Ma (Fig. 4b). This time scale may not be unreasonable for a duration of alteration on the CV parent asteroid. We note, however, that the duration for the presence of aqueous fluid on the CV parent asteroid is still poorly constrained.
Figure 4:
(a) Arrhenius plot of oxygen diffusion coefficients in spinel, åkermanite, gehlenite, diopside, forsterite, and anorthite under dry and wet conditions. Data from GILETTI et al (1978), OISHI & ANDO (1984), FARVER (1989), YURIMOTO et al (1989), and RYERSON & MCKEEGAN (1994). (b) Temperature and time dependencefor a complete oxygen isotopic exchange in a 10 μm-sized anorthite (typical size of plagioclase grains in Kaba chondrules) under dry (red line) and wet (blue line) conditions. Under the estimated conditions of metasomatic alteration ofKaba (200–300°C), it takes between 0.1 and 0.001 Ma to exchange oxygen isotopic cormposition of plagioclase ivith an aqueous fuid.
Conclusions
We reported on the mineralogy, petrography, and in situ measured oxygen-isotope compositions of primary high-temperature minerals in chondrules (olivines and plagioclase/feldspathic mesostasis) and of secondary fayalite and magnetite in chondrules and matrix of the Kaba (CVoxB3.1) carbonaceous chondrite.
Oxygen isotopic compositions of fayalite and magnetite appear to have equilibrated with external reservoir(s) having nearly the same Δ17O: on a three-isotope oxygen diagram, their compositions plot along mass-dependent fractionation line with Δ17O of ~−1.5‰. Magnetite and fayalite are in isotope disequilibrium with chondrule olivines, which plot along a slope-1 line and show a range of Δ17O, from ~−8‰ to ~−5‰. These data and the observed spread of δ18O of magnetite and fayalite (from ‒1 to +9‰) are inconsistent with high-temperature formation of magnetite during crystallization of chondrule melts (MARROCCHI et al. 2016), instead, they are consistent with formation of magnetite and fayalite during relatively low-temperature aqueous fluid-rock interaction (KROT et al. 1998; CHOI et al. 2000; DOYLE et al. 2017). Compositions of chondrule plagioclase/feldspthic mesostases are in isotopic disequilibrium with chondrule olivines: their Δ17O are similar to those of fayalite and magnetite.
Based on the mineralogy, petrography, oxygen isotopic compositions of fayalite and magnetite, and thermodynamic analysis of the fayalite-bearing assemblages in chondrites (ZOLOTOV et al. 2006), we conclude that fayalite and magnetite in Kaba formed during aqueous fluid-rock interaction at low water/rock ratio (0.1–0.2) and elevated temperatures (~200–300°C) on the CV chondrite parent asteroid. Therefore, the Δ17O values of the fayalite and magnetite correspond to Δ17O of aqueous fluid that operated on the CV chondrite parent asteroid and resulted in its alteration. Plagioclase and feldspathic mesostasis in Kaba chondrules experienced postcrystallization oxygen isotopic exchange with this aqueous fluid; olivine grains retained their original compositions. The inferred oxygen isotopic exchange in Kaba chondrules appear to have not affected their Al-Mg isotope systematics (NAGASHIMA et al. 2017).
Acknowledgements:
We would like to thank all organizers of the Conference at the University of Debrecen. We thank Dr. S. S. Russell (Natural History Museum, London), Dr. M. Gounelle (Natural History Museum, Paris), Dr. M. I. Petaev (Smithsonian Institution), and Dr. M. Wadhwa (Arizona State University) for providing polished thin sections of Kaba. We thank Drs. A. E. Rubin and J. T. Wasson for their comments and suggestions which helped to improve the paper. Handling the paper by Dr. R. McIntosh is highly appreciated. This work was supported by the NASA Emerging Worlds program grant NNX17A22G (A. N. Krot, P.I.).
References
- ARMSTRONG JT 1988: Quantitative Analysis of Silicate and Oxide Minerals: Comparison of Monte Carlo, ZAF, and Phi-Rho-Z Procedures In: NEWBURY DE. ed.: Microbeam Analysis. San Francisco Press, San Francisco, pp. 239–246. [Google Scholar]
- BONAL L, BOUROT-DENISE M, QUIRICO E, MONTAGNAC G 2006: Determination of the petrologic type of CV3 chondrites by Raman spectroscopy of included organic matter. Geochimica et Cosmochimica Acta 70, 1849–1863. [Google Scholar]
- BREARLEY AJ, KROT AN 2013: Metasomatism in the Early Solar System: The Record from Chondritic Meteorites In: Harlow DE, Austrheim H. eds.: Metasomatism and the Chemical Transformation of Rock — Lecture Notes in Earth System Sciences, Springer-Verlag, Berlin-Heidelberg, pp. 659–789. [Google Scholar]
- CHOI B-G, KROT AN, WASSON JT 2000: Oxygen-isotopes in magnetite and fayalite in CV chondrites Kaba and Mokoia. Meteoritics & Planetary Sáence 35, 1239–1249. [Google Scholar]
- CLAYTON RN, ONUMA N, GROSSMAN L, MAYEDA TK 1977: Distribution of the pre-solar component in Allende and other carbonaceous chondrites. Earth & Planetary Science Letters 34, 209–224. [Google Scholar]
- DE LAETER JR, BÖHLKE JK, DE BIÈVRE P, HIDAKA H, PEISER HS, ROSMAN KJR, TAYLOR PDP 2003: Atomic weights of the elements: Review 2000 (IUPAC Technical Report). Pure and Applied Chemistry 75, 683–800. [Google Scholar]
- DOYLE PM, JOGO K, NAGASHIMA K, KROT AN, WAKITA S, CIESLA FJ, HUTCHEON ID 2015: Early aqueous activity on the carbonaceous and ordinary chondrite parent asteroids recorded by secondary fayalite. Nature Communications 6, 1–10. [DOI] [PubMed] [Google Scholar]
- FARVER JR 1989: Oxygen self-diffusion in diopside with application to cooling rate determinations. Earth & Planetary Science Letters 92, 386–396. [Google Scholar]
- FARVER JR 2010: Oxygen and Hydrogen Diffusion in Minerals. Reviews in Mineralogy and Geochemistry 72, 447–507. [Google Scholar]
- GILETTI BJ, SEMET MP, YUND RA 1978: Studies in diffusion - III. Oxygen in feldspars: an ion microprobe determination. Geochimica et Cosmochimica Acta 42, 45–57. [Google Scholar]
- GRESHAKE A 1997: The primitive matrix components of the unique carbonaceous chondrite Acfer 094; a TEM study. Geochimica et Cosmochimica Acta 61, 437–452. [DOI] [PubMed] [Google Scholar]
- HERTWIG A, DEFOUILLOY C, KITA NT 2016: SIMS oxygen isotope study of chondrules in the least metamorphosed CV3 chondrite Kaba. 79th Annual Meeting of the Meteoritical Society, abstract number: 6472. [Google Scholar]
- HUA X, BUSECK PR 1995: Fayalite in the Kaba and Mokoia carbonaceous chondrites. Geochimica et Cosmochimica Acta 59, 563–578. [Google Scholar]
- HUA X, HUSS GR, TACHIBANA S, SHARP TG 2005: Oxygen, silicon, and Mn-Cr isotopes of fayalite in the Kaba oxidized CV3 chondrite:Constraints for its formation history. Geochimica et Cosmochimica Acta 69, 1333–1348. [Google Scholar]
- KROT AN, PETAEV MI, SCOTT ERD, CHOI B-G, ZOLENSKY ME, KEIL K 1998: Progressive alteration in CV3 chondrites: More evidence for asteroidal alteration. Meteoritics & Planetary Sáence 33, 1065–1085. [Google Scholar]
- KROT AN, ALEXANDER CMO’D, NAGASHIMA K, CIESLA FJ, FUJIYA W, BONAL L 2015: Aqueous Activity and Sources of Water on the Chondrite Parent Asteroids In: MICHEL P, DEMEO FE, BOTTKE WF. eds.: Asteroids IV, pp. 635–661. [Google Scholar]
- MARROCCHI Y, CHAUSSIDON M, PIANI L, LIBOUREL G 2016: Early scattering of the solar protoplanetary disk recorded in meteoritic chondrules. Science Advances 2, 11601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGASHIMA K, KROT AN, KOMATSU M 2017: 26Al-26Mg systematics in chondrules from Kaba and Yamato 980145 CV3 chondrites. Geochimica et Cosmochimica Acta 201, 303–319. [Google Scholar]
- OISHI Y, ANDO K 1984: Oxygen self-diffusion coefficient in single-crystal forsterite In: Sunagawa I. ed.: Materials science of the Earth’s interior Terra Scientific, Tokyo, pp. 271–280. [Google Scholar]
- RYERSON FJ, MCKEEGAN KD 1994: Determination of oxygen self-diffusion in åkermanite, anorthite, diopside, and spinel: Implications for oxygen isotopic anomalies and the thermal histories of Ca-Al-rich inclusions. Geochimica et Cosmochimica Acta 58, 3713–3734. [Google Scholar]
- TENNER TJ, USHIKUBO T, KURAHASHI E, KITA NT, NAGAHARA H 2013: Oxygen isotope systematics of chondrule phenocrysts from the CO3.0 chondrite Yamato 81020: evidence for two distinct oxygen isotope reservoirs. Geochimica et Cosmochimica Acta 102, 226–245. [Google Scholar]
- TENNER TJ, NAKASHIMA D, USHIKUBO T, KITA NT, WEISBERG MK 2015: Oxygen isotope ratios of FeO-poor chondrules in CR3 chondrites: Influence of dust enrichment and H2O during chondrule formation. Geochimica et Cosmochimica Acta 148, 228–250. [Google Scholar]
- USHIKUBO T, KIMURA M, KITA NT, VALLEY JW 2012: Primordial oxygen isotope reservoirs of the solar nebula recorded in chondrules in Acfer 094 carbonaceous chondrite. Geochimica et Cosmochimica Acta 90, 242–264. [Google Scholar]
- YURIMOTO H, MORIOKA M, NAGASAWA H 1989: Diffusion in single crystals of melilite: I. Oxygen. Geochimica et Cosmochimica Acta 53, 2387–2394. [Google Scholar]
- ZHENG Y-F 1991: Calculation of oxygen isotope fractionation in metal oxides. Geochimica et Cosmochimica Acta 55, 2299–2307. [Google Scholar]
- ZHENG Y-F 1993: Calculation of oxygen isotope fractionation in anhydrous silicate minerals. Geochimica et Cosmochimica Acta 57, 1079–1091. [Google Scholar]
- ZOLOTOV MYU, MIRONENKO MV, SHOCK EL 2006: Thermodynamic constraints on fayalite formation on parent bodies of chondrites. Meteoritics & Planetary Science 41,1 775–1796. [Google Scholar]