Abstract
To elucidate the mechanism of a biochemical process it is often essential to reconstitute the reaction in vitro using the minimal set of factors required to drive the reaction to completion. Here, we describe a method to reconstitute the folding and membrane integration of bacterial outer membrane (OM) proteins that have a characteristic β-barrel structure. In this method the BAM complex, a heteroligomer that catalyzes the membrane integration of β-barrel proteins, is first purified and inserted into small lipid vesicles. Denatured OM proteins are then assembled and integrated into the vesicles in the presence of a molecular chaperone called SurA.
Keywords: BAM complex, β-barrel proteins, Escherichia coli, Membrane proteins, Molecular chaperones, Protein folding, Protein purification, SurA
1. Introduction
The mechanism by which proteins are integrated into the bacterial outer membrane (OM) is a long-standing mystery. While a single hydrophobic α-helix can serve as a membrane-spanning domain for bacterial inner membrane (IM) proteins, the membrane-spanning domains of OM proteins generally fold into a structure known as a β-barrel. A β-barrel is an amphipathic β-sheet wrapped into a closed cylindrical structure that exposes a hydrophobic surface only after it is fully folded. Because of their unique architecture, OM proteins lack extended hydrophobic segments that would presumably cause them to be retained in the IM. Although pioneering genetic and biochemical studies conducted in the 1980s provided strong evidence that OM proteins are transported across the IM through the Sec machinery [1 , 2], their subsequent fate was the subject of much debate. Early experiments suggested that OM proteins fold in the periplasm through interactions with lipopolysaccharide (LPS) and then insert, possibly spontaneously, into the OM [3 , 4]. Other evidence, however, raised the possibility that OM proteins do not pass through the periplasm but instead remain associated with membranes throughout the localization process [5].
The discovery that the assembly of bacterial β-barrel proteins requires a multiprotein complex known as the β -barrel a ssembly m achine (BAM) complex that is comprised of an integral OM protein (BamA) and four lipoproteins (BamB-E) represented a major breakthrough in our understanding of OM protein biogenesis [6 , 7]. More recently, it has been shown that the purified E. coli BAM complex and a periplasmic chaperone called SurA that has been implicated in OM protein biogenesis in vivo [8 , 9] are sufficient to promote the integration of model OM proteins into proteoliposomes [10 – 12]. While this observation supports a model in which OM proteins pass through the periplasm and are then handed off to the BAM complex, it is not yet clear how the BAM complex catalyzes the membrane integration of its substrates. The crystal structure of BamA and disulfide bond cross-linking experiments strongly suggest that substrates are released, possibly in a stepwise fashion, through the opening of a lateral gate in the BamA β-barrel domain [13 , 14]. Other experiments, however, have suggested that BamA catalyzes the integration of prefolded β-barrels by lowering the kinetic barrier imposed by lipid head groups [15] and that the Bam lipoproteins may also play a role in the integration of at least some OM proteins [11 , 16].
In this chapter we describe a method to replicate the membrane integration of OM proteins in vitro using only the purified BAM complex, which is first reconstituted into small lipid vesicles, and SurA. Our method is based on the procedure developed by Kahne and co-workers [10], but differs in that we express all of the Bam subunits together instead of reconstructing the BAM complex from separate BamAB and BamCDE subcomplexes. At least in our hands the BAM complex appears to be more active when it is formed in vivo [12]. We demonstrate the use of the method to replicate the assembly of a model E. coli OM protein called EspP (which is a so-called autotransporter protein; see Ref. 17), but in principle it can be used to replicate the assembly of many other OM proteins. This method should provide a valuable tool to analyze the function of the BAM complex.
2. Materials
2.1. Protein Purification Buffers and Chromatography Materials
Ampicillin (sodium salt) (Sigma Chemical Company, St. Louis, MO, USA): 50 mg/mL stock solution in water. Store at −20 °C.
Isoproylthiogalactoside (IPTG) (MP Biomedical, Santa Ana, CA, USA): 100 mM stock solution in water. Sterile filter and store at −20 °C.
N -dodecyl-β- D -maltoside (DDM) (Anatrace, Maumee, OH, USA): 10 % solution in water. Store at −20 °C.
Membrane protein extraction buffer: 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 % DDM.
Buffer B: 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.03 % DDM.
Ni-NTA agarose (Qiagen, Hilden, Germany).
HiLoad Superdex 200 PG column used in conjunction with an AKTA FPLC chromatographic system (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Amicon Ultra-15 centrifugal filter units with Ultracel-10 membrane (EMD Millipore, Billerica, MA, USA) (see Note 1).
DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA).
Kanamycin (Sigma Chemical Company, St. Louis, MO, USA): 50 mg/mL solution in water. Store at −20 °C.
TALON metal affinity resin (Clontech, Mountain View, CA, USA).
Tris-buffered saline, pH 7.4 (TBS) (KD Medical, Columbia, MD, USA): 25 mM Tris–HCl, 137 mM NaCl, 3 mM KCl.
2.2. Reagent to Prepare Proteoliposomes
E. coli Polar Lipid Extract (Avanti Polar Lipids, Alabaster, AL, USA).
3. Methods
3.1. Expression and Purification of the BAM Complex
Transform an ompT - strain such as HDB150 (MC4100 ompT::spc ΔaraBAD leuD::kan) with plasmid pJH114 [12] and plate on LB agar containing 100 μg/mL ampicillin (see Note 2). Inoculate 12 mL LB/100 μg/mL ampicillin with a single colony from the transformation plate and grow overnight in a bacterial shaker at 37 °C (see Note 3).
Centrifuge the overnight culture at 2500 × g at room temperature for 10 min. Wash the cells by resuspending them in 12 mL LB and repeating the centrifugation step. Resuspend the cell pellet in 12 mL LB.
Add the cells to 1 L LB/100 μg/mL ampicillin in a 2.8 L Fernbach flask and shake at 37 °C.
When the culture reaches OD 600 = 0.5–0.6, add 0.4 mM IPTG to induce synthesis of the BAM complex and shake at 37 °C for an additional 1.5 h (see Note 4).
Centrifuge cultures at 4000 × g for 15 min (see Note 5).
Resuspend the cell pellet in 10 mL cold 20 mM Tris–HCl, pH 8.0. Samples should be kept cold in all subsequent steps.
Lyse the cells using a French press or other appropriate cell disruption instrument.
Remove unbroken cells by centrifuging the lysate at 6000 × g at 4 °C for 10 min.
Centrifuge the supernatant in a Beckmann 70 Ti rotor (250,000 × g , 4 °C, 30 min).
Discard the supernatant and resuspend the pellet in membrane protein extraction buffer. Incubate on ice for 1 h.
Repeat the ultracentrifugation step (step 8).
Mix the supernatant containing the soluble membrane proteins with 2 mL Ni-NTA agarose in a 50 mL conical tube and rotate for 1.5 h at 4 °C (see Note 6).
Pour the slurry into a small gravity flow column. After unbound proteins flow through, wash with 1 column volume buffer B containing 50 mM imidazole.
Elute the BAM complex in 3.5 mL buffer B containing 500 mM imidazole. Add the elution buffer in 1 mL increments.
Inject the eluate onto a Superdex 200 column washed with one column volume (120 mL) water and equilibrated with 1 column volume buffer B.
Run the column at 0.5 mL/min and collect 1 mL fractions.
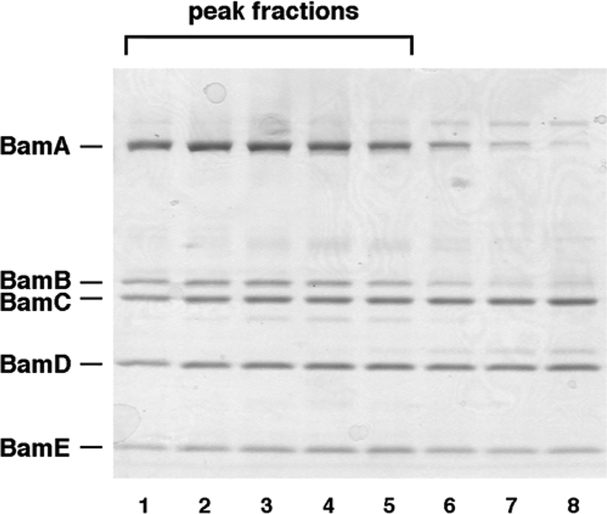
The BAM complex should be highly enriched in the fractions that form the major absorbance peak at λ = 280 nm (A 280). Analyze a small aliquot (typically 10 μL) of each peak fraction by SDS-PAGE and Coomassie Blue staining. The relative staining of each of the five BAM complex subunits should be similar in all of the peak fractions (Fig. 1) (see Note 7).
Pool the fractions that contain the purified BAM complex (typically ~5–7 fractions) and concentrate ~tenfold using Amicon Ultra-15 centrifugal filters or equivalent ultrafiltration devices.
Determine the concentration of the BAM complex using the Bio-Rad DC Protein Assay or equivalent detergent- compatible assay according to the manufacturer’s instructions (see Note 8). Adjust concentration to 20 μM (see Note 8). The protein can be stored briefly at 4 °C (see Note 9).
Fig. 1.
Analysis of peak fractions eluted from Superdex 200 gel filtration column by SDS-PAGE. Aliquots (10 μL) of peak fractions and side fractions were subjected to SDS-PAGE on an 8–16 % Tris–glycine minigel. The peak fractions (lanes 1–5) were highly enriched in the BAM holocomplex. These fractions were pooled and used to generate proteoliposomes. As illustrated here, the side fractions (lanes 6–8) often appear to contain incomplete BAM complexes
3.2. Insertion of the BAM Complex into Liposomes
Suspend E. coli phospholipids in water at a concentration of 20 mg/mL and sonicate until well dispersed.
Add 40 μL of the phospholipid suspension to 200 μL of the purified BAM complex in a 15 mL tube and incubate on ice for 5 min.
Dilute the mixture with 4 mL of 20 mM Tris–HCl, pH 8.0, and incubate on ice for 30 min to reduce the detergent concentration and promote proteoliposome formation.
Pellet the proteoliposomes in an ultracentrifuge (135,000 × g , 4 °C, 30 min) (see Note 10).
Resuspend the pellet in 200 μL 20 mM Tris–HCl, pH 8.0 (see Note 11).
Flash freeze aliquots of the proteoliposomes in liquid nitrogen and store at −80 °C (see Note 12).
3.3. Expression and Purification of SurA
Transform BL21(DE3) with pSK257 [10], a plasmid that encodes a hexahistidine tagged version of SurA lacking its signal peptide (see Note 13). Plate cells on LB agar containing 50 μg/mL kanamycin.
Inoculate 10–15 mL LB/50 μg/mL kanamycin with a single colony from the transformation plate and grow overnight at 37 °C in a bacterial shaker.
Dilute the overnight culture into 1 L LB/50 μg/mL kanamycin in a Fernbach flask and incubate at 37 °C.
At OD 600 = 1.0, add 0.1 mM IPTG. Transfer the culture to a 16 °C shaking water bath and incubate overnight.
Harvest and lyse the cells in 10 mL cold 20 mM Tris–HCl, pH 8.0, as described in Subheading 3.1 , steps 5 – 7 .
Centrifuge the lysate at 35,000 × g for 20 min at 4 °C.
Mix the supernatant with 1 mL pre-equilibrated TALON metal affinity resin. Wash the resin and elute the tagged SurA protein following the manufacturer’s instructions.
Dialyze the purified SurA overnight against 20 mM Tris–HCl, pH 8.0 (see Note 14). Determine the protein concentration using the formula C = A 280 /ε × l (Beer–Lambert law) where C is the concentration, ε is the extinction coefficient, and l is the path length. The extinction coefficient can be easily determined using the ProtParam tool (web.expasy.org/protparam/). Flash freeze aliquots in liquid nitrogen and store at −80 °C.
3.4. Expression and Purification of Denatured OM Proteins from Inclusion Bodies
Clone the gene that encodes the OM protein of interest without its signal peptide into an appropriate pET vector [18] (see Note 15). Transform BL21(DE3) with the resulting plasmid and plate cells on LB agar containing 50 μg/mL kanamycin.
Inoculate 12 mL LB supplemented with 50 μg/mL kanamycin with a single colony from the transformation plate and grow overnight in a bacterial shaker at 37 °C.
Dilute the overnight culture into 1 L LB/50 μg/mL kanamycin in a Fernbach flask and incubate at 37 °C.
At OD 600 = 0.7, add 0.5 mM IPTG and continue the incubation at 37 °C for 3 h.
Harvest and lyse the cells in 10 mL cold TBS as described in Subheading 3.1 , steps 5 – 7 .
Centrifuge lysate at 5000 × g for 10 min at 4 °C.
Discard the supernatant and resuspend the pellet in 10 mL TBS (see Note 16).
Repeat steps 6 and 7 twice, but after the final centrifugation resuspend the pellet in 5 mL 8 M urea.
Incubate at room temperature for 1 h.
Chill the sample briefly on ice and then centrifuge 37,000 × g for 20 min at 4 °C (see Note 10). Discard the pellet. At this point the protein of interest should be extremely highly enriched in the supernatant.
Determine the concentration of the denatured OM protein in the supernatant as described in Subheading 3.3 , step 8 .
Flash freeze aliquots of the OM protein in liquid nitrogen and store at −80 °C.
3.5. Assay for OM Protein Assembly
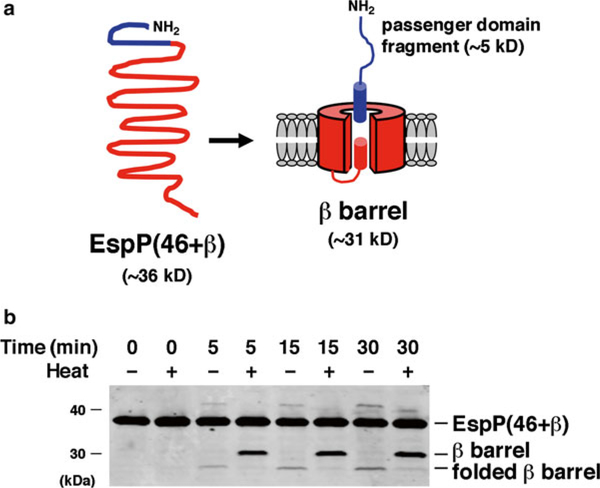
Add SurA, the denatured OM protein and the proteoliposomes containing the BAM complex to 20 mM Tris, pH 8.0, in that order (see Note 17). Mix after the addition of each component. To examine the assembly of EspP(46 + β), a derivative of EspP consisting of a β-barrel plus an embedded α-helical segment that is cleaved in an autocatalytic reaction following membrane integration (Fig. 2a ; see Ref. 19), we typically use 1–2 μM SurA, 0.1–0.2 μM substrate, and 0.2 μM reconstituted BAM complex. The optimal concentration of each component may vary, however, and should be determined empirically (see Notes 18 and 19). The volume of the reaction also depends on the nature of the experiment and the sensitivity of the readout assay.
Incubate the reaction at 30 °C for 1–30 min. Assembly of EspP(46 + β) can be observed within 1 min and typically peaks within ~15 min, but the kinetics of assembly of other OM proteins may differ.
Monitor the assembly and membrane integration of the OM protein using one or more readout assays. Because many folded β-barrel proteins (including EspP) are resistant to SDS denaturation in the absence of heat [3], the presence of a rapidly migrating form of an OM protein on an SDS gel or a Western blot is indicative of complete assembly (Fig. 2b). Resistance to protease digestion can also often be used to assess integration of an OM protein into proteoliposomes [12]. More specialized assays may also be useful to examine the assembly of specific types of OM proteins. The folding of enzymes like OmpT, for example, can be monitored by activity assays [10 , 12]. Likewise, the assembly of EspP (and a subset of other autotransporters) can be assessed by measuring the extent of autoproteolytic processing (Fig. 2b).
Fig. 2.
Assembly of a model OM protein catalyzed by the BAM complex and SurA. (a). EspP(46 + β) is a ~36 kD truncated form of the autotransporter EspP. Upon insertion into the OM EspP(46 + β) folds into a β-barrel structure. An α-helix traverses and protrudes through the extracellular side of the β-barrel pore. The protein is then rapidly cleaved into a 46-residue (~5 kD) “passenger domain” fragment and a ~31 kD β-barrel through an autocatayltic intrabarrel reaction. (b) EspP(46 + β) was incubated at 30 °C with proteoliposomes containing the BAM complex and SurA. Aliquots were removed at the indicated time points and either heated at 95 °C or maintained at room temperature. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose. Western blotting was then conducted using an antiserum raised against a C-terminal EspP peptide [12]. Because proteolytic maturation requires membrane integration and folding of the protein, the accumulation of the free β-barrel demonstrates that a fraction of the protein was assembled during the incubation. The presence of a rapidly migrating form of the β-barrel in the absence of heat provides further evidence that it was properly folded. Part (b) was reproduced from Ref. 12 under a Creative Commons Attribution license
4. Notes
Units with Ultracel-30, −50 or −100 membranes can presumably also be used.
A strain that lacks OmpT is recommended because the BAM complex proteins may be sensitive to the action of this protease. Readily available rec + strains (e.g., MG1655, MC4100) grow faster than the rec - strains that are commonly used for cloning (e.g., DH5α) and may also be preferable. Plasmid pJH114 is a pBR322-based plasmid that contains the genes encoding all of the E. coli BAM complex proteins under the control of the trc promoter. To aid in the purification of the BAM complex, an octahistidine tag was attached to the C- terminus of BamE. The addition of IPTG drives the expression of moderately high levels of the BAM complex. We have not used higher level expression vectors, but these vectors may be useful to increase the yield of the BAM complex.
We have found that it is often desirable to double the size of the prep described here. For the larger prep we grow two 12 mL overnight cultures and use each overnight culture to inoculate a 1 L culture. Cells are pooled and resuspended in twice the volume of Tris buffer prior to lysis. The volume of the membrane solubilization buffer and Ni-NTA agarose is also doubled.
Longer incubation times are not recommended. We have found that the BamCDE subcomplex tends to accumulate if the cultures are incubated for more than 1.5 h in the presence of IPTG.
The use of a Beckman JLA-8.1000 rotor or equivalent rotor that accepts 1 L bottles is recommended.
Prior to use the Ni-NTA beads should be washed with water and then buffer B.
We have found that 8–16 % Tris–glycine minigels (Life Technologies, San Diego, CA, USA) resolve the BAM complex proteins effectively, but other gel systems should also work well. The integrity of the BAM complex in the peak fractions can be confirmed using Blue Native PAGE.
At this stage the concentration of the BAM complex should be ≥20 μM if the protein was purified from 2 L of cells and if the yield is optimal. We have found, however, that the BAM complex can insert into liposomes and facilitate OM protein assembly even if the concentration is considerably lower.
We have found that the BAM complex tends to aggregate and lose activity if it is frozen in detergent solution prior to reconstitution into proteoliposomes. For that reason we recommend inserting the BAM complex into liposomes immediately after purification, but the purified complex can also be stored overnight at 4 °C.
It is convenient to use a tabletop ultracentrifuge with a rotor that holds >2 mL tubes (e.g., Beckman TLA-100.4 rotor) for this purpose.
Most of the BAM complex should insert into the lipid vesicles, so the concentration of the protein should not change significantly.
Once reconstituted into proteoliposomes the BAM complex can be thawed and refrozen at least a few times without apparent loss of activity.
BL21(DE3) competent cells are available commercially (two vendors are New England Biolabs, Ipswich, MA, USA and Life Technologies, San Diego, CA, USA). In pSK257, E. coli surA is expressed under the control of a T7 promoter. The gene is expressed upon the addition of IPTG, which activates transcription of a chromosomal copy of the T7 polymerase gene.
SurA should be nearly homogeneously pure at this stage.
pET vectors can be obtained commercially (two vendors are Clontech, Mountain View, CA, USA and EMD Millipore, Billerica, MA, USA). We recommend using a vector such as pET- 28 that facilitates the attachment of an N-terminal hexahistidine tag to the protein of interest. Although the protein is typically very highly enriched in inclusion bodies, in some cases it may be desirable to further achieve a higher degree of purity using a Ni-NTA column.
The pellet containing the inclusion bodies should have a chalky appearance and consistency.
We and others have found that the assembly of EspP derivatives and other model OM proteins including OmpT, OmpA and BamA does not require the addition of salt [10 – 12 , 20], but the optimal buffer composition may vary.
Kahne and co-workers have used different concentrations of the components to examine the assembly of OmpT, OmpA and BamA [10 , 11 , 20].
If the readout assay involves measuring the enzyme activity of the folded OM protein, it may be desirable to add an appropriate substrate molecule before starting the reaction.
Acknowledgment
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Emr S, Hanley-Way S, Silhavy TJ (1981) Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23:79–88 [DOI] [PubMed] [Google Scholar]
- 2.Brundage L, Hendrick JP, Schiebel E et al. (1990) The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62:649–657 [DOI] [PubMed] [Google Scholar]
- 3.Freudl R, Schwarz H, Stierhof YD et al. (1986) An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J Biol Chem 261:11355–11361 [PubMed] [Google Scholar]
- 4.Sen K, Nikaido H (1990) In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli . Proc Natl Acad Sci U S A 87: 743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese PN, Silhavy TJ (1998) Targeting and assembly of periplasmic and outer-membrane proteins in Escherichia coli . Annu Rev Genet 32:59–94 [DOI] [PubMed] [Google Scholar]
- 6.Voulhoux R, Bos MP, Geurtsen J et al. (2003) Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265 [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Malinverni J, Ruiz N et al. (2005) Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli . Cell 121:235–245 [DOI] [PubMed] [Google Scholar]
- 8.Lazar SW, Kolter R (1996) SurA assists the folding of Escherichia coli outer membrane proteins. J Bacteriol 178:1770–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouviere PE, Gross CA (1996) SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev 10:3170–3182 [DOI] [PubMed] [Google Scholar]
- 10.Hagan CL, Kim S, Kahne D (2010) Reconstitution of outer membrane protein assembly from purified components. Science 328:890–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagan CL, Westwood DB, Kahne D (2013) Bam lipoproteins assembly BamA in vitro . Biochemistry 52:6108–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman-Hernandez G, Peterson JH, Bernstein HD (2014) Reconstitution of bacterial auto-transporter assembly using purified components. ELife 3:04234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noinaj N, Kuszak AJ, Gumbart JC et al. (2013) Structural insight into the biogenesis of β-barrel membrane proteins. Nature 501: 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noinaj N, Kuszak AJ, Balusek C et al. (2014) Lateral opening and exit pore formation are required for BamA function. Structure 22:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessman D, Chung YH, Danoff EJ et al. (2014) Outer membrane β barrel protein folding is physically controlled by periplasmic lipid head groups and BamA. Proc Natl Acad Sci U S A 111:5878–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ieva R, Tian P, Peterson JH et al. (2011) Sequential and spatially restricted interactions of assembly factors with an autotransporter β domain. Proc Natl Acad Sci U S A 108: E383–E391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dautin N, Bernstein HD (2007) Protein secretion in Gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol 61:89–112 [DOI] [PubMed] [Google Scholar]
- 18.Studier FW, Rosenberg AH, Dunn JJ et al. (1990) Use of T7 polymerase to direct expression of cloned genes. Methods Enzymol 185:60–89 [DOI] [PubMed] [Google Scholar]
- 19.Dautin N, Barnard TJ, Anderson DE et al. (2007) Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J 26:1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagan CL, Kahne D (2011) The reconstituted Escherichia coli BAM complex catalyzes multiple rounds of β-barrel assembly. Biochemistry 50:7444–7446 [DOI] [PMC free article] [PubMed] [Google Scholar]