Abstract
Objective: Photodynamic therapy (PDT) using 10% 5-aminolevulinic acid (ALA) gel (GEL) has been shown to be highly effective for treating actinic keratosis (AK) but has only been studied using red-light activation. The goal of this study was to compare GEL and a 20% ALA solution (SOL) using blue-light activation under typical clinical conditions. Design: This double-blind, split-face study randomized subjects to GEL or SOL application to contiguous 25cm2 fields containing 4 to 8 AK lesions on either side of the face or scalp (no curettage, 1-hour incubation, no occlusion) followed by blue light exposure (1,000 seconds, 417nm, 10J/cm2). Participants: Forty adult subjects were treated on either the face (n=20) or scalp (n=20). Measurements: Primary outcomes included change in baseline AK lesions. Secondary outcomes included local skin reaction (LSR) scores and visual analog scale (VAS) pain scores. Results: Lesions treated with GEL were 97.1 percent cleared at Day 84 versus 94.9 percent for lesions treated with SOL (p<0.001 vs. baseline); additionally, 86.8 percent of areas treated with GEL and 78.9 percent of areas treated with SOL showed 100-percent clearance (p<0.001 vs. baseline). Mean VAS pain scores were minimal for the SOL and the GEL (25.4 vs. 28.5 and 16.1 vs. 19.3, respectively; p=nonsignificant). At three days after the first and second treatments, more significant LSRs were noted in areas treated with SOL, including erythema, crusting, and scaling/dryness. There were no significant adverse events observed. Conclusion: GEL was equivalent to SOL for clearing AK lesions on the face and scalp with blue-light PDT; however, SOL caused significantly more local skin reactions.
Keywords: actinic keratosis, photodynamic therapy, 5-aminolevulinic acid, blue light
Actinic keratosis (AK) is a condition frequently encountered by dermatologists. AK presents as rough, scaly, erythematous lesions on sun-damaged areas of the face, scalp, trunk, and extremities. Chronic sun exposure causes abnormal growth of atypical epidermal keratinocytes and subsequent AK development. AK is a precancerous lesion that can progress to squamous cell skin cancer (SCC).1,2 In one study, more than 40 percent of patients with a prior diagnosis of multiple AKs developed a nonmelanoma skin cancer or melanoma during 5 to 11 years of follow-up.3
Photodynamic therapy (PDT) is a highly effective means for treating precancerous skin lesions.4 It is currently considered the method of choice for treating AK5 and is being used with increasing frequency for this purpose.6 Many AK lesions are clinically detectible; however, a larger area of skin is frequently affected by subclinical lesions in the deeper layers of the epidermis.7 Consequently, PDT can be especially helpful where there are multiple or confluent lesions.8,9 PDT has also demonstrated effectiveness in treating field cancerization associated with AK lesions, which is important for preventing the occurrence of additional AK lesions and subsequent development of SCC.10,11
The most commonly used photosensitizer in the United States (US) is 5-aminolevulinic acid (ALA). When topically applied, ALA is preferentially absorbed by dysplastic AK lesions, where it is metabolized intracellularly to protoporphyrin IX, a highly potent photosensitizer.12 When exposed to the appropriate wavelength of light in the presence of oxygen, the activation of protoporphyrin IX results in the production of reactive oxygen species and cell death. Surrounding normal tissues are minimally affected.6 A new photosensitizer (10% ALA gel; GEL) is the only PDT product that is approved by the US Food and Drug Administration for both lesion- and field-directed PDT.13
The GEL was developed for use with red illumination,13 while a 20% ALA solution (SOL) was developed for use with blue illumination,14 which is the predominant light source used in the US for PDT. Consequently, a frequent question posed by dermatologists is whether GEL can be used together with a blue illuminator for the treatment of AK. The objective of this randomized, double-blind, parallel-group study was to compare the efficacy and tolerability of GEL to SOL for treating adult subjects with AK lesions on the face and scalp following blue-light illumination under typical clinical conditions.
METHODS
Subjects. Eligible subjects (N=40) included men and women who were at least 18 years of age with 4 to 8 discrete AK lesions within a contiguous 25cm2 field on each side of the face (n=20) or scalp (n=20). Female subjects were postmenopausal, surgically sterile, or using an effective method of birth control. Women of childbearing potential were required to have a negative urine pregnancy test result at the respective screening and baseline visits. Enrolled subjects expressed their willingness to comply with all study requirements.
Subjects were excluded from participation if they presented with an incompletely healed wound, hypertrophic or hyperkeratotic lesions, cutaneous horns, or lesions that had not, to date, responded to repeated cryosurgery within the planned treatment area. Those with a history of recent use of medications or treatments that could interfere with evaluating the treatment area, including but not limited to topical medications, oral retinoids, immunomodulating agents, cytotoxic drugs, ultraviolet B phototherapy, or other therapies for AK, were also excluded.
Treatment. The screening visit occurred at 1 to 14 days prior to the baseline visit. During the screening visit, demographic information and a medical history including concomitant medications were obtained, a physical examination including vital signs and urine pregnancy tests was performed, and the treatment area was identified. During the baseline visit (Day 0), vital signs and the urine pregnancy test were repeated and the target treatment area was photographed.
Subjects were treated bilaterally either on the face or the scalp. For each group, subjects were randomized to receive treatment with GEL for topical use (Ameluz®; Biofrontera, Inc., Wakefield, Massachusetts) or SOL for topical use (Levulan® Kerastick®; DUSA Pharmaceuticals, Inc., Wilmington, Massachusetts) either on the right or left side of the face or scalp. After washing with soap and water and degreasing the skin with alcohol, GEL or SOL was applied to cover the 25cm2 area on each side, per the randomization, without curettage or occlusion, for one hour followed by 1,000 seconds of blue light illumination (10J/cm2) (BLU-U® Blue Light Photodynamic Therapy Illuminator; DUSA Pharmaceuticals, Inc., Wilmington, Massachusetts). The application of study treatments and study assessments were performed by different staff members.
Subjects returned to the study site, and the following procedures and assessments were performed at the indicated times:
Treatment discomfort was measured by visual analog scale (VAS) scores for both the left and right sides of the face or scalp on Days 0 and 28 following the treatment.
Treatment areas were photographed and local skin reactions (LSRs) and other adverse events (AEs) were recorded on Days 0, 3, 14, 28, 31, 42, 56, and 84.
AK lesions were assessed, including lesion counts, mapping, size, and grade on Days 0, 14, 28, 42, 56, and 84 (in addition to the screening visit).
Vital signs were recorded, and urine pregnancy tests were completed on Days 0 and 28 (in addition to the screening visit).
All subjects were treated at Day 0 and were retreated per the original right or left randomization scheme on Day 28.
Primary endpoint. The primary endpoint was a comparison of the effectiveness of GEL and SOL regarding the ability to decrease the percentage of AK lesions on the face and scalp after illumination with a blue-light PDT illuminator as measured in percent reduction from baseline.
Secondary endpoints. The secondary endpoints were to assess global photodamage and determine the comparative tolerability of GEL and SOL followed by blue-light PDT illumination with respect to erythema, edema, oozing/vesiculation/crusting, dryness/scaling, hyperpigmentation, and hypopigmentation. Subjects were issued daily diaries for recording LSRs and other possible AEs.
Safety endpoints. Other safety assessments included obtaining a clinical history and recording physical findings and adverse events. Product tolerability was assessed using LSR scale scores and treatment discomfort was measured using a 100-mm VAS.
Statistical analysis. A sample size of 40 subjects randomized to receive treatment for AK on the face (n=20) and scalp (n=20) was determined to be sufficient for the planned efficacy endpoints. Endpoints were evaluated using a Student’s t-test at the 0.05 significance level. Continuous data were summarized by treatment group using descriptive statistics (number, mean, median, standard deviation, minimum, and maximum). Categorical data were summarized by treatment group using frequency tables (frequencies and percentages), and 95-percent confidence intervals (CIs) were constructed for proportion of successes. VAS scores were analyzed continuously.
The frequency and proportion of subjects with AEs were summarized by body system and preferred term using the Medical Dictionary for Regulatory Activities (MSSO, McLean, Virginia). Subjects were counted only once at each summary level if they reported one or more AE at that level. Tabular summaries presented AEs by severity and relatedness. Descriptive statistics for vital signs were presented by visit and compared with baseline data within treatment groups.
Ethics. The study was conducted in compliance with the Declaration of Helsinki (revised, 2000), the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use harmonized tripartite guideline regarding good clinical practice (E6 Consolidated Guidance, April 1996), the United States Code of Federal Regulation, and relevant local laws and regulations. The protocol used in the study was approved by a commercial institutional review board (US Investigational Review Board, Inc., Miami, Florida). Enrolled subjects provided informed consent, including photoconsent, prior to participation in any study-related activities.
RESULTS
Enrolled subjects were randomized to undergo treatment with GEL (n=20) applied to one side of the face and SOL (n = 20) applied to the other side. The clinical observation period occurred from July 2017 through November 2017. Subject demographic and baseline characteristics are summarized in Table 1.
TABLE 1.
Demographic characristics of study subjects
| Sex, n(%) | |
| Male | 36 (90) |
| Female | 4 (10) |
| Age, n(%) | |
| 50–59 years | 5 (12.5) |
| 60–69 years | 17 (42.5) |
| 70–79 years | 14 (35) |
| 80–89 years | 4 (10) |
| Race, n(%) | |
| White | 40 (100) |
| Ethnicity, n(%) | |
| Hispanic | 2 (5) |
| Non-Hispanic | 38 (95) |
| Fitzpatrick skin type, n(%) | |
| I | 3 (7.5) |
| II | 21 (52.5) |
| III | 14 (35) |
| IV | 2 (5) |
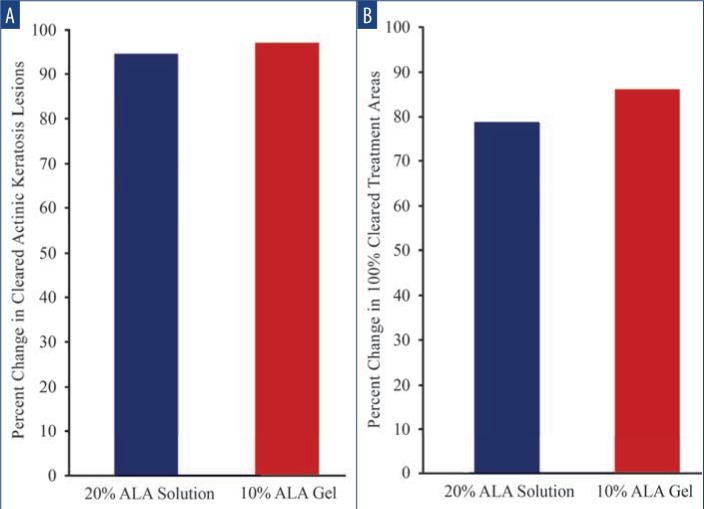
Efficacy. Among lesions treated with SOL, 57.7 percent were cleared at Day 28, increasing to 94.9 percent by Day 84 (p<0.001 vs. baseline). Among lesions treated with GEL, 52.3 percent of AK lesions were cleared at Day 28, increasing to 97.1 percent at Day 84 (p<0.001 vs. baseline) (Figure 1A). All subjects received a second treatment at Day 28. At the final follow-up visit on Day 84, 78.9 percent of areas treated with SOL and 86.8 percent of areas treated with GEL showed 100-percent clearance (for both groups, p<0.001 vs. baseline) (Figure 1B).
FIGURE 1.
Change in cleared AK lesions—A) Among lesions treated with SOL, 57.7% were cleared at Day 28; by Day 84, cleared areas had increased to 94.9% (p<0.001 from baseline). Among lesions treated with GEL, 52.3% of AK lesions were cleared at Day 28; by Day 84, these had increased to 97.1% (p<0.001 from baseline). B) At the Day 84 follow-up visit, 78.9% of areas treated with SOL and 86.8% of areas treated with GEL showed 100% clearance (for both groups, p<0.001 from baseline).
ALA: 5-aminolevulinic acid; AK: actinic keratosis; SOL: 20% ALA solution; GEL: 10% ALA gel
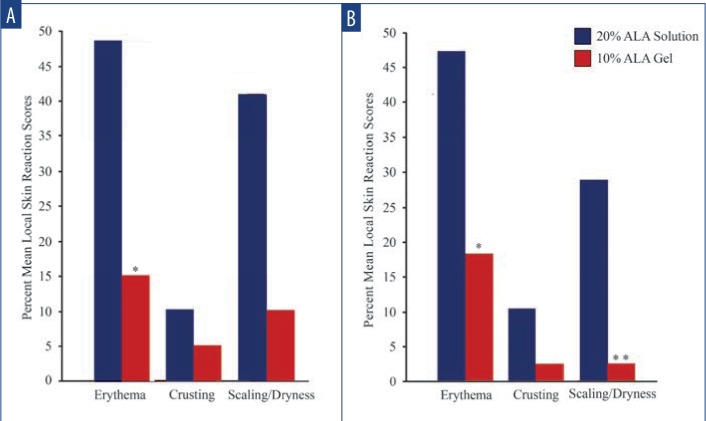
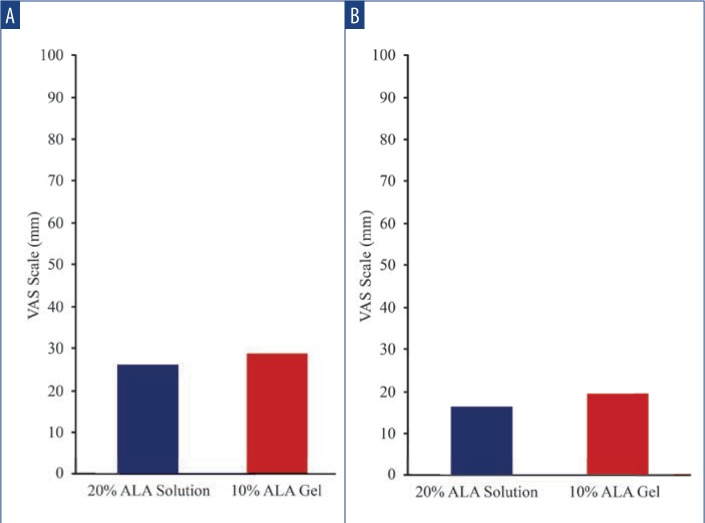
Safety and tolerability. LSR scores were higher among subjects treated with SOL at Days 3 and 31 (3 days after each treatment). At Day 3, increased LSRs associated with areas treated with SOL vs. GEL, including erythema (48.7% vs. 15.4%; p=0.002), crusting (10.3% vs. 5.1%; p=nonsignificant), and scaling/dryness (41.0% vs. 10.3%; p= 0.002) (Figure 2A) were noted. At Day 31, increased LSRs associated with areas treated with SOL included erythema (47.4% vs. 18.4%; p=0.008), crusting (10.5% vs. 2.6%; p=nonsignificant), and scaling/dryness (29.0% vs. 2.6%; p=0.002) (Figure 2B). Mean VAS pain scores for SOL and GEL were minimal and not significantly different between the first treatment (25.4 vs. 28.5) (Figure 3A) and the second treatment (16.1 vs. 19.3) (Figure 3B), respectively.
FIGURE 2.
Mean local skin reaction scores—A) Three days after the first PDT treatment, a higher percentage of local skin reactions was associated with areas treated with SOL vs. GEL, including erythema (48.7% vs. 15.4%), crusting (10.3% vs. 5.1%), and scaling/dryness (41.0% vs. 10.3%) (* denotes p=0.002). B) Three days after the second PDT, a higher percentage of local skin reactions was associated with areas treated with SOL vs. 10% GEL, including erythema (47.4% vs. 18.4%), crusting (10.5% vs. 2.6%), and scaling/dryness (29.0% vs. 2.6%) (* denotes p=0.008; ** denotes p=0.002).
ALA: 5-aminolevulinic acid; PDT: photodynamic therapy; SOL: 20% ALA solution; GEL: 10% ALA gel
FIGURE 3.
Mean VAS pain scores—A) Mean VAS pain scores for SOL and GEL were minimal and not significantly different for the first treatment (25.4 vs. 28.5, respectively). B) Mean VAS pain scores for SOL and GEL were minimal and not significantly different for the second treatment (16.1 vs. 19.3, respectively).
ALA: 5-aminolevulinic acid; VAS: visual analog scale; SOL: 20% ALA solution; GEL: 10% ALA gel
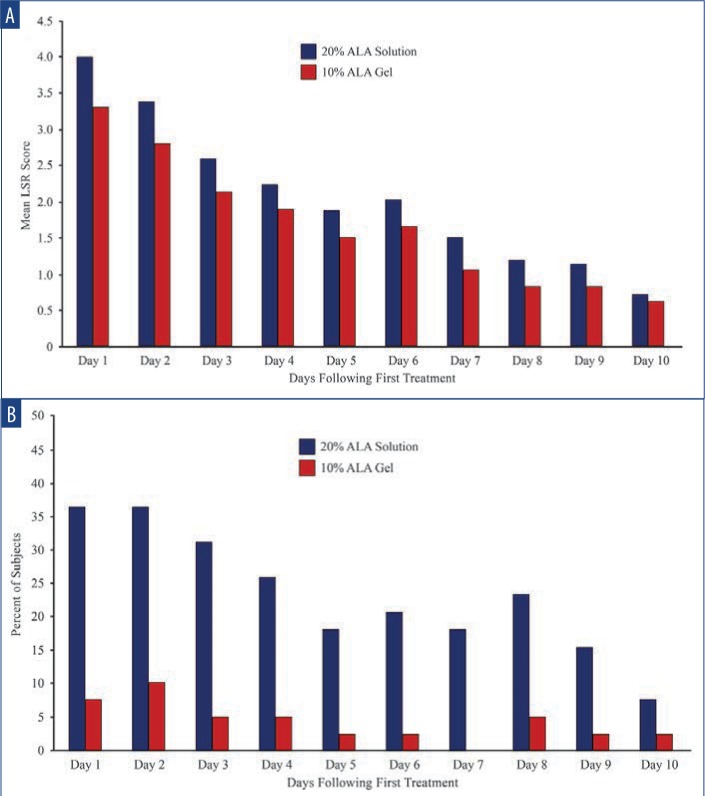
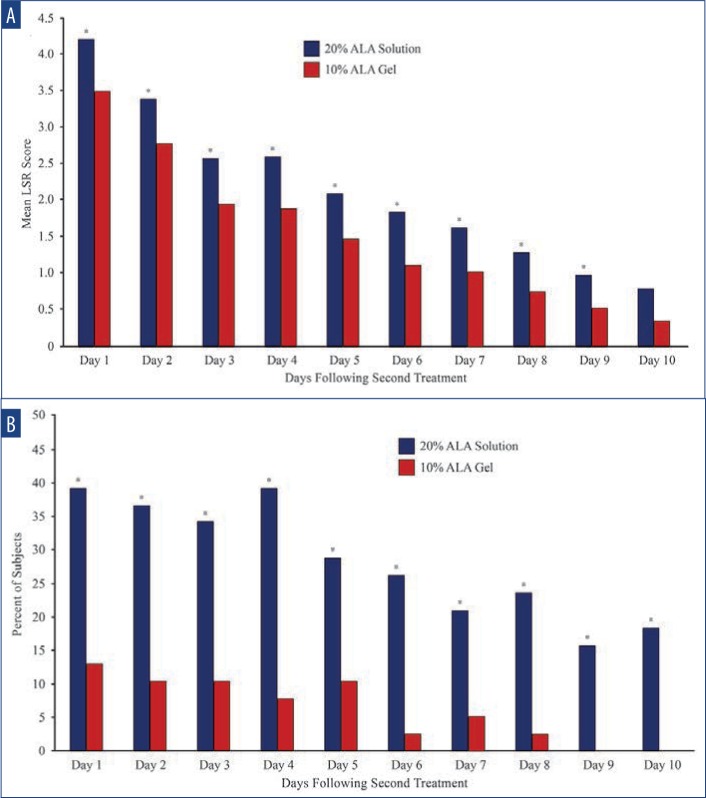
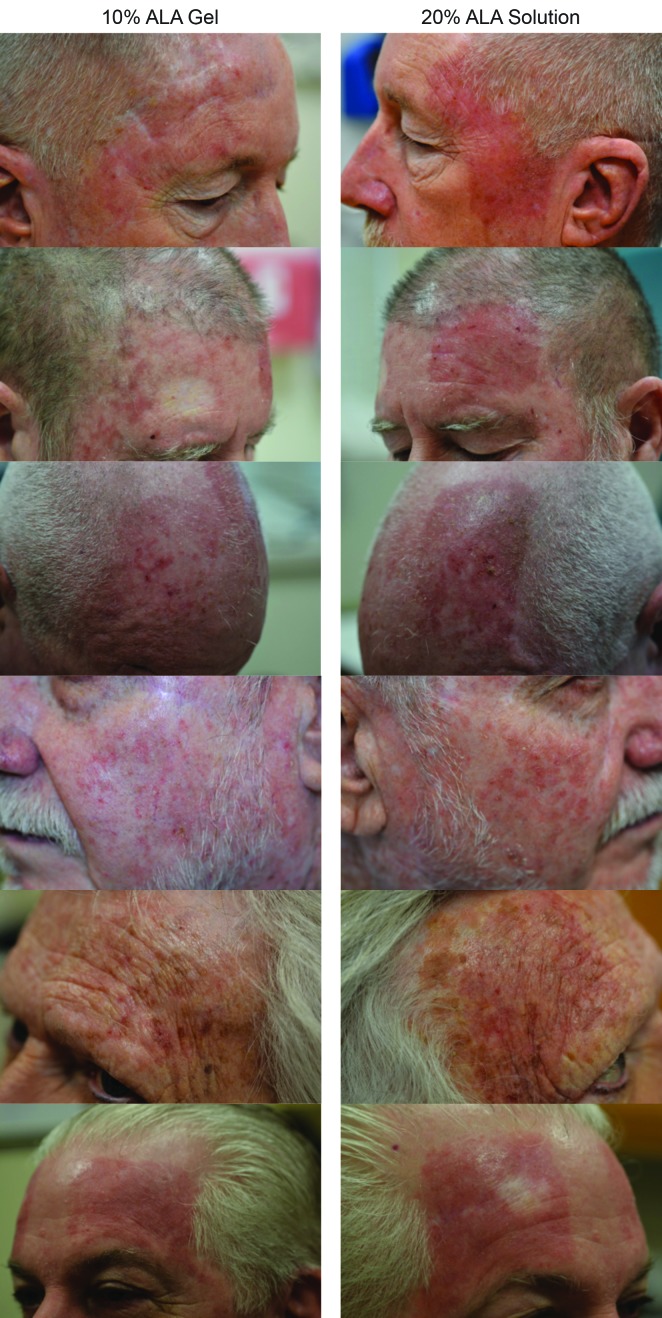
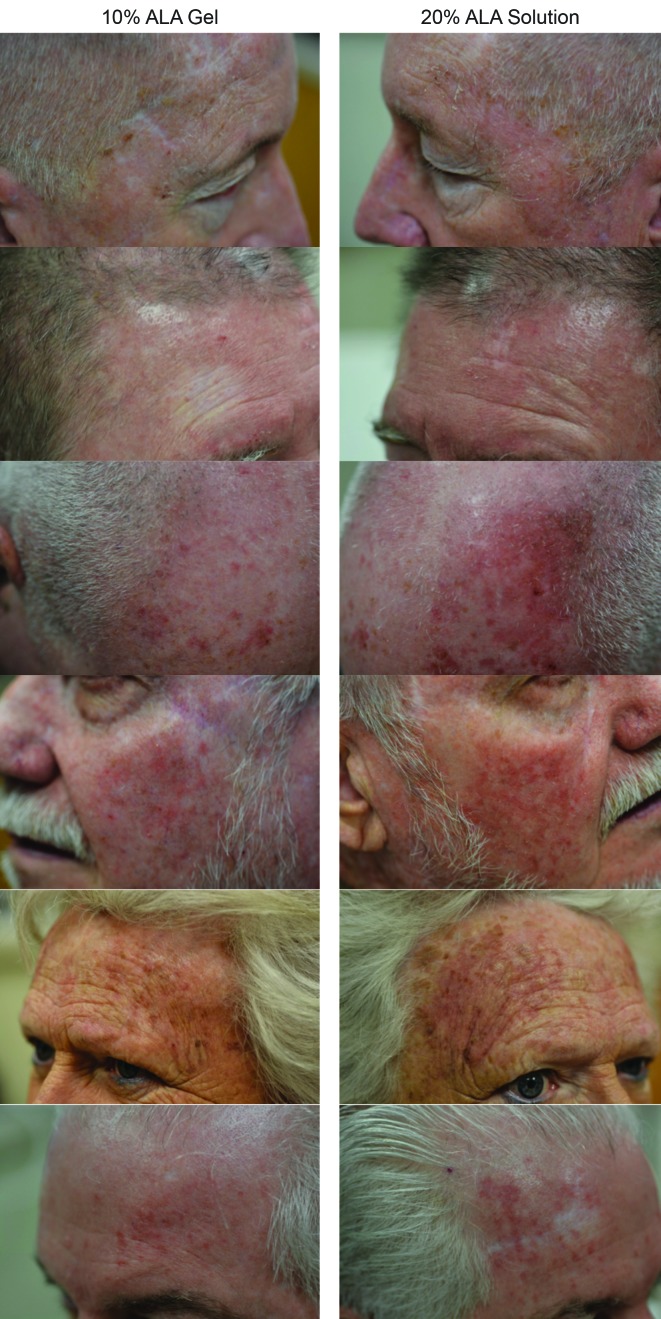
The mean self-reported LSR scores based on daily diaries of the subjects are shown following the first treatment in Figure 4A and after the second treatment in Figure 4B. Mean LSR scores were consistently higher among subjects treated with SOL. The mean percent increases in self-reported LSR scores based on subject daily diaries are shown following the first treatment in Figure 5A and after the second treatment in Figure 5B. The mean percent increase in LSR scores was significantly higher on the side treated with SOL (p˙0.05) except on Day 10 following the first treatment. The difference in LSR severity among subjects treated with SOL is evident among the subject images on Days 3 (Figure 6) and 31 (Figure 7), which were obtained at three days after the first treatment (Day 0) and second treatment (Day 28).
FIGURE 4.
Self-reported LSR scores following the first treatment—A) Mean local skin reaction scores were consistently higher among subjects treated with SOL. B) Mean percent change in subject local skin reaction scores remained consistently higher among subjects treated with SOL.
ALA: 5-aminolevulinic acid; LSR: local skin reaction; SOL: 20% ALA solution; GEL: 10% ALA gel
FIGURE 5.
Subject self-reported LSR scores following the second treatment—A) Mean LSR scores were consistently higher on the side treated with SOL. B) Mean percent change in subject LSR scores remained consistently higher on the side treated with SOL (* denotes p≤0.05).
ALA: 5-aminolevulinic acid; LSR: local skin reaction; SOL: 20% ALA solution; GEL: 10% ALA gel
FIGURE 6.
LSR severity after the first treatment—Three days after the first PDT treatment (Day 3), local skin reactions are noticeably more severe among subjects treated with SOL.
LSR: local skin reaction; PDT: photodynamic therapy; ALA: 5-aminolevulinic acid; SOL: 20% ALA solution; GEL: 10% ALA gel
FIGURE 7.
Local skin reaction severity after the second treatment—three days after the second PDT treatment (Day 31), local skin reactions are noticeably more severe among subjects treated with SOL.
LSR: local skin reaction; PDT: photodynamic therapy; ALA: 5-aminolevulinic acid; SOL: 20% ALA solution; GEL: 10% ALA gel
DISCUSSION
PDT is a highly effective means for treating precancerous skin lesions15 and is currently considered the method of choice for treating AK.5 In addition to its effectiveness, PDT has demonstrated superior cosmetic outcomes compared to surgery.12 PDT can be especially helpful where there are multiple or confluent lesions.8,9 PDT has been shown to be an effective treatment for field cancerization associated with AK lesions,16,17 which is important for preventing the occurrence of additional AK lesions and subsequent development of SCC.10,11 PDT has also been shown to be a cost-effective treatment for AK,18 and its use is described in numerous guidelines for treating AK.5,8,9,19,20
GEL is intended for use with a red-light source with a spectrum of approximately 635nm,13 while SOL was developed for use with a blue-light illuminator with a spectrum of approximately 417nm.21 As dermatologists frequently inquire about the use of GEL with blue light, the objective of this study was to attempt to answer this question by comparing the efficacy and safety of both photosensitizers for the treatment of AK when used in conjunction with a blue-light illuminator.
Both ALA products were found to be effective for treating AK lesions on the face and scalp; however, SOL was associated with more severe LSRs. The greater amount of skin reactions associated with SOL might be due to its higher concentration of aminolevulinic acid or greater specificity of GEL for dysplastic AK lesions. The GEL product is a unique oil-in-water nanoemulsion of vesicles with a mean diameter of 20nm. These vesicles are composed of a lipid core surrounded by an emulsifying monolayer of phospholipids.22 The nanoemulsion aids in the delivery of ALA directly to the epidermal layers. The ability of ALA to penetrate the skin is believed to result from the fusion of nanoemulsion vesicles with the lipid components of the stratus corneum. In an ex-vivo model, GEL displayed more intense protoporphyrin IX fluorescence than a comparator 20% ALA cream.23 Nanoemulsion formulations of this type are widely used in dermatology, as their biophysical properties permit rapid tissue penetration.24 This formulation contrasts with SOL, which is a simple alcoholic solution.
This study also provided an opportunity to assess GEL under typical clinical conditions. This is the first time GEL has demonstrated efficacy after only one hour of incubation, versus three hours, without occlusion or curettage. A longer study will be required to determine if long-term clearance rates are similar to those in the phase III GEL studies.25
GEL offers several advantages over SOL; specifically, illumination of the treatment area can be performed at three hours after the application of GEL to AK lesions13 versus waiting 14 to 18 hours following the application of SOL.14 In addition to enhancing skin penetration, the nanoemulsion technology also greatly increases product stability. Separately, GEL can be stored for up to 12 weeks in a refrigerator after opening,13 while SOL must be discarded at two hours after mixing ALA and vehicle.14
Limitations. The results of this study are limited by the relatively small population, which made it difficult to determine any statistical difference in efficacy between the two different formulations of ALA.
CONCLUSION
The primary objective of the study was to compare the effectiveness of a 20% ALA solution and a 10% ALA gel in conjunction with a blue-light photodynamic therapy illuminator for treating AK lesions on the face or scalp. Both products were effective for clearing AK lesions on the face and scalp after one-hour incubation, without occlusion or curettage, and exposure to blue light (10 J/cm2); however, the use of the 20% ALA solution was associated with significantly more severe LSRs.
ACKNOWLEDGMENT
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt with Apothekon, Inc. (funded by Biofrontera, manufacturer of the ALA gel product evaluated in this study).
REFERENCES
- 1.Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol. 2013;169(3):502–518. doi: 10.1111/bjd.12420. [DOI] [PubMed] [Google Scholar]
- 2.Czarnecki D, Meehan CJ, Bruce F, et al. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J Cutan Med Surg. 2002;6(3):207–209. doi: 10.1007/s10227-001-0041-x. [DOI] [PubMed] [Google Scholar]
- 3.Dika E, Fanti PA, Misciali C, et al. Risk of skin cancer development in 672 patients affected by actinic keratosis. G Ital Dermatol Venereol. 2016;151(6):628–633. [PubMed] [Google Scholar]
- 4.Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–827. doi: 10.1097/DSS.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 5.Braathen LR, Szeimies RM, Basset-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. International Society for Photodynamic Therapy in Dermatology, 2005. J Am Acad Dermatol. 2007;56(1):125–143. doi: 10.1016/j.jaad.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145–163. doi: 10.2147/CCID.S35334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torezan LA, Festa-Neto C. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88(5):775–786. doi: 10.1590/abd1806-4841.20132300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Berker D, McGregor JM, Mohd Mustapa MF, et al. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]
- 9.Werner RN JA, Rosumeck S, Erdmann R, Sporbeck B, Nast A. Methods and results report—evidence and consensus-based (S3) guidelines for the treatment of actinic keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum. J Eur Acad Dermatol Venereol. 2015;29(1):e1–e66. doi: 10.1111/jdv.13179. [DOI] [PubMed] [Google Scholar]
- 10.Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol. Venereol. 2017;31(Suppl 2):8–11. doi: 10.1111/jdv.14092. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):5–7. doi: 10.1111/jdv.14151. [DOI] [PubMed] [Google Scholar]
- 12.Cohen DK, Lee PK. Photodynamic therapy for nonmelanoma skin cancers. Cancers (Basel). 2016;8(10):E90. doi: 10.3390/cancers8100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biofrontera Pharmaceuticals. Wakefield, MA, USA.: Ameluz® (aminolevulinic acid HCl) gel 10% [prescribing information] [Google Scholar]
- 14.DUSA Pharmaceuticals. Wilmington, MA, USA.: Levulan® Kerastick® (aminolevulinic acid HCl) for topical solution 20% [prescribing information] [Google Scholar]
- 15.Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9(6):e96829. doi: 10.1371/journal.pone.0096829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz®) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED® lamp. Br J Dermatol. 2016;175(4):696–705. doi: 10.1111/bjd.14498. [DOI] [PubMed] [Google Scholar]
- 17.Green AC. Epidemiology of actinic keratoses. Curr Probl Dermatol. 2015;46:1–7. doi: 10.1159/000366525. [DOI] [PubMed] [Google Scholar]
- 18.Vale SM, Hill D, Feldman SR. Pharmacoeconomic considerations in treating actinic keratosis: an update. Pharmacoeconomics. 2017;35(2):177–190. doi: 10.1007/s40273-016-0462-4. [DOI] [PubMed] [Google Scholar]
- 19.Morton CA, Szeimies RM, Sidorofi A, et al. European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications - actinic keratoses, Bowen’s disease, basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27(5):536–544. doi: 10.1111/jdv.12031. [DOI] [PubMed] [Google Scholar]
- 20.Morton C, Szeimies RM, Sidorofi A, et al. European Dermatology Forum Guidelines on topical photodynamic therapy. Eur J Dermatol. 2015;25(4):296–311. doi: 10.1684/ejd.2015.2570. [DOI] [PubMed] [Google Scholar]
- 21.DUSA Pharmaceuticals, Inc. BLU-U® Blue Light Photodynamic Therapy Illuminator Model 4170. [Accessed March 18, 2018]. http://www.dusapharma.com/using-blu-u.html Available at.
- 22.Maisch T, Santarelli F, Schreml S, et al. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp Dermatol. 2010;19(8):e302–e305. doi: 10.1111/j.1600-0625.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz L, Novak B, Hoeh AK, et al. Epidermal penetration and protoporphyrin IX formation of two different 5-aminolevulinic acid formulations in ex vivo human skin. Photodiagnosis Photodyn Ther. 2016;14:40–46. doi: 10.1016/j.pdpdt.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Li YH, Gao XH, et al. The application of nanoemulsion in dermatology: an overview. J Drug Target. 2013;21(4):321–327. doi: 10.3109/1061186X.2013.765442. [DOI] [PubMed] [Google Scholar]
- 25.Dirschka T, Radny P, Dominicus R, et al. Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis. Br J Dermatol. 2013;168(4):825–836. doi: 10.1111/bjd.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]