Abstract
Cancer testis antigens (CTA) are expressed in testis and placenta and anomalously activated in a variety of tumors. The mechanistic contribution of CTAs to neoplastic phenotypes remains largely unknown. Using a chemigenomics approach, we find that the CTA HORMAD1 correlates with resistance to the mitochondrial complex I inhibitor piericidin A in non–small cell lung cancer (NSCLC). Resistance was due to a reductive intracellular environment that attenuated the accumulation of free radicals. In human lung adenocarinoma (LUAD) tumors, patients expressing high HORMAD1 exhibited elevated mutational burden and reduced survival. HORMAD1 tumors were enriched for genes essential for homologous recombination (HR), and HORMAD1 promoted RAD51-filament formation, but not DNA resection, during HR. Accordingly, HORMAD1 loss enhanced sensitivity to γ-irradiation and PARP inhibition, and HORMAD1 depletion significantly reduced tumor growth in vivo. These results suggest that HORMAD1 expression specifies a novel subtype of LUAD, which has adapted to mitigate DNA damage. In this setting, HORMAD1 could represent a direct target for intervention to enhance sensitivity to DNA-damaging agents or as an immunotherapeutic target in patients.
Introduction
Cancer testis antigens (CTA) are genes defined by their expression pattern, which is normally found in testis or placenta, but activated in nearly every tumor type. Given that the testis is immune-privileged, the expression of these proteins has long been prized for their immunotherapeutic potential. More recently, a number of reports from our lab and others indicate that these anomalously expressed proteins are not merely bystanders in the tumor cell–regulatory environment, but can be engaged to promote neoplastic phenotypes. Specifically, CTAs are reported to promote mitotic fidelity, degrade tumor-suppressor proteins, confer tolerance to DNA damage, and reprogram transcriptional networks (1–9). These reports suggest the provocative hypothesis that CTAs may represent direct intervention targets with an extraordinarily broad therapeutic window.
A cohort of CTAs is essential for recombination of homologous chromosomes during meiosis. These include components of the synaptonemal complex (SYCE1 and SYCP1), the meiotic topoisomerase that catalyzes DNA double-strand breaks (DSB; SPO11), as well as multiple proteins that mediate homologue alignment and recombination (HORMAD1, HORMAD2, and TEX15; refs. 10–20). Mice lacking these genes are healthy, but infertile (12, 18–22). In all cases, spermatocytes arrest during Prophase I due to defective synapse formation or an inability to undergo recombination and chromosome segregation (12, 18–22). In some cases, females are also infertile due to defects in chromosome segregation and quality control (16, 17, 23). Expression of each of these genes has been reported in human cancer, but scant information exists regarding the tumorigenic function of meiotic CTAs and whether they have any context selectivity (24–27).
Through a large-scale effort to identify antitumor natural products collected from marine-derived bacteria, we have identified non–small cell lung cancer (NSCLC) cell lines that are resistant to the electron transport chain (ETC) Complex I (NADH dehydrogenase) inhibitor, piericidin A (PA). Strikingly, we found that this resistance correlates with expression of the CTA, HORMAD1. We find that the basis for PA resistance is a highly reductive environment that prevents accumulation of H2O2. Mechanistic follow-up studies indicate that HORMAD1 is not complicit in this resistance, but is essential for remediating DNA DSBs. These data suggest that HORMAD1 expression specifies a subtype of NSCLC that has evolved cellular mechanisms to both avoid and mitigate excessive DNA damage.
Materials and Methods
Natural product fraction library
The natural product fraction (NPF) library contains extracts from 300 marine-derived bacterial strains and 20 marine invertebrates. Fermentation of each bacterial strain gave rise to a total of 20 NPFs/strain (Nomenclature: SNX-###-F1 through SNX-###-F20, where F1 is the most polar and F20 the least polar). All NPFs in the library are standardized to 10 mg/mL in DMSO. See Supplementary Methods for details of library and fraction generation and PA purification.
NPF viability screening
Each of the 4,358 natural products fractions was screened at 4 doses (0.18, 0.55, 1.65, and 5 mg/mL) across 26 cell lines. Activity scores at each dose were used as a response vector, resulting in 17,432 total input response vectors. Activity scores were calculated as the percent cell death relative to control (0 = no death; 100 = 100% cell death), and the equation is: −1 × [100 − (value/DMSO) × 100]. Dose–response curves for PA were performed at 12 half-log doses with concentration ranging from 50 μmol/L to 50 pmol/L in triplicate in two independent runs for each cell line.
Elastic net analysis
To discover RNA sequencing (RNA-seq) expression features predictive of response to PA, an elastic net analysis was employed as previously described using a publically available RNA-seq dataset (28, 29). ED50 values were used as a response vector. Elastic net parameters were fit with a 5-fold cross-validation analysis.
Cell lines and chemicals
All NSCLC cell lines were obtained from John Minna (UT Southwestern) between 2004 and 2015 (30). Cells were cultured in RPMI medium supplemented with 5% FBS at 37°C and 5% CO2 and were not passaged more than 25 times after thawing. Cells were authenticated upon receipt and during this study in 2016 and 2017 using short tandem repeat profiling. Cells were periodically evaluated for mycoplasma contamination by DAPI stain for extra-nuclear DNA. Chemicals (and manufacturer) used were as follows: PA (Enzo), rotenone (Sigma-Aldrich), 6-aminonicotinamide (6-AN; Alfa Aesar), and 5-aza-2′deoxycytidine (5-aza; MP Biomedicals).
Immunoblotting
Immunoblotting was performed as previously described (5). Antibodies used were as follows: HORMAD1 (HPA037850, 1:1,000, Sigma-Aldrich, RRID: AB_10696358), Actin (sc-8432, 1:1,000, Santa Cruz Biotechnology, RRID: AB_626630), and GADPH (G8795, 1:5,000, Sigma-Aldrich, RRID: AB_1078991).
Immunohistochemistry
NSCLC tumor microarrays were stained using a Leica Bond Max automated stainer (Leica Biosystems). Tissue sections were deparaffinized and rehydrated following the Leica Bond protocol. Antigen retrieval was performed with Bond Solution #2 (Leica Biosystems, equivalent to EDTA buffer pH 9.0) for 20 minutes, then HORMAD1 antibody (HPA037850, 1:500, Sigma-Aldrich) was employed for 15 minutes at room temperature. The primary antibody was detected using the Bond Polymer Refine Detection Kit (Leica Biosystems) with diaminobenzidine as chromogen. The slides were counterstained with hematoxylin, dehydrated, and coverslipped. Nuclear expression of HORMAD1 was evaluated by pathologists using the H-score system.
Immunofluorescence
Immunofluorescence was performed as previously described (5). Antibodies used were as follows: RAD51 (398587, 1:800, Santa Cruz Biotechnology) and RPA2 (NA19L, 1:500, Millipore, RRID: AB_565123). Cells were imaged using a Zeiss LSM510 confocal microscope or Keyence Fluorescence Microscope BZ-X710.
Cell quantification assays
Cells were plated into 96-well plates (Corning, #3904) at 30% to 50% confluency, treated for 72 hours, trypsinized, and then quantified using a hemocytometer.
EdU incorporation assays
Cells were plated into 96-well format (Corning, #3904) at 30% to 50% confluency, treated for 72 hours, and then exposed to EdU for 2 hours before fixing the cells in 3.7% formaldehyde. Cells were stained using the protocol for Click-iT EdU Alexa Fluor 488 Imaging Kit (Invitrogen) and costained with Hoechst 3342 (Invitrogen). Cells were quantified using fluorescence microscopy.
Stable cell lines
sgCTRL and sgHORMAD1 lines were generated using pLX-sgRNA and pCW-Cas9 constructs (Addgene plasmid #50662, #50661; ref. 31).
Clonogenic cell survival assays
Cells were plated at 30% to 50% confluency. Forty-eight hours after plating, cells were irradiated using a Caseium137 source, trypsinized, and replated at various densities (escalating with dose). For olaparib assays, cells were replated directly into drug. Cells were cultured for 10 to14 days, fixed, and stained in a 0.5% crystal violet, 10% acetic acid, and 90% methanol solution for 10 minutes. A cluster of 50 cells was considered a colony. SF = (PE-treated samples)/(PE of control), where SF is surviving fraction and PE is plating efficiency.
FACS analyses
For superoxide assays, cells were plated at approximately 50% confluency and treated 24 hours later for 1 hour. Cells were subsequently treated with 5 μmol/L MitoSOX (Invitrogen) in Hank’s Balanced Salt Solution (HBSS; Invitrogen) for 10 minutes at 37 C and 5% CO2. For H2O2 assays, cells were plated at 30% to 50% confluency and treated for 72 hours. Cells were subsequently treated with 5 μmol/L CM-H2DCFDA (Invitrogen) in HBSS for 20 minutes at 37°C and 5% CO2, washed with HBSS, and then recovered in fresh medium for 15 minutes. Cells were immediately analyzed by flow cytometry using a BD LSR Fortessa instrument and BD FACSDiva 6.2 software. A minimum of 1.0 × 104 cells were analyzed per condition. FlowJo software was used to generate flow charts and calculate KS-Max difference.
NADPH/NADP+ assays
Cells were plated into 96-well tissue culture plates (Corning, #3904) and treated for 72 hours. Cells were processed according to the manufacturer’s protocol for NADP/NADPH-Glo Assay (Promega).
siRNA transfection
siRNA studies were performed as previously described (5).
Dose curves
Cells were plated into 96-well tissue culture plates (Corning, #3904) at 30% to 50% confluency, treated for 72 hours with drug, and then analyzed using Cell-Titer Glo (Promega). Curves were fitted and ED50s calculated as previously described (29).
Kaplan–Meier and mutation analyses
RNA-seq expression values from The Cancer Genome Atlas (TCGA) provisional datasets for lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) were used to bifurcate patient tumors into high and low HORMAD1-expressing groups. Bifurcation was made at the point that generated the highest HR. Cox regression analysis was used to obtain HRs and P values. Mutations were assessed from TCGA whole-exome sequencing data. The number of mutations reflects the number of genes that are mutated.
Signal-to-noise and GSEA analyses
Datasets from TCGA for provisional LUAD were used (data downloaded March 2017, 517 tumors). Patients were bifurcated based on the following criteria: HORMAD1 low: HORMAD1 fragments per kilobase of exon per million fragments mapped (FPKM) <1 and <150 mutations, HORMAD1 high: HORMAD1 FPKM >300 and >500 mutations. Genes that did not have an FPKM >1 in at least one tumor sample were excluded. Data were log2-transformed and genes that did not have >2 fold change (log(a) − log(b) > 1 OR log(a) − log(b) < − 1) were also excluded. S2N values were based on the following equation: (mean(A) − mean(B))/(sd(A) + sd(B)). For gene set enrichment analysis (GSEA), the top 10% of genes most differentially expressed in HORMAD1-high tumors based on S2N analysis (251 genes) were analyzed using the Broad Institute’s Molecular Signatures Database Hallmark and Kyoto Encyclopedia of Genes and Genomes (KEGG) sets (32, 33).
Xenograft experiments
All animal experiments were conducted with Institutional Animal Care and Use Committee approval of protocol 2016–101795. For the 5-aza experiments, 6- to 8-week-old female NOD. cg-PRKDCSCIDIl2rgtm1Wjl/SzJ (NSG; RRID:IMSR_JAX:005557) mice (weighing 19–24 grams) were subcutaneously injected in the flank with 1 million cells (HCC44 and H2122) in 200 μL PBS. Once tumors reached 175 mm3, mice were intraperitoneally injected with 2 mg/kg of InSolution 5-aza (Millipore 189826) daily for 5 days, after which, tumors were extracted and flash frozen. For the CRISPR experiments, 6- to 8-week-old female Hsd: Athymic Nude-Foxn1nu (RRID:MGI:5652489) mice (weighing 15–20 grams) were subcutaneously injected in the flank with 2 million cells (A549 Cas9/sgCTRL or A549 Cas9/sgHORMAD1) in 100 μL PBS. Once tumors were visible, volume was measured by calipers twice per week.
Statistical analysis
GraphPad Prism (GraphPad Software) was used to perform statistical analyses. Data were assessed by two-tailed, unpaired t tests, Mann–Whitney or Shapiro–Wilk tests as indicated. P values less than 0.05 were considered significant. For xenograft experiments, simple randomization was used to assign mice to control or experimental groups.
Results
PA resistance correlates with HORMAD1 expression in NSCLC
We devised a discovery pipeline to identify natural products with selective antitumor activity in NSCLC (Fig. 1A). A NPF library with >4,000 fractions from marine-derived bacteria was screened at four doses (a total of 17,432 fraction/dose combinations) for effects on viability in each of 25 tumor-derived NSCLC lines and 1 normal, immortalized lung line that were previously annotated for gene expression, mutation status, and copy-number variation. We prioritized further investigation of fractions that exhibited activity against at least one NSCLC cell line at ng/mL concentration, were not pan-toxic, and exhibited a distinctive chemical signature by LC/MS analysis. From this analysis, we identified 63 fractions that reached our criteria (Supplementary Fig. S1A and S1B). Among these fractions was SNB-051–14, which was a selective, nonpolar fraction that originated from a strain of Streptomyces variabilis (Supplementary Fig. S1C). We cultured the S. variabilis strain, SNB-051, on a large scale and obtained fractions used in iterative bioassay analysis with two sensitive NSCLC cell lines (HCC44 and H2122) to identify a single, potent fraction: SNB-051-F36-H7 (see Supplementary Methods; Supplementary Fig. S1D). High-resolution ESI-MS analysis of SNB-051-F36-H7 identified a mass/charge consistent with the molecular formula C25H37NO4 (m/z [M+H] 416.2794). Further 1H NMR analysis indicated that the active constituent was PA (see Supplementary Methods; Supplementary Fig. S1E). As a quinone analog, PA binds to Complex I and inhibits the oxidation of ubiquinone and generation of the proton gradient essential for generation of ATP (34). PA also enhances reactive oxygen species (ROS) production by Complex I (34). Although Complex I inhibitors have been suggested as anticancer compounds, the selective toxicity observed for PA in NSCLC has not been previously observed (35, 36).
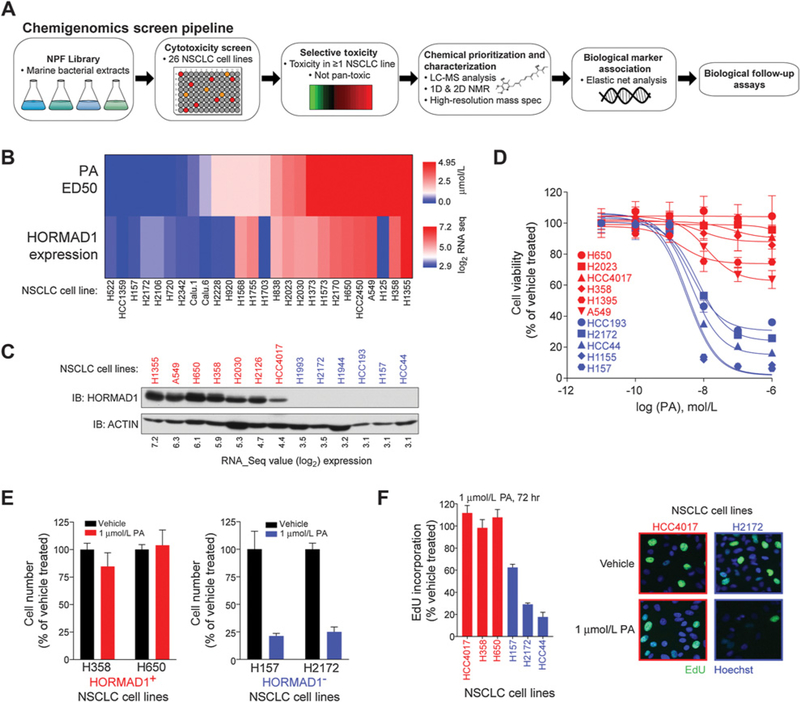
Figure 1.
Natural compound screen identifies correlation between HORMAD1 and PA sensitivity. A, Schematic for chemigenomics screen pipeline. B, Heat maps of HORMAD1 expression (log2 RNA-seq values) and PA (ED50) in a panel of NSCLC cell lines. C, Whole-cell lysates of indicated cell lines were immunoblotted with indicated antibodies. D, Indicated cell lines were exposed to indicated doses of PA for 72 hours. HORMAD1-positive cell lines, red; HORMAD1-negative cell lines, blue. Points indicate viability as measured by Cell-Titer Glo (n = 3). Bars, SD. Curves are nonlinear fits to the respective data. E, HORMAD1-positive cells (left) and HORMAD1-negative cells (right) were exposed to PA for 72 hours and quantified using a hemocytometer. Bars represent the average (n = 2) ± range. F, Left, indicated cell lines were exposed to PA for 72 hours, followed by EdU incorporation. Bars represent the mean (n = 2) ± range. Right, representative images of EdU staining (EdU, green; Hoechst, blue) of HORMAD1-positive HCC4017 and HORMAD1-negative H2172 cells.
We next generated a 12-point dose–response curve for PA in 26 NSCLC cell lines. A regularized linear regression algorithm was applied to determine if distinct gene expression features from whole-genome transcript profiles were predictive of PA response (29). This revealed a correlation of HORMAD1 with resistance to PA (Fig. 1B). HORMAD1 is a meiotic chromatin-binding protein that is essential for synaptonemal complex formation, generation of DSBs, and the meiotic silencing of unsynapsed chromatin checkpoint (17). HORMAD1 is expressed in human cancer, which classifies it as a CTA (26). HORMAD1 expression is normally restricted to the testis (data accessed from GTEx Portal on April 04, 2018; Supplementary Fig. S1F). The functional role of HORMAD1 in NSCLC has not been previously evaluated; however, a report in triple-negative breast cancer indicates a possible tumorigenic function in DNA repair (1). Thus, we chose to further focus on the significance of HORMAD1 in NSCLC.
We examined HORMAD1 protein expression in NSCLC and found that RNA-seq expression values above 4.0 corresponded with robust protein expression (Fig. 1C; Supplementary Fig. S1G; ref. 29). We refer to HORMAD1 mRNA expression > 4.0 as HORMAD1 positive (+), whereas < 4.0 are classified as HORMAD1 negative (–). Using these cutoffs, we recapitulated the differential sensitivity profile with independently synthesized PA and expanded our analysis to additional cell lines not in the original screening set (Fig. 1D). The endpoint assay in these experiments, CellTiter-Glo, measures cellular ATP, which is likely depleted following inhibition of the ETC by PA. Thus, we quantitated viable cells at the experimental end point. Treatment of HORMAD1(–) cells led to a greater than 50% decrease in cell number; however, no such reduction was observed in the HORMAD1(+) samples (Fig. 1E). Furthermore, EdU incorporation indicated that only HORMAD1(–) cells reduced proliferation (Fig. 1F). This data indicates that a subset of NSCLC cells is highly sensitive to PA, and expression of the CTA protein, HORMAD1, correlates with resistance.
HORMAD1-expressing NSCLCs exhibit elevated reductive capacity
We next assessed superoxide (O2–) generation following PA exposure in both the HORMAD1(–) and HORMAD1(+) cell lines. In this setting, all NSCLC lines tested, irrespective of PA sensitivity, induced O2– (Fig. 2A). Superoxide is subsequently converted to hydrogen peroxide (H2O2) by superoxide dismutase (37). We assayed accumulation of H2O2 and observed little, if any, increase in HORMAD1(+) cells following PA exposure (Fig. 2B). In contrast, HORMAD1(–) cells exhibited a robust accumulation. This data indicates that PA-resistant NSCLC lines are not reliant on the ETC for ATP production and can mitigate the accumulation of H2O2, which may otherwise inhibit cellular proliferation (38).
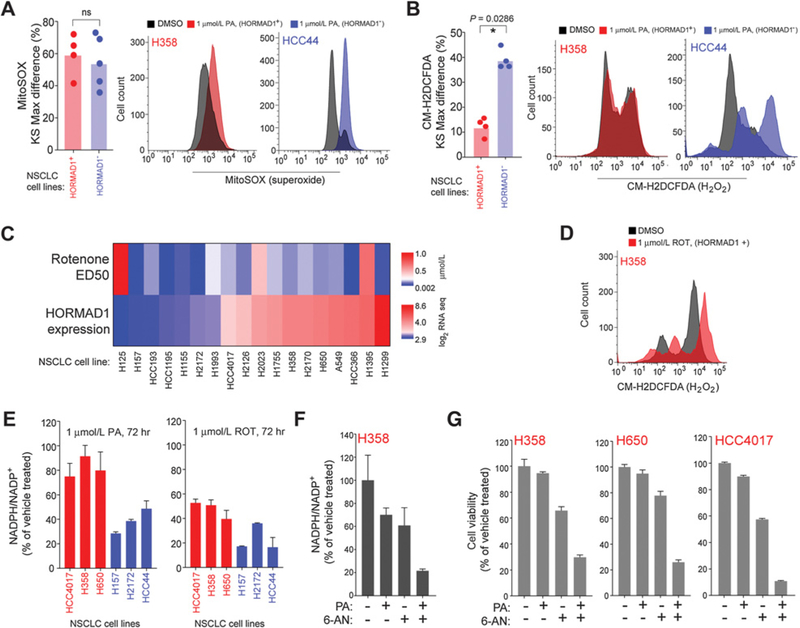
Figure 2.
HORMAD1-positive lines escape oxidative stress induced by PA. A, Left, HORMAD1-positive (red) and HORMAD1-negative (blue) cells exposed to PA (1 μmol/L) were stained with MitoSOX prior to FACS analysis. Bars represent the mean KS-Max difference (compared with vehicle treated) of all cell lines, and dots indicate mean values for individual cell lines (n = 3). HORMAD1-positive cell lines from top to bottom: HCC4017, A549, H650, H358. HORMAD1-negative cell lines from top to bottom: HCC44, H157, H1155, H2172, H2122. P value calculated by Mann–Whitney test (ns, nonsignificant). Right, representative flow cytometry distributions for PA-treated HORMAD1-positive H358 (red) and HORMAD1-negative HCC44 (blue) cells. B, Left, HORMAD1-positive (red) and -negative (blue) cell lines exposed to PA (1 μmol/L) for 72 hours and stained with CM-H2DCFDA prior to FACS analysis. Bars represent the mean KS-Max difference (compared with vehicle treated) for all cell lines, and dots indicate mean values for individual cell lines (n ≥ 2). HORMAD1-positive cell lines from top to bottom: HCC4017, H358, A549, H650. HORMAD1-negative cell lines from top to bottom: HCC193, HCC44, H2172, H157. P value calculated by Mann–Whitney test. Right, representative flow cytometry distributions for PA-treated HORMAD1-positive H358 (red) and HORMAD1-negative HCC44 (blue) cells. C, Heat maps of HORMAD1 expression (log2 RNA-seq values) and rotenone (ED50) in a panel of NSCLC cell lines. D, H358 cells were exposed to rotenone (ROT) for 72 hours and stained with CM-H2DCFDA prior to FACS analysis. E, HORMAD1-positive (red) and HORMAD1-negative (blue) cell lines exposed to PA or rotenone for 72 hours prior to NADPH/NADP+ ratio assay. Bars represent the mean (n ≥ 2) ± range. F, H358 cells were exposed to PA (10 μmol/L) and/or 6-AN (5 μmol/L) for 96 hours prior to NADPH/NADP+ ratio assay. Bars represent the mean (n = 3) ± SD. G, Indicated cell lines were exposed to PA (10 μmol/L) and/or 6-AN (three treatments at 5 μmol/L, 2.5 μmol/L for HCC4017) for 96 hours prior to viability readout by Cell-Titer Glo. Bars represent the mean (n = 3) ± SD.
To determine whether resistance to Complex I inhibition was a general phenomenon of HORMAD1(+) NSCLC cells, we evaluated sensitivity to rotenone, another Complex I inhibitor (39–41). HORMAD1(+) and HORMAD1(–) cells exhibit similar sensitivities to rotenone, although we observed slightly enhanced sensitivity in HORMAD1(–) cells (Fig. 2C). Importantly, exposure of HORMAD1(+) cells to rotenone led to an accumulation of H2O2 (Fig. 2D). Unlike PA, rotenone is a noncompetitive inhibitor of Complex I and is more potent at inducing ROS in vitro (42, 43). The PA resistance in HORMAD1(+) cells may reflect differences in the properties of these inhibitors that lead to differential induction of ROS.
H2O2 is well known to induce oxidation of lipids, proteins, and DNA (37). Indeed, exposure of HORMAD1(–) cells to PA led to an accumulation of the DNA damage response indicator, 53BP1 (Supplementary Fig. S2A). Reduction of cellular H2O2 is highly dependent upon the NADPH/NADP+ ratio, which maintains a pool of the reductant, glutathione. We measured NADPH/ NADP+ ratios in HORMAD1(+) and (–) cells to monitor cellular oxidative stress. In HORMAD1(+) NSCLC cells exposed to PA, NADPH/NADP+ was unchanged, whereas it decreased by more than half in HORMAD1(–) cells, indicating an exhaustion of the antioxidant machinery (Fig. 2E). Glucose-6-phosphate-dehydrogenase (G6PD) is the main cellular source of NAPDH. We next coinhibited HORMAD1(+) cells with the G6PD antagonist, 6-AN (44). This combination resulted in a decrease in NADPH/NADP+ when HORMAD1+ cells were exposed to PA (Fig. 2F). Further-more, we observed a combinatorial loss of viability in three HORMAD1(+) NSCLC cell lines (Fig. 2G). We conclude that resistance to PA in HORMAD1(+) tumors is due to an enhanced capacity to reduce ROS. Conversely, HORMAD1(–) cells may have defects in reductive pathways that enhance their sensitivity to ROS accumulation.
To determine functional consequences of HORMAD1 expres-sion on PA resistance, we performed a series of gain- and loss-of-function experiments. HORMAD1 was stably expressed in HORMAD1(–) NSCLC cells; however, we did not observe PA resistance (Supplementary Fig. S2B). We generated HORMAD1-knockdown cell lines by stably transducing HORMAD1-specific shRNAs into four HORMAD1(+) NSCLC cell lines. We also used CRISPR/Cas9 to knockdown HORMAD1 in A549 and H358 cell lines (sgHORMAD1). In none of these settings did we observe enhanced PA sensitivity (Supplementary Fig. S2C and S2D). Based on these observations, we conclude that the observed PA resistance may be a by-product of the regulatory environment of HORMAD1-expressing cells, which has been selected to attenuate free radical damage that could otherwise prevent survival.
HORMAD1 portends poor survival and high mutation burden in LUAD
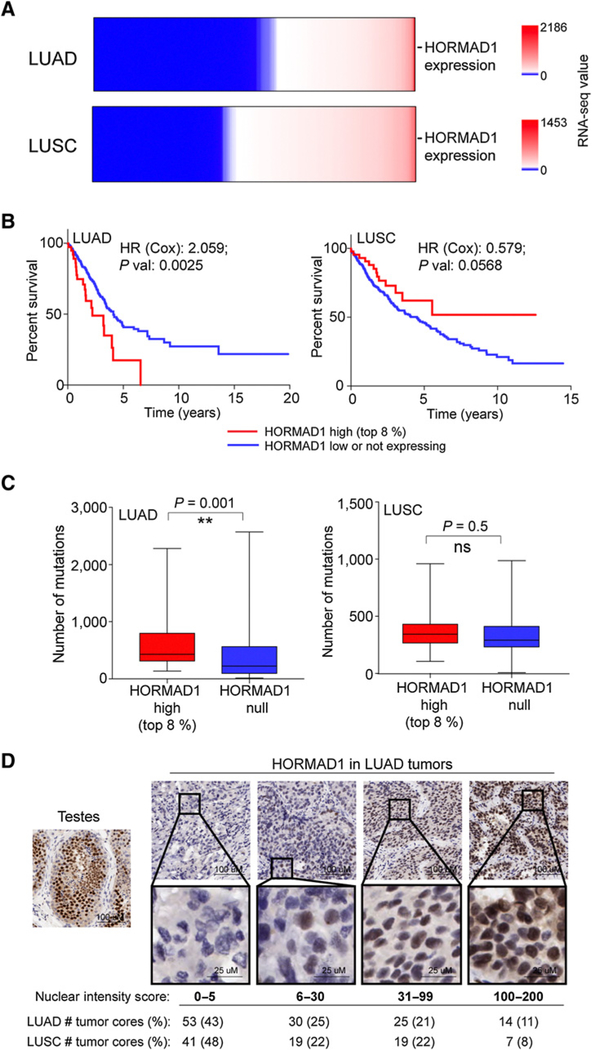
We next evaluated the significance of HORMAD1-expressing tumors in human NSCLC cancer. HORMAD1 mRNA expression is present in approximately 50% of both LUAD and LUSC tumors (Fig. 3A). In patients with LUAD, the highest approximately 10% of HORMAD1-expressing patients had significantly reduced overall survival (HR = 2.059, P = 0.0025; Cox Regression). However, we did not detect this correlation in LUSC (HR = 0.579, P = 0.0568; Cox Regression; Fig. 3B). Furthermore, high HORMAD1 expression also correlated with elevated mutation burden exclusively in the LUAD patient population but not in the LUSC tumors (Fig. 3C). To evaluate expression of HORMAD1 protein in human tissue samples, we developed an immunohistochemical staining protocol. Consistent with the expression data, over half of NSCLC tumor cores stained positive for HORMAD1 protein (Fig. 3A and D). HORMAD1 was detected primarily in the nucleus of tumor cells, consistent with possible chromatin association in tumors as in its native tissue (Fig. 3D; ref. 17).
Figure 3.
HORMAD1 indicates poor prognosis and elevated mutation burden in LUAD. A, Heat maps of HORMAD1 mRNA expression (log2 RNA-seq values) derived from TCGA data. B, Kaplan–Meier survival curves from TCGA for patients with LUAD and LUSC. HRs and P values calculated by Cox regression analysis. C, Box plots indicate median and interquartile range, and bars represent min to max. P values calculated by Mann–Whitney test. D, Representative images of IHC staining and nuclear intensity scores (as scored by pathologist) for HORMAD1 in LUAD and LUSC tumor cores. ns, nonsignificant.
Activation of HORMAD1 in lung cancer cells
The transcriptional regulatory mechanisms that activate expression of CTAs in cancers remain relatively obscure. However, inhibitors of methylation have been successfully used to induce CTA expression in patient populations to boost responses to CTA-based immunotherapy (45). To test whether HORMAD1 is also regulated by DNA methylation, we exposed NSCLC cells to 5-aza for 72 hours. We observed a robust induction of HORMAD1 mRNA in the HORMAD1(–) cells to levels of 5% to 20% of HORMAD1(+) cells (Fig. 4A and B). This increase in expression was sufficient for HORMAD1 protein accumulation as observed by immunoblot (Fig. 4C). The mRNA expression was stable for up to 19 days after withdrawal of 5-aza (Fig. 4D). We also tested the capacity of 5-aza to induce HORMAD1 expression in vivo. Here, we xenografted HORMAD1(–) NSCLC cell lines into immuno-compromised mice. Similar to the human clinical treatment regimen, mice were treated once daily for 5 days with a subcu-taneous dose of 5-aza (45). In both xenografted tumors we evaluated, robust induction of HORMAD1 was observed in this setting (Fig. 4E). Thus, 5-aza exposure may be sufficient to boost presentation of HORMAD1 antigenic peptides for immune-targeting. However, due to the nonspecificity of demethylating agents, it is difficult to conclude that 5-aza exposure leads to acquisition of phenotypes associated with the HORMAD1 sub-type classification.
Figure 4.
HORMAD1 activated by demethylation in NSCLC. A, HORMAD1-negative cell lines were exposed to 5-aza (1 μmol/L) for 48 hours, and mRNA expression was quantified by qPCR. Bars represent the mean (n = 3) ± SD. B, HORMAD1-positive cell lines were exposed to 5-aza (1 μmol/L) for 48 hours, and mRNA expression was quantified by qPCR. Bars represent the mean (n = 3) ± SD. C, Indicated cell lines were exposed to 5-aza (1 μmol/L) for 48 hours, and whole-cell lysates collected at 72 hours and immunoblotted with indicated antibodies. D, H1993 and HCC44 cells were exposed to 5-aza (1 μmol/L) for 48 hours, and mRNA expression was quantified by qPCR at indicated time points. Bars represent the mean (n = 2) range. E, Top, xenograft experiment schematic. Middle, mRNA expression from xenograft tumors quantified by qPCR. Bars represent the mean, and outlined circles indicate individual tumors (n ≥ 3). Bottom, immunoblots with indicated antibodies for representative tumor xenografts.
HORMAD1 is required for DNA repair by homologous recombination
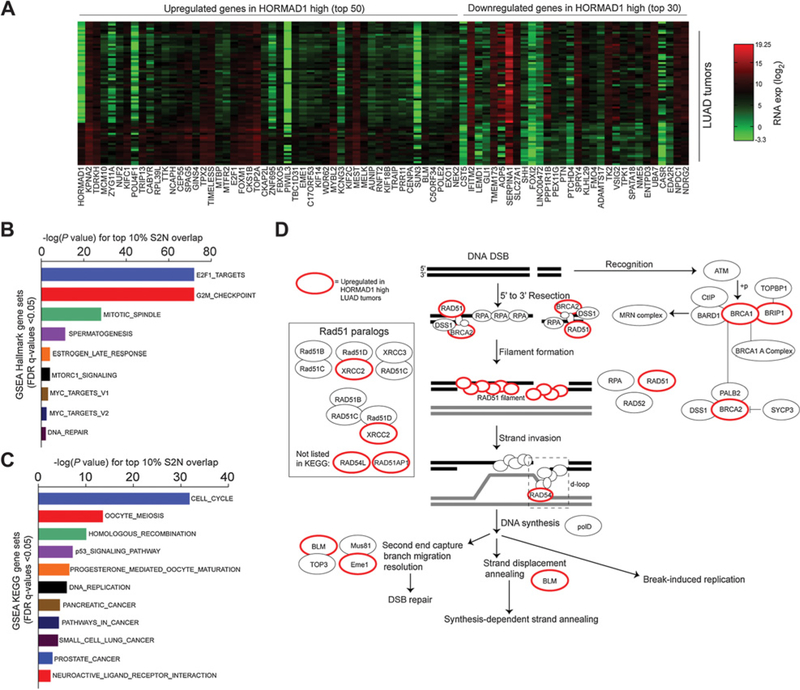
To determine whether the HORMAD1(+) LUAD subtype contains significant molecular differences from HORMAD1(–) tumors, we applied a signal-to-noise (S2N) expression profiling analysis on LUAD NSCLC from the TCGA expression datasets (Fig. 5A). Upregulated genes were subjected to GSEA Hallmark analysis, which indicated a significant enrichment of cell cycle, G2–M checkpoint, and mitotic genes (Fig. 5B; Supplementary Fig. S3A). GSEA KEGG pathway analysis revealed genes involved in cell cycle, meiosis, and homologous recombination (HR) pathways (Fig. 5C; Supplementary Fig. S3B). By manual inspection of the top 10% of upregulated genes, we identified a number of genes associated with mitosis, indicating that HORMAD1 tumors are highly proliferative (Supplementary File S1). In addition, we noted elevated expression of genes previously classified as CTAs, suggesting that HORMAD1 may be induced as part of a broader spermatogenic program in LUAD. We also observed activation of multiple components of the E2F1 transcriptional network that promotes DNA damage signaling (Supplementary Fig. S3A; Supplementary File S1). Significantly, we identified many of the key factors involved in HR (including BRCA1, BRCA2, RAD54L, RAD51, and EME1) as well as components involved in DSB checkpoint signaling (CHEK1, CHEK2) as upregulated in HORMAD1(+) tumors (Fig. 5D; Supplementary Fig. S3B). Together, these findings suggest that HORMAD1 expression is correlated with an elevated expression of DNA damage repair proteins, particularly those involved in HR.
Figure 5.
DNA repair genes upregulated in HORMAD1-high tumors. A, S2N analysis heat map for genes differentially expressed based on HORMAD1 mRNA expression (log2 RNA-seq values) in patients with LUAD. B, Gene sets with significant overlap (FDR q < 0.05) between the top 10% of genes identified by S2N (HORMAD1-high expressing tumors) and GSEA Hallmark Gene Sets. Bars indicate the –log of the P for overlap between the two gene sets. C, Gene sets with significant overlap (FDR q < 0.05) between the top 10% of genes identified by S2N (HORMAD1-high expressing tumors) and GSEA KEGG Gene Sets. Bars indicate the –log of the P value for overlap between the two gene sets. D, KEGG pathway schematic for HR pathway in human. Genes outlined in red are among top 10% of genes identified by S2N (HORMAD1-high expressing tumors).
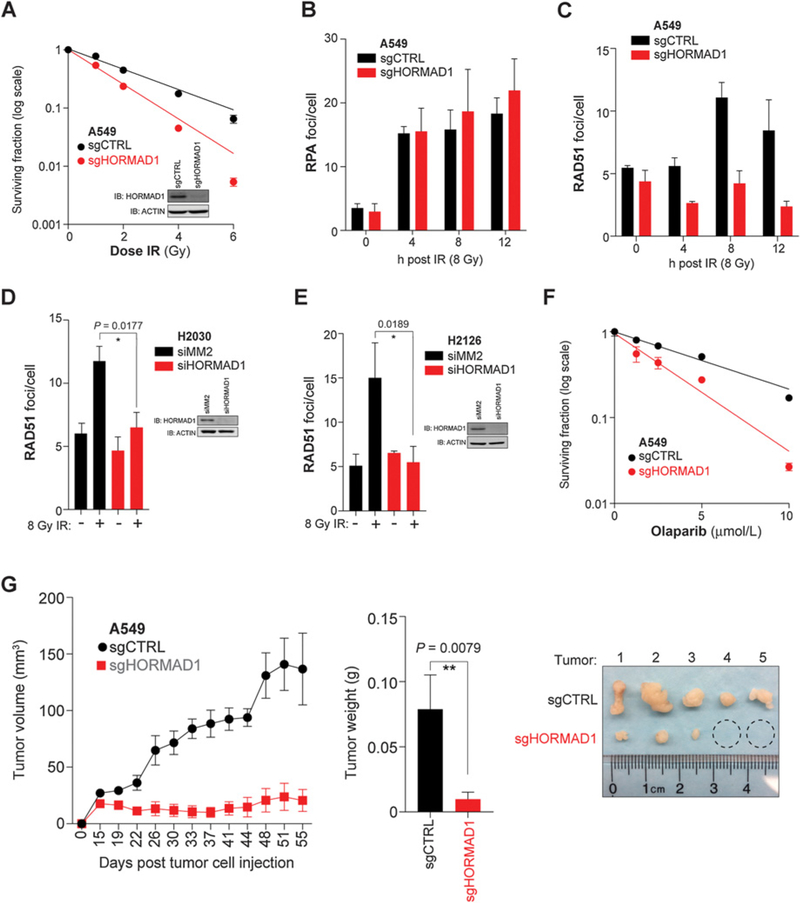
Given HORMAD1’s functional role in meiotic HR and the upregulation of a number of DNA repair genes in HORMAD1(+) human LUAD tumors, we evaluated the ability of HORMAD1 to modulate this DNA DSB repair pathway. We performed clonogenic survival assays following irradiation (IR) using sgCTRL and sgHORMAD1 A549 cells. In this setting, A549 sgHORMAD1 cells exhibited enhanced radiosensitivity as compared with control cells, suggesting that HORMAD1 may be instrumental in the repair of IR-induced DSBs (Fig. 6A). We next asked whether HORMAD1 plays a direct role in HR by examining DNA end resection and recruitment of HR factors to IR-generated DSBs. The repair process is initiated by resection of the DSB end, which produces ssDNA ends that are quickly bound by the RPA protein. We did not observe a significant difference in RPA foci formation following IR in sgHORMAD1 A549 cells as compared with control, which indicates that HORMAD1 is not required for initiation of HR (Fig. 6B). The ATPase, RAD51, replaces RPA on the ssDNA and is required for strand invasion for HR to occur. Thus, RAD51 nucleofilament formation is a well-established marker to measure on-going HR. Strikingly, in sgHORMAD1 cells, we observe a significant decrease in RAD51 focus formation at multiple time points after IR (Fig. 6C). A549 cells are p53 wild-type and KRAS mutant; thus, we tested additional cell lines with opposite mutations: H2126 (p53 mutant, KRAS wild-type) and H2030 (p53 mutant, KRAS mutant). Importantly, the loss of RAD51 loading following HORMAD1 depletion was recapitulated in these different genetic backgrounds (Fig. 6D and E).
Figure 6.
HORMAD1 is required for HR DNA repair. A, Clonogenic survival in A549 cells at doses (Gy) indicated. Each data point represents the mean (n = 3) ± SD. Curves are nonlinear (semi-log) fits to the respective data. Whole-cell lysates were immunoblotted with indicated antibodies. B, A549 cells were irradiated with 8 Gy. RPA2 foci in EdU-positive cells were quantified at indicated time points (at least 60 cells per condition). Bars represent the mean (n ≥ 2) ± range. C, A549 cells were irradiated with 8 Gy. RAD51 foci in EdU-positive cells were quantified at indicated time points (at least 60 cells per condition). Bars represent the mean (n = 2) ± range. D, H2030 cells were transfected with indicated siRNA for 72 hours, fixed, and stained 8 hours after IR (8 Gy). RAD51 foci in EdU-positive cells were quantified (at least 90 cells per condition). Bars represent the mean (n = 4) ± SD. P value calculated by two-tailed, unpaired t test, and normality assessed by Shapiro–Wilk test. Whole-cell lysates were immunoblotted with indicated antibodies. E, H2126 cells were transfected with indicated siRNA for 72 hours, fixed, and stained 8 hours after IR (8 Gy). RAD51 foci in EdU-positive cells were quantified (at least 45 cells per condition). Bars represent the mean (n = 3) ± SD. P value calculated by two-tailed, unpaired t test, and normality assessed by Shapiro–Wilk test. Whole-cell lysates were immunoblotted with indicated antibodies. F, Clonogenic survival in A549 cells at doses of olaparib indicated. Each data point represents the mean (n = 3) ± SD. Curves are nonlinear fits (semi-log) to the respective data. G, A549 xenograft experiment. Left, tumor volume measurements were taken by caliper on indicated days. Each data point represents the mean (n = 5) ± SEM. Middle, mass of excised tumors. Bars represent the mean (n = 5) ± sem. P value calculated by Mann–Whitney test. Right, images of individual tumors (ruler indicates cm) and dashed circles indicate tumors that were not large enough to collect.
HR-defective cell lines and tumors exhibit increased sensitivity to small-molecule inhibitors of PARP1. Given that HORMAD1 appears essential for HR, we asked whether HORMAD1-depleted cells exhibit enhanced sensitivity to PARP1 inhibition. We performed clonogenic survival assays on sgCTRL and sgHORMAD1 A549 cells exposed to olaparib. HORMAD1 loss led to a dramatic decrease in tumor cell survival (Fig. 6F). Collectively, the data suggest that HORMAD1 directly regulates HR in the steps between DNA end resection and RAD51 nucleofilament formation.
We next sought to determine the significance of HORMAD1 inhibition to DNA damage–inducing agents in vivo. We attempted to establish xenograft tumors with sgCTRL and sgHORMAD1 A549 cells. However, the sgHORMAD1 cells exhibited limited, if any, in vivo tumor growth (Fig. 6G). This finding indicates that HORMAD1 is required for tumor establishment and growth, a phenotype that was not observed in vitro.
Discussion
We have demonstrated the utility of NPF toxicity profiling to reveal unique phenotypic subtypes in NSCLC. We discovered differential sensitivities to the Complex I inhibitor, PA, in NSCLC. PA inhibits oxidative phosphorylation and promotes the generation of ROS. Although Complex I inhibitors have previously been suggested as anticancer agents, the inhibition of ATP production and induction of ROS would likely lead to toxicity in normal tissues. However, we find a subset of NSCLC that exhibits enhanced sensitivity to the PA-containing crude NPF fraction, SNB-051–14, as compared with nontransformed human bronchial epithelial cells (Supplementary Fig. S1C). This suggests the possibility that a therapeutic window may exist for treatment with PA, perhaps due to an acute dependence on the ETC for ATP production and/or a deficiency in oxidizing H2O2 in a subset of NSCLC tumors.
Availability of comprehensive molecular annotations of NSCLC cell lines allowed us to connect chemical sensitivity to functional biomarkers for downstream analysis. Surprisingly, HORMAD1 expression correlated with PA resistance. In our studies, HORMAD1 does not appear to be necessary or sufficient to alter PA sensitivity. However, we discovered a novel function for HORMAD1 in DNA repair, specifically by stabilizing RAD51 filaments during HR. The requirement for HORMAD1 in HR may reflect a requirement for enhanced DNA repair for survival. The activation of oncogenes is well known to induce the generation of DNA damage, which could pose a bottleneck to survival due to the activation of the DNA damage response pathways and subsequent apoptosis or senescence (46). Engagement of HORMAD1 could promote HR efficiency and escape from cell death. In support of this hypothesis, HORMAD1-high tumors have a significantly elevated mutation burden that may otherwise engage DNA damage checkpoints and limit survival. Thus, we propose that in the context of LUAD, HORMAD1 may be conscripted to promote efficient and accurate DNA repair, thereby conferring a selective advantage by preventing further accumulation of deleterious mutations. One source of this DNA damage could be cellular ROS, which is frequently increased in tumor cells. As the HORMAD1(+) subtype appears to reduce ROS more efficiently, we hypothesize that these cells have evolved mechanisms to both mitigate ROS and ensuing DNA damage. Significantly, a growing body of evidence indicates that DNA repair capacity is influenced by the redox state of a cell as reduced forms of certain proteins are essential for activating repair pathways (47). Future studies detailing HORMAD1’s mechanism of action in HR could reveal vulnerabilities related to DNA damage machinery present in this subtype. Moreover, the capacity of HORMAD1 to promote HR also suggests that the expression of HORMAD1 could serve as a biomarker for response to chemotherapy and/or radiosensitivity.
The association of HORMAD1 with mutation burden and poor survival exclusively in LUAD, but not LUSC, suggests that its function could be lineage dependent. Well-documented differences, particularly in sensitivity to folate metabolism inhibitors, suggest that these two histologic subtypes exhibit divergent dependencies for DNA repair (48). Furthermore, a study by Watkins and colleagues has indicated that HORMAD1 correlates with elevated chromosomal scarring in triple-negative breast cancer. However, these authors found that HORMAD1 promotes nonhomologous end joining in this disease setting (1). The discrepancies in reported function may reflect selectivity with respect to where and when a meiotic CTA is activated and thus how it may be engaged to promote tumorigenesis. Significantly, study of HORMAD1’s molecular mechanism of action in these different contexts could reveal novel biology associated with lineage-specific preferences for DNA repair.
CTAs are well-established targets for adoptive T-cell therapies (ATC). The safety and efficacy of ATC are highly dependent on both a tumor-specific and abundant expression of the antigen target. We hypothesize that HORMAD1 may represent an ideal immunotherapeutic target for a number of reasons. First, HORMAD1 expression appears to be highly restricted to testis. Significantly, mice lacking HORMAD1 exhibit no defects aside from a loss of fertility, indicating the dispensability of HORMAD1 in adult tissues (18). Thus, HORMAD1 antigen presentation may be highly restricted to tumor cells, which is essential for the safety and specificity of antigen-based ATC. Second, our study demonstrates that exposure to 5-aza may boost HORMAD1 protein expression, particularly in the HORMAD1(–) state. Thus, this treatment may generate sufficient antigen presentation to provoke T-cell–mediated engagement (49). Third, the elevated mutation burden observed in tumors expressing extremely high levels of HORMAD1 suggests an enhanced neoantigen load. Neoantigen load has recently been correlated with response to both ATC and immune-checkpoint blockade (50). HORMAD1 itself may be a target for ATC in these tumors and may be able to predict responsiveness to PD-1 blockade. Importantly, HORMAD1 may predict sensitivity to PD-1 inhibitors in patients with NSCLC that are typically classified as nonresponders due to ROS1 amplification, ALK4 translocation, or EGFR mutation. Prospective studies associating HORMAD1 mRNA or IHC expression with sensitivity to these agents will be important for assessing the capacity of HORMAD1 to serve as an immunotherapy biomarker. These studies will be complemented by those predicting neoantigen load and tumor-infiltrating lymphocytes in HORMAD1-positive tumors.
Supplementary Material
Significance:
This study uses a chemigenomics approach to demonstrate that anomalous expression of the CTA HORMAD1 specifies resistance to oxidative stress and promotes HR to support tumor cell survival in NSCLC.
Acknowledgments
The authors would like to thank Michael A. White for helpful discussions and Melanie Cobb for critical review of the article. A.W. Whitehurst was supported by Department of Defense (LC130495), NCI (R01CA196905), and AACR-SU2C (SU2C-AACR-IRG1211). B.A. Nichols was supported by NCI (R01CA196905) and CPRIT (RP140100). B.A. Nichols and E.A. McMillan were supported by NIH (5T32GM008203). J.D. Minna, I.I. Wistuba, A.W. Whitehurst, P.A. Villalobos, and J. Rodriguez-Canales were supported by University of Texas Lung SPORE (P50CA070907). I.I. Wistuba, P.A. Villalobos, and J. Rodriguez-Canales were supported by NCI (P30CA016672). Screening studies were supported through NIH (U01 CA176284) to J.B. MacMillan, J.D. Minna, and B.A. Posner. J.B. MacMillan and J.D. Minna were also supported by the Margot Johnson Foundation. J.B. MacMillan was supported by NIH (R01CA149833) and the California Tobacco-Related Disease Research Program (271R-0033). A.J. Davis was supported by NCI (CA092584 and CA162804). These studies were supported by the Simmons Cancer Center Core grant NCI P30CA142543.
Disclosure of Potential Conflicts of Interest
B.A. Posner reports receiving commercial research grant from Pfizer, Inc. J.D. Minna receives licensing fees from the NIH and University of Texas Southwestern Medical Center for lung cancer cell lines. No potential conflicts of interest were disclosed by the other authors.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Watkins J, Weekes D, Shah V, Gazinska P, Joshi S, Sidhu B, et al. Genomic complexity profiling reveals that HORMAD1 overexpression contributes to homologous recombination deficiency in triple-negative breast cancers. Cancer Discov 2015;5:488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappell KM, Sinnott R, Taus P, Maxfield K, Scarbrough M, Whitehurst AW. Multiple cancer testis antigens function to support tumor cell mitotic fidelity. Mol Cell Biol 2012;32: 4131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pineda CT, Ramanathan S, Fon Tacer K, Weon JL, Potts MB, Ou YH, et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 2015;160:715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Mutter-Rottmayer E, Greenwalt AM, Goldfarb D, Yan F, Yang Y, et al. A neomorphic cancer cell-specific role of MAGE-A4 in trans-lesion synthesis. Nat Commun 2016;7:12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxfield KE, Taus PJ, Corcoran K, Wooten J, Macion J, Zhou Y, et al. Comprehensive functional characterization of cancer-testis antigens defines obligate participation in multiple hallmarks of cancer. Nat Commun 2015;6:8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 2005;122:835–47. [DOI] [PubMed] [Google Scholar]
- 7.Michael AK, Harvey SL, Sammons PJ, Anderson AP, Kopalle HM, Banham AH, et al. Cancer/testis antigen PASD1 silences the circadian clock. Mol Cell 2015;58:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitehurst AW, Xie Y, Purinton SC, Cappell KM, Swanik JT, Larson B, et al. Tumor antigen acrosin binding protein normalizes mitotic spindle function to promote cancer cell proliferation. Cancer Res 2010;70:7652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pineda CT, Potts PR. Oncogenic MAGEA-TRIM28 ubiquitin ligase down-regulates autophagy by ubiquitinating and degrading AMPK in cancer. Autophagy 2015;11:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schramm S, Fraune J, Naumann R, Hernandez-Hernandez A, Hoog C, Cooke HJ, et al. A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination, and fertility. PLos Genet 2011;7:e1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa Y, Speed R, Ollinger R, Alsheimer M, Semple CA, Gautier P, et al. Two novel proteins recruited by synaptonemal complex protein 1 (SYCP1) are at the centre of meiosis. J Cell Sci 2005;118:2755–62. [DOI] [PubMed] [Google Scholar]
- 12.Bolcun-Filas E, Hall E, Speed R, Taggart M, Grey C, de Massy B, et al. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLos Genet 2009;5:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz RL, Alcid AD, Berger JM, Keeney S. Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation. Mol Cell Biol 2002;22:1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellani MA, Boateng KA, McLeod D, Camerini-Otero RD. The expression profile of the major mouse SPO11 isoforms indicates that SPO11beta introduces double strand breaks and suggests that SPO11alpha has an additional role in prophase in both spermatocytes and oocytes. Mol Cell Biol 2010;30:4391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carofiglio F, Sleddens-Linkels E, Wassenaar E, Inagaki A, van Cappellen WA, Grootegoed JA, et al. Repair of exogenous DNA double-strand breaks promotes chromosome synapsis in SPO11-mutant mouse meiocytes, and is altered in the absence of HORMAD1. DNA Repair (Amst) 2018;63:25–38. [DOI] [PubMed] [Google Scholar]
- 16.Shin YH, McGuire MM, Rajkovic A. Mouse HORMAD1 is a meiosis i checkpoint protein that modulates DNA double- strand break repair during female meiosis. Biol Reprod 2013;89:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, et al. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 2011;13:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, et al. Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLos Genet 2010;6: e1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Eckardt S, Leu NA, McLaughlin KJ, Wang PJ. Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J Cell Biol 2008;180:673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kogo H, Tsutsumi M, Inagaki H, Ohye T, Kiyonari H, Kurahashi H. HORMAD2 is essential for synapsis surveillance during meiotic prophase via the recruitment of ATR activity. Genes Cells 2012;17:897–912. [DOI] [PubMed] [Google Scholar]
- 21.Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 2000;6:975–87. [DOI] [PubMed] [Google Scholar]
- 22.de Vries FA, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, et al. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Gen Develop 2005;19:1376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC. The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell 2017;67:1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tureci O, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. PNAS 1998;95:5211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atanackovic D, Blum I, Cao Y, Wenzel S, Bartels K, Faltz C, et al. Expression of cancer-testis antigens as possible targets for antigen-specific immunotherapy in head and neck squamous cell carcinoma. Cancer Biol Ther 2006;5:1218–25. [DOI] [PubMed] [Google Scholar]
- 26.Chen YT, Venditti CA, Theiler G, Stevenson BJ, Iseli C, Gure AO, et al. Identification of CT46/HORMAD1, an immunogenic cancer/testis antigen encoding a putative meiosis-related protein. Cancer Immun 2005;5:9. [PubMed] [Google Scholar]
- 27.Liu M, Chen J, Hu L, Shi X, Zhou Z, Hu Z, et al. HORMAD2/CT46.2, a novel cancer/testis gene, is ectopically expressed in lung cancer tissues. Mol Hum Reprod 2012;18:599–604. [DOI] [PubMed] [Google Scholar]
- 28.Potts MB, McMillan EA, Rosales TI, Kim HS, Ou YH, Toombs JE, et al. Mode of action and pharmacogenomic biomarkers for exceptional responders to didemnin B. Nat Chem Biol 2015;11:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillan EA, Ryu M-J, Diep CH, Mendiratta S, Clemenceau JR, Vaden RM, et al. Chemistry-first approach for nomination of personalized treatment in lung cancer. Cell 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelps RM, Johnson BE, Ihde DC, Gazdar AF, Carbone DP, McClintock PR, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem Suppl 1996;24:32–91. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science (New York, NY) 2014;343: 80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. PNAS 2005;102: 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Fenical W. The unique chemistry and biology of the piericidins. J Antibiot (Tokyo) 2016;69:582–93. [DOI] [PubMed] [Google Scholar]
- 35.Palorini R, Simonetto T, Cirulli C, Chiaradonna F. Mitochondrial complex I inhibitors and forced oxidative phosphorylation synergize in inducing cancer cell death. Int J Cell Biol 2013;2013:243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urra FA, Munoz F, Lovy A, Cardenas C. The mitochondrial Complex(I)ty of cancer. Front Oncol 2017;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer 2014;14:709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell 2015;27:156–7. [DOI] [PubMed] [Google Scholar]
- 39.Naguib A, Mathew G, Reczek CR, Watrud K, Ambrico A, Herzka T, et al. Mitochondrial complex I inhibitors expose a vulnerability for selective killing of pten-null cells. Cell Rep 2018;23:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W, Tian H, Yue W, Li L, Li S, Gao C, et al. Rotenone induces apoptosis in human lung cancer cells by regulating autophagic flux. IUBMB Life 2016;68:388–93. [DOI] [PubMed] [Google Scholar]
- 41.Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol Carcinog 2010;49:141–51. [DOI] [PubMed] [Google Scholar]
- 42.Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, et al. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta 2009;1787:384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedrich T, van Heek P, Leif H, Ohnishi T, Forche E, Kunze B, et al. Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I). Relationship of one site with the ubiquinone-binding site of bacterial glucose:ubiquinone oxidoreductase. Eur J Biochem 1994;219:691–8. [DOI] [PubMed] [Google Scholar]
- 44.Johnson WJ, McColl JD. 6-Aminonicotinamide–a potent nicotinamide antagonist. Science (New York, NY) 1955;122:834. [DOI] [PubMed] [Google Scholar]
- 45.Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 2015;64:1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006;444:633–7. [DOI] [PubMed] [Google Scholar]
- 47.Luo M, He H, Kelley MR, Georgiadis MM. Redox regulation of DNA repair: implications for human health and cancer therapeutic development. Antioxidants Redox Signal 2010;12:1247–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen MH, Justice R, Pazdur R. Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist 2009;14:930–5. [DOI] [PubMed] [Google Scholar]
- 49.Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res 2014;2:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun 2017;8:1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.