Key Points
Question
What are the spectrum, timing, and incidence of fatal toxic effects associated with immune checkpoint inhibitors?
Findings
A broad range of regimen-specific toxic effects caused fatalities in 0.3% to 1.3% of treated patients; fatal toxic effects tended to occur very early in treatment (median of 40 and 14.5 days for monotherapy and combination immunotherapy, respectively).
Meaning
Fatal toxic effects from immune checkpoint inhibitors are rare but have diverse causes; awareness is needed among clinicians across disciplines given the increase in use of these agents.
This systematic review and meta-analysis analyzes the incidence of regimen-specific fatal toxic effects associated with the use of immune checkpoint inhibitors for cancer treatment.
Abstract
Importance
Immune checkpoint inhibitors (ICIs) are now a mainstay of cancer treatment. Although rare, fulminant and fatal toxic effects may complicate these otherwise transformative therapies; characterizing these events requires integration of global data.
Objective
To determine the spectrum, timing, and clinical features of fatal ICI-associated toxic effects.
Design, Setting, and Participants
We retrospectively queried a World Health Organization (WHO) pharmacovigilance database (Vigilyze) comprising more than 16 000 000 adverse drug reactions, and records from 7 academic centers. We performed a meta-analysis of published trials of anti–programmed death-1/ligand-1 (PD-1/PD-L1) and anti–cytotoxic T lymphocyte antigen-4 (CTLA-4) to evaluate their incidence using data from large academic medical centers, global WHO pharmacovigilance data, and all published ICI clinical trials of patients with cancer treated with ICIs internationally.
Exposures
Anti–CTLA-4 (ipilimumab or tremelimumab), anti–PD-1 (nivolumab, pembrolizumab), or anti–PD-L1 (atezolizumab, avelumab, durvalumab).
Main Outcomes and Measures
Timing, spectrum, outcomes, and incidence of ICI-associated toxic effects.
Results
Internationally, 613 fatal ICI toxic events were reported from 2009 through January 2018 in Vigilyze. The spectrum differed widely between regimens: in a total of 193 anti–CTLA-4 deaths, most were usually from colitis (135 [70%]), whereas anti–PD-1/PD-L1–related fatalities were often from pneumonitis (333 [35%]), hepatitis (115 [22%]), and neurotoxic effects (50 [15%]). Combination PD-1/CTLA-4 deaths were frequently from colitis (32 [37%]) and myocarditis (22 [25%]). Fatal toxic effects typically occurred early after therapy initiation for combination therapy, anti–PD-1, and ipilimumab monotherapy (median 14.5, 40, and 40 days, respectively). Myocarditis had the highest fatality rate (52 [39.7%] of 131 reported cases), whereas endocrine events and colitis had only 2% to 5% reported fatalities; 10% to 17% of other organ-system toxic effects reported had fatal outcomes. Retrospective review of 3545 patients treated with ICIs from 7 academic centers revealed 0.6% fatality rates; cardiac and neurologic events were especially prominent (43%). Median time from symptom onset to death was 32 days. A meta-analysis of 112 trials involving 19 217 patients showed toxicity-related fatality rates of 0.36% (anti–PD-1), 0.38% (anti–PD-L1), 1.08% (anti–CTLA-4), and 1.23% (PD-1/PD-L1 plus CTLA-4).
Conclusions and Relevance
In the largest evaluation of fatal ICI-associated toxic effects published to date to our knowledge, we observed early onset of death with varied causes and frequencies depending on therapeutic regimen. Clinicians across disciplines should be aware of these uncommon lethal complications.
Introduction
Immune checkpoint inhibitors (ICIs) targeting cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1/ligand-1 (PD-1/PD-L1) have transformed the treatment landscape of many different cancers. Responses occur in a substantial fraction of patients and are frequently durable and even curative. Combined ICI blockade appears to further improve clinical outcomes compared with monotherapies.1,2,3,4,5
Toxic effects associated with ICIs may affect any organ, and stem from activation of autoreactive T cells damaging host tissues. Most frequently, these immune-related adverse events (irAEs) affect the colon, liver, lungs, pituitary, thyroid, and skin, although uncommon events involving the heart, nervous system, and other organs also occur.6,7 Combined PD-1 plus CTLA-4 blockade triggers substantially more irAEs than anti–PD-1 alone (55%-60% vs 10%-20% high-grade events).1,8 These toxic effects remain a major challenge in clinical care and a barrier for developing even more active combinations.
Although irAEs are typically manageable with corticosteroids or other immune modulators, uncommon fatal events have been reported.9,10,11,12,13,14 Individual clinical trial reports, however, are unable to comprehensively characterize these rare toxic effects, and to our knowledge no single study has reported more than 9 fatal events.15 Furthermore, it is not clear how the rates of fatal toxic effects compare with other common oncologic interventions (eg, chemotherapy, targeted therapy, surgery). The extremely high prevalence of ICI use, movement toward adjuvant therapy, and more aggressive combinations in development ensures that life-threatening and fatal complications will increase as a problem for clinicians across disciplines.14 The frequency, spectrum, and clinical features of fatal irAEs, however, are not well-defined. Herein, we draw on multiple sources, including the World Health Organization (WHO) pharmacovigilance database (Vigibase-Vigilyze),16 international multi-institutional treatment data, and all published clinical trials to characterize more than 750 fatal irAEs.
Methods
The study was conducted under institutional review board approval at all institutions. Waiver of consent for retrospective data review was obtained.
Vigilyze Database
Vigilyze-Vigibase (http://www.vigiaccess.org/), the WHO database of individual safety case reports and adverse drug reactions, comprises more than 16 000 000 case reports. Vigilyze was accessed and queried on January 30, 2018 for ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab (eMethods in the Supplement for full description of search and database).16 To avoid capturing cancer-related deaths, we included only reports where known irAEs occurred (eTable 1 in the Supplement shows a list of the irAEs included). Patients with resolving toxic effects, unknown outcomes, or known/presumed cancer-related deaths were excluded; only reports with fatal events attributed to drug toxicity were included. Fatality rates were assessed as the number of fatal events divided by total events for each toxic effect.
Multicenter Analysis
All patients treated with ICIs at participating institutions (Vanderbilt-Ingram Cancer Center, Massachusetts General Hospital, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Melanoma Institute of Australia, Westmead Hospital, National Center for Tumor Diseases Heidelberg, Moffitt Cancer Center) were evaluated from existing treatment databases. This included patients with malignant skin cancers at all institutions, as well as lung and kidney cancers at Vanderbilt. Patients with deaths that were judged as probably or definitely treatment-related by the treating physician were included; all other deaths (including cancer-related and unclear situations) were excluded. Patient demographics (age, sex, comorbidities), treatment regimens (agent, dose), and outcomes were collected. Data regarding toxic effects were collected, including type, symptoms, onset, immunosuppressive treatments, hospitalizations, concurrent irAEs, and time of death. Age and sex of patients with fatal toxic effects were compared with a subset of 1348 patients without (all patients from 3 centers) using Mann-Whitney U and χ2 tests.
Meta-analysis
Pubmed was searched for clinical trials using the following terms: “ipilimumab,” “tremelimumab,” “nivolumab,” “pembrolizumab,” “atezolizumab,” “avelumab,” “durvalumab,” “PD-1,” “PD-L1,” and “CTLA-4” on March 24, 2018 (eFigure 2 in the Supplement).17 English-language trials were included and spanned from 2003 to 2018. All 514 studies were screened; nonprospective clinical trials or trials testing other agents were excluded (n = 349). Trials evaluating only pediatric patients, combinations including other agents (other than anti–PD-1, PD-L1, or CTLA-4), accrual of 10 or fewer patients, single dosing, or treatment following hematopoietic stem cell transplant were excluded (n = 44). When 2 publications reported the same trial, the article with the longer follow-up time was selected (eg, a phase 3 study that was initially reported and then with prolonged follow-up). The remaining trials (n = 112) were assessed individually for drug regimen (combined PD-1/CTLA-4, anti–PD-1, anti–PD-L1, anti-CTLA-4) and dose, total number of patients treated, and number and type of fatal drug-related events. The incidence of fatal irAEs with each drug regimen was compared with other regimens. Similarly, the rate of fatal irAEs were evaluated and compared with different doses of ipilimumab (3 mg/kg vs 10 mg/kg for monotherapy; 1 mg/kg vs 3 mg/kg for combination therapy).
Statistics
Time to toxic event for all evaluable cases was evaluated using the Kaplan-Meier method and compared using the log-rank test. Fatal toxic effects rates between different drugs or doses were compared using χ2 testing. Other clinical variables of interest were evaluated descriptively. Statistical analyses were performed in GraphPad Prism (version 6, Graphpad Software); the meta-analysis was performed using R statistical software (packages metafor and meta, R Foundation). Event rates and their corresponding 95% confidence intervals were estimated using both fixed effect model and random effects model. Forest plots were constructed to summarize data for each analysis group with incidence rate and to provide a visual analysis of studies evaluating fatal drug-related events.
Results
Global Pharmacovigilance Analysis
To evaluate the spectrum of fatal ICI-related toxic effects, we interrogated a global adverse drug reaction database (Vigilyze-Vigibase) that contains more than 16 000 000 case reports. From screening 31 059 individual ICI-related case reports, we identified 613 fatal irAEs (Table 1). Among these, 193 received ipilimumab monotherapy, 333 received anti–PD-1/PD-L1, and 87 received the combination of anti–PD-1/PD-L1 plus anti–CTLA-4 (combination). Patients treated with anti–CTLA-4 (all with ipilimumab) largely had melanoma (136 [96%] and 49 [66%] for monotherapy and combination, respectively), whereas most patients treated with anti–PD-1/PD-L1 had lung cancer (152 [54%]), melanoma (50 [18%]), or genitourinary cancers (10%). Most patients had a single toxic effect causing death, although combination therapy had multiple concurrent irAEs present more often than either monotherapy (27% for combination vs 14% [ipilimumab] and 15% [anti-PD-1/PD-L1]; P = .01).
Table 1. Spectrum of Fatal Immune-Related Adverse Events in Vigilyze.
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Ipilimumab (n = 193) | Anti–PD-1/PD-L1 (n = 333) | Combination (n = 87) | ||
| Types of cancera | <.001 | |||
| Melanoma | 136 (96) | 50 (18) | 49 (66) | |
| Lung cancer | 0 | 152 (54) | 17 (23) | |
| Other | 5 (4) | 78 (28) | 8 (11) | |
| Type of fatal irAE | ||||
| Colitis | 135 (70) | 58 (17) | 32 (37) | <.001 |
| Pneumonitis | 15 (8) | 115 (35) | 12 (14) | <.001 |
| Hepatitis | 31 (16) | 74 (22) | 19 (22) | .23 |
| Hypophysitis | 10 (5) | 3 (1) | 2 (2) | .01 |
| Cardiac | 3 (2) | 27 (8) | 22 (25) | <.001 |
| Myositis | 1 (0.5) | 22 (7) | 11 (13) | <.001 |
| Nephritis | 1 (0.5) | 7 (2) | 3 (4) | .19 |
| Adrenal | 8 (4) | 6 (2) | 3 (4) | .26 |
| Neurologic | 11 (6) | 50 (15) | 7 (8) | .003 |
| Hematologic | 3 (2) | 14 (4) | 2 (2) | .22 |
| Other (skin, thyroid, diabetes, other gastrointestinal) | 13 (7) | 24 (8) | 7 (8) | .93 |
| Other clinical features | ||||
| Median time to irAE, days | 40 | 40 | 14 | .01 |
| >1 concurrent irAE, % | 27 (14) | 51 (15) | 24 (28) | .01 |
| Reporting year | ||||
| 2014 or before | 98 (51) | 3 (1) | 2 (2) | <.001 |
| 2015 | 45 (23) | 20 (6) | 9 (10) | <.001 |
| 2016 | 21 (11) | 88 (28) | 17 (20) | .001 |
| 2017 | 26 (13) | 192 (58) | 44 (51) | <.001 |
| 2018 (up to January 15) | 3 (2) | 30 (9) | 15 (17) | <.001 |
Abbreviations: irAE, immune-related adverse event; PD-L1, programmed death ligand-1; PD-1, programmed death-1.
Percent of known (52 patients treated with ipilimumab, 53 with anti–PD-1/PD-L1, and 13 with combination did not list cancer types).
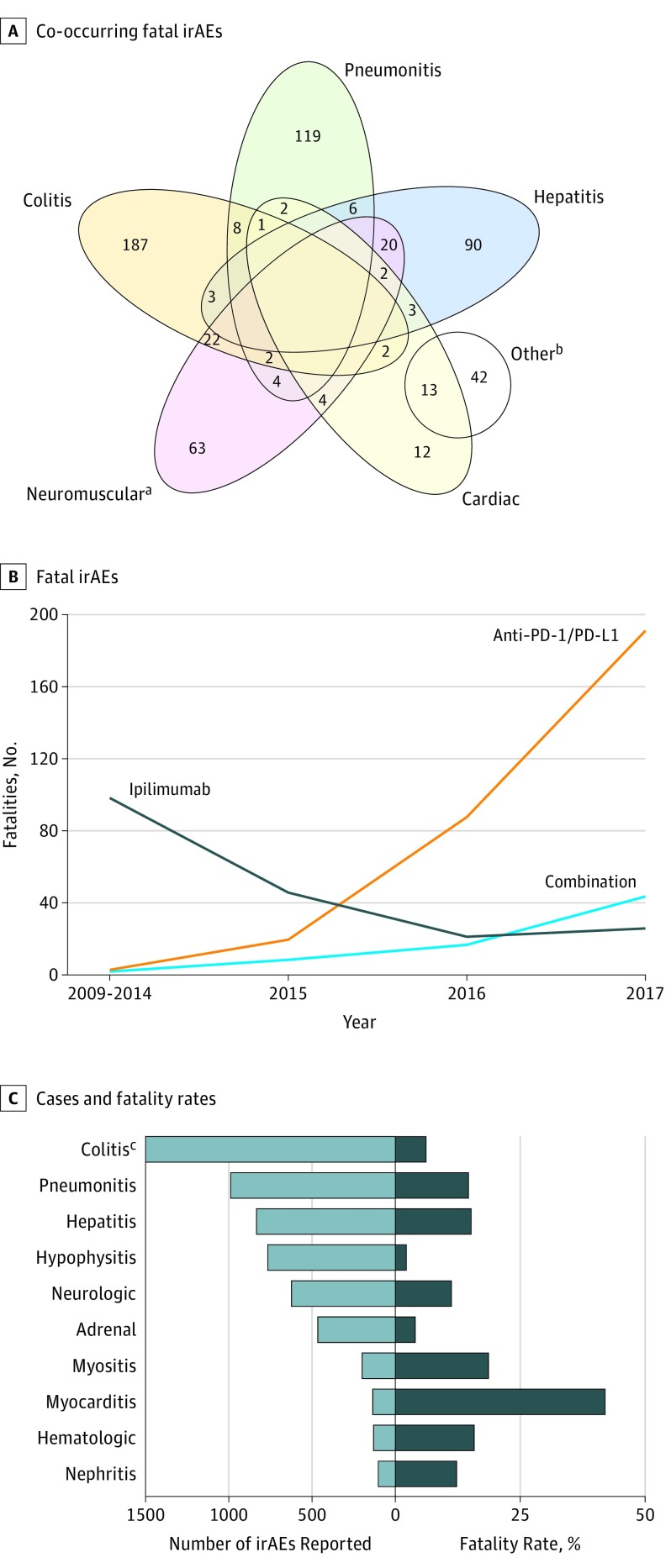
The types of fatal irAEs differed markedly between regimens (Table 1) (eTable 2 in the Supplement). With ipilimumab monotherapy, colitis/diarrhea was highly predominant (135 [70%]); hepatitis (31 [16%]) and pneumonitis (15 [8%]) occurred in smaller proportions. Anti–PD-1/PD-L1 monotherapy, by contrast, had a wide distribution of fatal irAEs including pneumonitis (115 [35%]), hepatitis (74 [22%]), colitis (58 [17%]), neurologic events (50 [15%]), and myocarditis (27 [8%]). Combination therapy deaths were most often owing to colitis (32 [37%]), myocarditis (22 [25%]), hepatitis (19 [22%]), pneumonitis (12 [14%]), and myositis (11 [13%]). Myositis and myocarditis frequently co-occurred (Figure 1A). Notably, myasthenia gravis also co-occurred in 5 (10% ) of 52 myocarditis cases; other co-occurring events appeared sporadic.18 Neurologic events were most commonly encephalitis and myasthenia gravis. Infrequent deaths were observed across all regimens from dermatologic (eg, toxic epidermal necrolysis; 1.5%), hematologic (hemophagocytic lymphohistiocytosis, hemolytic anemia, idiopathic thrombocytopenic purpura; 3%), and endocrine toxic effects (hypophysitis, adrenal insufficiency; 5.5%).
Figure 1. Clinical Characteristics of Fatal Immune-Related Adverse Events (irAEs).
A, Overlap of co-occurring fatal irAEs including colitis, pneumonitis, hepatitis, cardiac, and neuromuscular. B, Number of fatal irAEsreported by year and by immune checkpoint inhibitor regimen. C, Number of cases (light blue) and fatality rate (dark blue) for each class of toxic effect.
aOther group also contains: 14 patients with 2 other irAEs, 11 patients with a categorized irAE and 2 other irAEs, 3 patients with 3 other irAEs, 2 patients with 2 categorized irAEs and 2 other irAEs, 1 patient with 1 categorized irAE and 4 other irAEs.
bIncludes myasthenia gravis, myositis, and Guillain-Barre syndrome. All other concurrent irAEs (eg, noncardiac) are reflected in the Other group.
cIncludes 3905 total cases.
We noted increased reporting of fatal toxic effects over time. The absolute numbers of anti–PD-1/PD-L1 and combination therapy deaths increased substantially, with more than 65% of all deaths occurring in 2017 and January, 2018 (Figure 1B). The types of fatal toxic effects were largely stable over time, although myocarditis increased with all regimens (eFigure 1 in the Supplement).
To determine the risk of fatality associated with particular toxic effects, we assessed fatality rates for different classes of toxic effects (Figure 1C). Myocarditis appeared to present the highest risk of death, with 52 (39.7%) deaths among 131 cases. Pneumonitis, hepatitis, myositis, nephritis, neurologic, and hematologic toxic effects all had fatalities in 10% to 17% of reported cases. Hypophysitis, adrenal insufficiency, and colitis had the lowest reported fatality rates (2%, 3.7%, and 5%, respectively).
Multicenter Analysis
To probe deeper into the clinical characteristics of fatal irAEs, we reviewed all patients treated with ICIs from 7 international academic centers. Among 3545 patients (3228 [91%] with melanoma), we observed 21 (0.59%) fatal irAEs. These occurred in 7 patients treated with ipilimumab, 9 treated with anti–PD-1, and 5 treated with combined PD-1/CTLA-4 blockade (eTable 3 in the Supplement). Nineteen of 21 patients had melanoma or other malignant skin cancers (n = 2); the median age was 72 years (range, 37-84 years). Comorbidities were typical of an elderly population, including 12 with hypertension and 6 with other cardiac conditions; 2 patients had preexisting autoimmune disease (Graves disease). Patients who died of toxic effects were older than those without fatal toxic effects (median, 70 vs 62 years; absolute difference, 8; P = .009) with similar sex distribution (57% vs 60% male; χ2 = 0.09; P = .77).
Fatal toxic effects in these 21 patients included myocarditis (n = 6; including 3 with concurrent myositis), neurologic toxic effects (n = 5), colitis/enteritis (n = 6), and hepatitis (n = 5). Nine patients had more than 1 concurrent irAE, including myocarditis and myositis (n = 3), myocarditis and undiagnosed neurologic deterioration (n = 1), neurologic deterioration and colitis (n = 1), colitis and dermatitis (n = 1), enteritis and pure red cell aplasia (n = 1), and systemic multiorgan inflammation and hypophysitis (n = 2). Two unique patients who previously received organ transplants (allogeneic stem cell and renal transplants) died of hepatic failure following 1 dose of single-agent anti–PD-1.
The median time to irAE onset was 15 days following treatment initiation (range, 3-543 days), including 11 (52%) cases within 20 days. The median time from symptom onset to death was 32 days (range, 3-355 days). Thirteen (62%) patients had a more fulminant course with a single hospitalization before death, whereas 8 (38%) patients had a protracted course with improvement (or stabilization) and hospital discharge followed by subsequent worsening. Myocarditis (5 of 6 cases) and hepatic failure (4 of 5) tended to present in a fulminant fashion whereas neurologic cases tended to be more protracted in nature (4 of 5 cases). All 21 patients received high-dose steroids; 4 received infliximab and 5 intravenous immunoglobulin. The median time to receiving high-dose steroids was 5 days after symptom onset (range, 0-112 days). Two patients had long delays owing to difficulty diagnosing a vague neurologic weakness syndrome (112 days) and unreported diarrhea/colitis symptoms (60 days); all other patients received steroids within 8 days.
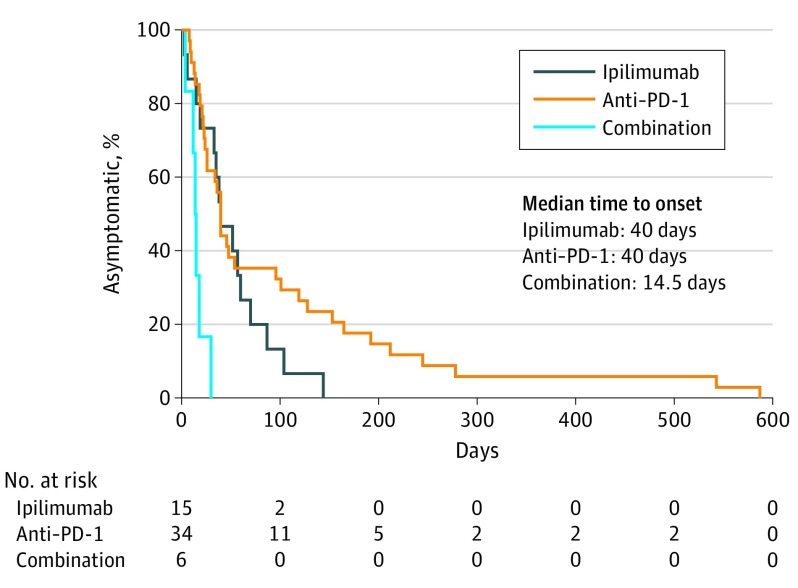
To determine the regimen-specific timing of these fatal toxic effects, we combined our multicenter data with Vigilyze reports. The median times to toxic event onset occurred early after therapy onset, regardless of treatment type (40 days, 40 days, and 14.5 days with ipilimumab, anti–PD-1/PD-L1, and combination, respectively, P < .001) (Figure 2). The median time to death following treatment initiation was 64, 43, and 35 days for ipilimumab, anti–PD-1/PD-L1, and combination, respectively (P = .27) (eFigure 1D-E in the Supplement).
Figure 2. Time to Symptom Onset of Fatal Toxic Effects by ICI Regimen.
Anti–PD-1 indicates anti–programmed death-1.
Meta-analysis
Although useful to define the clinical characteristics and spectrum of fatal irAEs, these databases are unable to conclusively define their frequency in individual ICI regimens. To evaluate the frequency of fatal toxic effects, we evaluated all published clinical trials of anti–PD-1 (nivolumab, pembrolizumab), anti–PD-L1 (atezolizumab, avelumab, durvalumab), anti–CTLA-4 (ipilimumab, tremelimumab) therapies, and combinations (PD-1/PD-L1/ plus CTLA-4 inhibition) (eFigure 2 and eTable 4 in the Supplement). This comprised 112 trials and 19 217 patients. In these trials, 122 fatal drug-related AEs occurred, in 0.36% (PD-1), 0.38% (PD-L1), 1.08% (CTLA-4), and 1.23% (PD-1/PD-L1 plus CTLA-4) of patients (eTable 4 in the Supplement). PD-1/PD-L1 inhibitors were associated with lower fatal toxic effects rates compared with either CTLA-4 monotherapy or combination (χ2 = 58.8; P < .001). By contrast, there was no difference in fatal toxic effects incidence between PD-1 and PD-L1 inhibitors (χ2 = 0.021; P = .88) or between anti–CTLA-4 monotherapy and combination therapy (χ2 = 0.23; P = .62). A formal meta-analysis revealed similar patterns, albeit with slightly higher estimates of fatality rates (0.8%-1.7%) (eFigures 3-6 in the Supplement).
To explore further, we asked whether fatal toxic effects occurred more often at higher doses of ipilimumab. We compared trials with ipilimumab monotherapy at 3 mg/kg (1438 patients) vs 10 mg/kg (3016 patients). Patients treated with the 3 mg/kg dose had fewer fatal AEs compared with 10 mg/kg (0.56% vs 1.29%; χ2 = 4.9; P = .03). By contrast, patients treated with ipilimumab plus anti–PD-1 had a similar incidence of fatal events when treated with ipilimumab 1 mg/kg (892 patients) compared with 3 mg/kg (545 patients) (1.0% vs 1.28%; χ2 = 0.26; P = .61).
To provide additional validation regarding the spectrum of irAEs, we assessed the types of fatal irAEs among the 122 published events (Table 2). Similar to the data in Vigilyze and in our retrospective cohort, colitis (including colitis with bowel perforation) was the most frequent cause of death with anti–CTLA-4 monotherapy (23 of 58 anti–CTLA-4-related deaths). Cardiac events, including myocarditis and sudden death occurred in 9 (16%) patients, hepatic failure in 5 (9%), and pneumonitis in 3 (5%). Of 45 anti–PD-1/PD–L1-associated deaths, 19 resulted from pneumonitis, 7 from cardiac events, and 2 from colitis/diarrhea. Of 19 combination therapy-associated deaths, 4 resulted from pneumonitis, 4 from cardiac events, 2 from hematologic events (aplastic anemia and HLH), and 3 from neurologic events. Among all groups, other, nonclassical irAEs were reported as drug-related causes of death, including infectious causes (most commonly pneumonia in 10 patients and sepsis in 3), 3 resulted from electrolyte imbalances, 4 from hemorrhagic or thrombotic events, and 3 from multiorgan failure. It was not reported whether these fatal events were direct complications of stereotypical irAEs (eg, electrolyte imbalance resulting from colitis, pneumonia following neurotoxic effects, etc.).
Table 2. Incidence and Types of Immune Checkpoint Inhibitor-Related Fatalities From Systematic Review and Meta-analysis.
| Variable | Anti–CTLA-4 (n = 5368) | Anti–PD-1 (n = 9136) | Anti–PD-L1 (n = 3164) | Anti–PD-1/PD-L1 Plus CTLA-4 (n = 1549) |
|---|---|---|---|---|
| Deaths, No. (%) | 58 (1.08) | 33 (0.36) | 12 (0.38) | 19 (1.23) |
| Type of fatal toxic effect | ||||
| Colitis | 23 (40) | 2 (6) | 0 | 2 (11) |
| Pneumonitis | 3 (5) | 14 (42) | 5 (42) | 4 (21) |
| Hepatitis | 5 (9) | 0 | 1 (8) | 2 (11) |
| Cardiac | 9 (16) | 4 (12) | 3 (25) | 4 (21) |
| Neurologic | 1 (2) | 1 (3) | 0 | 3 (16) |
| Nephritis | 1 (2) | 0 | 0 | 1 (5) |
| Hematologic | 2 (4) | 2 (6) | 0 | 2 (11) |
| Infectious | 8 (14) | 5 (15) | 2 (18) | 3 (16) |
| Hemorrhagic/thrombotic | 2 (4) | 1 (3) | 0 | 1 (5) |
| Electrolyte imbalance | 1 (2) | 2 (6) | 0 | 0 |
| Multiorgan failure | 3 (5) | 0 | 0 | 0 |
| Other | 1 (2) | 2 (6) | 1 (8) | 0 |
Abbreviations: CTLA-4, cytotoxic T lymphocyte antigen-4; PD-L1, programmed death ligand-1; PD-1, programmed death-1.
Discussion
We report the largest and most comprehensive analysis to our knowledge of fatal ICI-associated toxic effects published to date. We found that these events generally occur very early after therapy initiation and with marked distinctions between ICI regimens. Despite the impressive number of fatal events reported (>600 in Vigilyze), the risk of fatal irAEs remains very low for individual patients with advanced cancer, and should not dissuade use of these potentially curative therapies.
Instead, the global increase in ICI use across cancer types highlights the importance of defining the most serious toxic effects and developing awareness among oncologists, emergency department physicians, critical care providers, and other specialists. This study underscores that the risk of death associated with complications of ICI therapy is real, but within or well below fatality rates for common oncologic interventions; for example platinum-doublet chemotherapy (0.9%),19 allogeneic stem cell transplant (approximately 15%),20 targeted therapy with angiogenesis or tyrosine kinase inhibitors (0%-4%),5,21,22 and complex oncology surgeries (eg, Whipple procedure or esophagectomy, 1%-10%).23,24 Further, the number of deaths identified even by this global analysis is dwarfed by the numbers of cancer deaths (1.6 million from lung cancer alone annually). Similarly, a treatment-related death rate of 0.36% to 1.23% is dramatically lower than the near-100% fatality rate for metastatic solid tumors. However, recognizing the timing and spectrum of events is an important new set of concepts that need to be disseminated to the practice community. Furthermore, their expanding use as adjuvant or maintenance therapies (as currently approved in melanoma and non-small cell lung cancer)25,26 in patients already treated with curative intent underscores the need to understand and prevent these unusual events.
We observed variable patterns among ICI regimens. Ipilimumab deaths were dominated by colitis, whereas anti–PD-1 had a wide spectrum of events. Combination therapy had more frequent multiorgan involvement, and nearly one-third of all deaths were from myocarditis, myositis, and/or neurologic events. Hepatitis accounted for about 20% of deaths in each cohort. Each database largely validated the patterns of death from distinct regimens with a few exceptions. Intriguingly, our retrospective analysis at large, experienced academic centers suggested that neurologic and cardiac toxic effects comprised nearly half of deaths. Thus, we surmise that more optimized treatment of these events is urgently needed. In addition, persistent deaths from colitis and pneumonitis, events with standardized treatment algorithms and clear symptoms, suggests that patient and physician education remains a critical objective. Further analyses over time will help determine whether widespread awareness prevents these fatal events.
We observed that ipilimumab-based therapy was associated with higher mortality rates than anti–PD-1/PD-L1. Although ipilimumab monotherapy usage will likely decrease in the future, combined ipilimumab and anti–PD-1 has demonstrated impressive activity in several common cancers.1,2,3,4,5 Although a lower dose decreased death rates as monotherapy, a reduced dose of ipilimumab in combination regimens did not significantly decrease the rate of fatal toxic effects (1.0% vs 1.28%). More data are needed for definitive conclusions given the low absolute number of deaths in trials testing the lower dose (n = 9) and generally more favorable toxic effects rates for this regimen. Also, in our retrospective cohort, patients with fatal events were significantly older than those without fatal toxic effects. Although prior reports have suggested overall toxic effects rates are equivalent across age groups,27 we hypothesize that older patients are at higher risk of death owing to impaired functional reserve and increased medical comorbidities, although the absolute risk of toxicity-related deaths remains very low in this population. Finally, our retrospective series showed some patients did not receive steroids for more than 5 days, largely owing to lack of reporting or unusual clinical presentations. Delayed treatment could contribute to deaths in some cases.
Limitations
This study has several limitations. The rarity of these events makes them challenging to characterize. To our knowledge, the most fatal toxic effects reported previously in a single study was 9, hence our characterization of multiple databases marks the most thorough investigation to date. The multicenter analysis could be limited by inclusion of only academic centers with extensive ICI usage. Conceivably, rates and types of fatal irAEs may differ among providers less experienced with these agents. The Vigilyze analysis is limited by a lack of definitive causality, and lacks detailed clinical data. Frequently, the causes of fatal events in patients with metastatic cancer can be challenging to determine. For example, the diagnosis of immune-mediated hepatitis could be confounded by liver dysfunction from metastatic cancer infiltration or hypoperfusion (owing to distributive, hypovolemic, or cardiogenic shock). Most other events, however, (eg, colitis, myocarditis, pneumonitis) are more clearly drug-related, and unlikely to be confounded by other complications. In addition, because event reporting is voluntary, we suspect that severe toxic effects are over-represented in this database (thus toxicity-specific fatality rates may be overestimated), and we cannot rule out region-specific reporting patterns that could influence the data. Finally, the meta-analysis revealed that many fatal toxic effects were not reported as conventional irAEs. We surmise that these were largely complications of irAEs (eg, sepsis following colon perforation, sudden cardiac death from myocarditis), although this is speculative. Furthermore, because the follow-up for most published studies was fairly short, long-term monitoring to rule out delayed serious toxic effects is needed.
Conclusions
Fatal toxic effects associated with ICIs are uncommon and compare favorably with other oncologic interventions, but do occur at a rate of 0.3% to 1.3%. These tend to arise early in treatment, differ among various regimens, and often result in rapid clinical deterioration. Providers across specialties should be aware of these potentially lethal complications. Similar broad awareness and early treatment of other cancer therapy-related complications (eg, febrile neutropenia, anemia) has been paramount for effective multidisciplinary cancer care.
eMethods
eTable 1. List of irAEs included
eTable 2. Additional demographic of fatal immune related adverse events in Vigilyze
eTable 3. Clinical features of 21 ICI-related fatalities from six large academic centers
eFigure 1. Proportion of fatal toxicities, overall survival, and time to symptom onset
eFigure 2. PRISMA diagram for articles selected for meta-analysis
eFigure 3. Forest plot of incidence of death due to immune related adverse events with anti-CTLA-4 therapy
eFigure 4. Forest plot of incidence of death due to immune related adverse events with anti-PD-1 therapy
eFigure 5. Forest plot of incidence of death due to immune related adverse events with anti-PD-L1 therapy
eFigure 6. Forest plot of incidence of death due to immune related adverse events with combination anti-CTLA-4 and anti-PD-1 therapy
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345-1356. doi: 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 Study. J Clin Oncol. 2017;35(34):3851-3858. doi: 10.1200/JCO.2016.72.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31-41. doi: 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883-895. doi: 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 7.Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36-49. doi: 10.1016/j.ctrv.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol. 2018;4(1):98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi: 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709-717. doi: 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13. doi: 10.1186/s40425-016-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology. 2017;6(10):e1344805. doi: 10.1080/2162402X.2017.1344805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol. 2018;137(3):601-609. doi: 10.1007/s11060-018-2752-5 [DOI] [PubMed] [Google Scholar]
- 14.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi: 10.1016/S0140-6736(18)30533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40-47. doi: 10.1200/JCO.2016.69.1584 [DOI] [PubMed] [Google Scholar]
- 16.Lindquist M. VigiBase, the WHO Global ICSR Database System: basic facts. Drug Inf J. 2008;42(5):409-419. doi: 10.1177/009286150804200501 [DOI] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics. 2015;16:169. doi: 10.1186/s12859-015-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignon JP, Tribodet H, Scagliotti GV, et al. ; LACE Collaborative Group . Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552-3559. doi: 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 20.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091-2101. doi: 10.1056/NEJMoa1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long GV, Weber JS, Infante JR, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol. 2016;34(8):871-878. doi: 10.1200/JCO.2015.62.9345 [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, Ohe Y, Vansteenkiste J, et al. ; FLAURA Investigators . Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 23.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10-15. doi: 10.1097/01.sla.0000217673.04165.ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75(1):217-222. doi: 10.1016/S0003-4975(02)04368-0 [DOI] [PubMed] [Google Scholar]
- 25.Weber J, Mandala M, Del Vecchio M, et al. ; CheckMate 238 Collaborators . Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824-1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 26.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 27.Betof AS, Nipp RD, Giobbie-Hurder A, et al. Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist. 2017;22(8):963-971. doi: 10.1634/theoncologist.2016-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. List of irAEs included
eTable 2. Additional demographic of fatal immune related adverse events in Vigilyze
eTable 3. Clinical features of 21 ICI-related fatalities from six large academic centers
eFigure 1. Proportion of fatal toxicities, overall survival, and time to symptom onset
eFigure 2. PRISMA diagram for articles selected for meta-analysis
eFigure 3. Forest plot of incidence of death due to immune related adverse events with anti-CTLA-4 therapy
eFigure 4. Forest plot of incidence of death due to immune related adverse events with anti-PD-1 therapy
eFigure 5. Forest plot of incidence of death due to immune related adverse events with anti-PD-L1 therapy
eFigure 6. Forest plot of incidence of death due to immune related adverse events with combination anti-CTLA-4 and anti-PD-1 therapy