Abstract
This 5-year follow-up of a phase 2 clinical trial assesses long-term durability of minimal residual disease–negative remission and time to disease progression among patients with newly diagnosed multiple myeloma who received triplet-based induction therapy with lenalidomide maintenance therapy.
State-of-the art treatment of multiple myeloma (MM) involves induction with triplet-based regimens using combinations of immunomodulatory drugs and proteasome inhibitors, which have shown improved progression-free survival and overall survival compared with doublet regimens in the newly diagnosed (ND) and relapsed and refractory (RR) setting.1,2,3 Carfilzomib is a selective proteasome inhibitor approved by the US Food and Drug Administration for use in the carfilzomib, lenalidomide, and dexamethasone (KRd) regimen for the treatment of RRMM.3,4 We previously reported early data on this phase 2 study of 45 patients with NDMM.5 Herein, we expand on our initial results and present the long-term durability of minimal residual disease–negative complete remissions (MRD-negative CRs) and time to progression, the last characterized by depth of response, age, and cytogenetic risk profile.
Methods
This study of KRd with lenalidomide maintenance (KRd-r) enrolled patients with treatment-naive NDMM regardless of eligibility for autologous stem cell transplant (NCT01402284).5 Patients received eight 28-day cycles of carfilzomib, 20/36 mg/m2, with dexamethasone, 20/10 mg (days 1, 2, 8, 9, 15, 16), and lenalidomide, 25 mg (days 1-21), followed by 2 years of maintenance therapy with lenalidomide, 10 mg (days 1-21). Transplant-eligible patients underwent harvest after 4 cycles of therapy. Minimal residual disease–negative complete remission was assessed by multicolor flow cytometry (bone marrow aspirate; 10−5 sensitivity) after induction, at 1 and 2 years of lenalidomide maintenance therapy, and annually thereafter. The National Cancer Institute institutional review board approved the study, and participants provided written informed consent.
Results
Forty-five patients who met eligibility criteria were enrolled; among these, 27 (60%) were men and mean (SD) age was 61 (12) years5; median (95% CI) follow-up was 5.2 (4.7-5.6) years. Treatment with KRd-r led to rapid, deep, and durable overall response rate and sustained MRD-negative CRs. All but 1 patient (overall response rate, 98%; 95% CI, 88%-100%) experienced a partial response or better as the best objective response, with a mean (SD) of 4.4 (1.1) weeks to partial response or better. The median duration of response was 65.7 months (95% CI, 55.6 months to not estimable). Twenty-eight of the 45 patients (62%; 95% CI, 47%-76%) experienced MRD-negative CR. To date, durability of MRD-negative CR has been observed at up to 70 months, with a median duration of more than 4 years (52.4 months; 95% CI, 35.3-61.6). Patients who experienced MRD negativity by the end of cycle 8 had a 78% decrease in risk of progression (Figure 1). Moreover, treatment with KRd-r was associated with unprecedented progression-free duration and overall survival, with a median time to progression of more than 5.5 years (67.3 months; 95% CI, 51.0 months to not estimable). Median overall survival was not reached because, as of this writing,40 of the 45 patients are still alive. However, at a 6-year milestone, the probability of survival was 84%. Deep responses of MRD-negative CR and long progression-free durations occurred irrespective of age or adverse cytogenetic risk factors (Figure 2). In general, KRd-r was well tolerated, with manageable low-grade toxic effects and no grade 3 or greater peripheral neuropathy, the primary end point.5
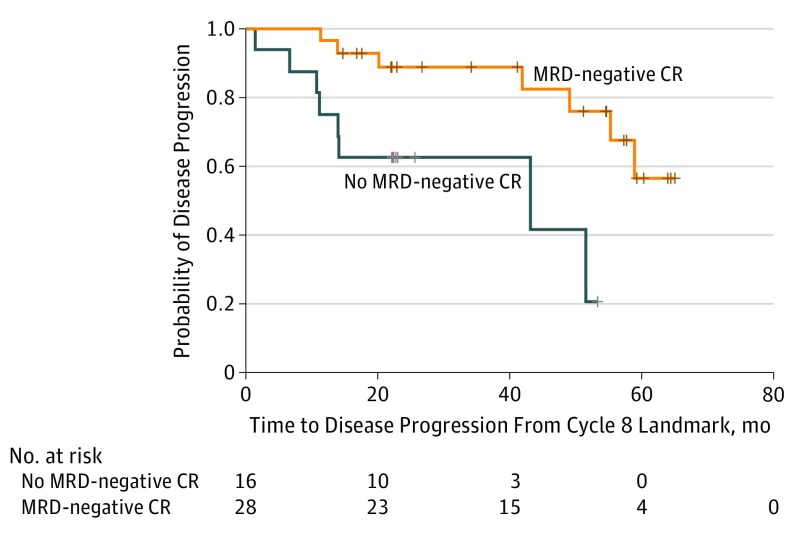
Figure 1. Time to Disease Progression Based on Minimal Residual Disease (MRD)–Negative Complete Remission (CR) Status.
Patients who experienced MRD-negative CR by the end of carfilzomib, lenalidomide, and dexamethasone induction (8 cycles) had a significantly longer time to disease progression, with a 78% reduction in risk of progression (hazard ratio, 0.22; 95% CI, 0.07-0.69; P = .005). Hatch marks on the curves indicate censored data.
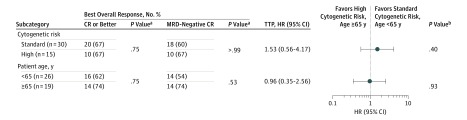
Figure 2. Minimal Residual Disease (MRD)–Negative Complete Remission (CR) and Time to Disease Progression (TTP) by Age and Cytogenetic Risk.
Deep and sustained MRD-negative responses and prolonged TTP after treatment with a drug regimen of carfilzomib, lenalidomide, and dexamethasone with lenalidomide maintenance therapy occurred irrespective of age group or adverse cytogenetic risk factors. This depiction of TTP for important myeloma subgroups of age and poor cytogenetic risk shows no difference in hazard ratios (HRs) for TTP for either subgroup. Patients older than 65 years derived a clinical benefit similar to that of younger patients in terms of MRD-negative CR and TTP. Moreover, patients with myeloma who had high cytogenetic risk factors of 17p deletion or translocations of (4;14), (14;16), and (14;20) derived the same levels of deep response and prolonged progression-free duration as those with standard risk.
aCalculated using the Fisher exact test.
bCalculated using the log-rank test.
Discussion
First-line treatment of NDMM with the modern and highly efficacious KRd regimen incorporating a “by default delayed” autologous stem cell transplant strategy led to high rates of MRD-negative CR, which were sustained with a median duration of more than 4 years. We confirm that attaining MRD negativity with at least 10−5 sensitivity is crucial for deriving long-term benefit, as shown previously.6 In the present study, MRD-negative CR was strongly associated with a delay in progression and with an estimated greater than 80% reduction in the risk of disease progression. Furthermore, and of clinical importance, our findings indicate that these deep responses and long progression-free durations are attainable regardless of patient age or cytogenetic risk category. Taken together, these findings stress the importance of using highly efficacious triplet-based regimens for these subcategories of NDMM and avoiding de-escalation of triplet regimen and dosage based on patient age or other factors.
Our results compare favorably with autologous stem cell transplant–based regimens. The major limitation is the single-arm nature of the study design, and cross-trial comparisons are limited. Given the impressive activity of KRd-r in NDMM, we subsequently designed a trial using KRd-r for patients with high-risk smoldering MM, which is currently enrolling participants (NCT01572480).
References
- 1.Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. doi: 10.1016/S0140-6736(16)31594-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazandjian D, Landgren O. A look backward and forward in the regulatory and treatment history of multiple myeloma: approval of novel-novel agents, new drug development, and longer patient survival. Semin Oncol. 2016;43(6):682-689. doi: 10.1053/j.seminoncol.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel DS, Dimopoulos MA, Ludwig H, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(8):728-734. doi: 10.1200/JCO.2017.76.5032 [DOI] [PubMed] [Google Scholar]
- 4.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ; ASPIRE Investigators . Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. doi: 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]
- 5.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746-754. doi: 10.1001/jamaoncol.2015.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant. 2016;51(12):1565-1568. doi: 10.1038/bmt.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]