Key Points
Question
Do topical agents applied just before radiotherapy alter the surface dose?
Findings
Surveys of 241 clinicians and patients found that avoiding topical agents prior to radiation treatments was widespread. In this dosimetric and preclinical study, there was no difference in the measured radiation dose at the skin surface with or without a 1- to 2-mm-thick layer of metallic or nonmetallic topical agents regardless of beam energy or beam incidence, although very thickly applied topical agents increased the surface dose; irradiated skin in mice showed no differences in phosphorylated histone H2AX–positive foci or in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining with or without topical agents of varying thickness.
Meaning
Use of topical agents just before radiotherapy may be safely liberalized, although it appears that very thick applications of topical treatments just before radiotherapy should be avoided.
Abstract
Importance
Radiation dermatitis is common and often treated with topical therapy. Patients are typically advised to avoid topical agents for several hours before daily radiotherapy (RT) out of concern that topical agents might increase the radiation dose to the skin. With modern RT’s improved skin-sparing properties, this recommendation may be irrelevant.
Objective
To assess whether applying either metallic or nonmetallic topical agents before radiation treatment alters the skin dose.
Design, Setting, and Participants
A 24-question online survey of patients and clinicians was conducted from January 15, 2015, to March 15, 2017, to determine current practices regarding topical therapy use. In preclinical studies, dosimetric effect of the topical agents was evaluated by delivering 200 monitor units and measuring the dose at the surface and at 2-cm depth in a tissue-equivalent phantom with or without 2 common topical agents: a petroleum-based ointment (Aquaphor, petrolatum 41%) and silver sulfadiazine cream, 1%. Skin doses associated with various photon and electron energies, topical agent thicknesses, and beam incidence were assessed. Whether topical agents altered the skin dose was also evaluated in 24 C57BL/6 mice by using phosphorylated histone (γ-H2AX) immunofluorescent staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Preclinical studies took place at the University of Pennsylvania.
Main Outcomes and Measures
Patient and clinician survey responses; surface radiation dose readings in tissue-equivalent phantom; and γ-H2AX and TUNEL intensity measured in mice.
Results
The 133 patients surveyed received RT for cancer and had a median (range) age of 60 (18-86) years; 117 (87.9%) were women. One hundred eight clinicians completed the survey with 105 reporting that they were involved in managing patient skin care during RT. One hundred eleven (83.4%) of the patients and 96 (91.4%) of the 105 clinicians received or gave the advice to avoid applying topical agents before RT treatments. Dosimetric measurements showed no difference in the delivered dose at either the surface or a 2-cm depth with or without a 1- to 2-mm application of either topical agent when using en face 6- or 15-megavoltage (MV) photons. The same application of topicals did not alter the surface dose as a function of beam incident angle from 15° to 60°, except for a 6% increase at 60° with the silver sulfadiazine cream. Surface dose for 6- and 15-MV beams were significantly increased with a thicker (≥3-mm) topical application. For 6 MV, the surface dose was 1.05 Gy with a thick layer of petroleum-based ointment and 1.02 Gy for silver sulfadiazine cream vs 0.88 Gy without topical agents. For 15 MV, the doses were 0.70 Gy for a thick layer of petroleum-based ointment and 0.60 Gy for silver sulfadiazine cream vs 0.52 Gy for the controls. With 6- and 9-MeV electrons, there was a 2% to 5% increase in surface dose with the use of the topical agents. There were no dose differences at 2-cm depth. Irradiated skin in mice showed no differences in γ-H2AX–positive foci or in TUNEL staining with or without topical agents of varying thickness.
Conclusions and Relevance
Thin or moderately applied topical agents, even if applied just before RT, may have minimal influence on skin dose regardless of beam energy or beam incidence. The findings of this study suggest that applying very thick amounts of a topical agent before RT may increase the surface dose and should be avoided.
This survey study of patients undergoing radiotherapy for cancer compares clinician advice against using topical agents before radiotherapy to avoid an increased radiation dose to the skin with the results of a dosimetric study and a preclinical study in an animal model.
Introduction
Up to 90% of patients undergoing radiotherapy (RT) will experience acute radiation dermatitis,1 with Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 grade 2 or higher dermatitis reported in 40% of patients undergoing whole-breast radiotherapy,2 64% of patients undergoing concurrent chemoradiotherapy for head and neck cancer, and 73% undergoing concurrent chemoradiotherapy for anal cancer.3,4 Although most patients experience mild to moderate dermatitis, some experience significant discomfort, pruritus, and infections.1 Topical agents are frequently used several times daily to ameliorate this condition, including creams, such as silver sulfadiazine, that contain heavy metals. Most patients undergoing daily RT to the breast, head and neck, cervix, anus, or extremities use topical agents. Patients are typically advised to avoid use of topical agents for several hours before RT based on the concern that these topical agents may increase the radiation dose to the skin.1 This recommendation came into widespread practice during the era of orthovoltage radiation, which lasted until the early 1950s. Orthovoltage radiation uses lower-energy photons that deliver a significantly higher dose to the skin than the modern linear accelerator. In the era of modern RT with improved sparing of dose to the skin, the typical recommendation to avoid applying topical agents before RT, although still popular, may no longer be relevant. We hypothesized that the application of either metallic or nonmetallic topical agents before RT would have minimal effect on dose to the skin.
In this study, we assessed current practices regarding use of topical agents before RT by conducting online surveys of both radiation oncology patients and clinicians. We performed dosimetric experiments to measure dose at given depths in a tissue-equivalent phantom by using different topical agents, different incident beam angles, and different beam energy levels to assess the association of topical agent use with dose to the skin. Last, we performed preclinical experiments to assess the association of topical agent use with dose to the skin in C57BL/6 mice by using phosphorylated histone (γ-H2AX) immunofluorescent staining to detect radiation-induced DNA damage and a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to test for radiation-induced apoptosis.
Methods
Online Survey
We conducted anonymous online surveys of patients and clinicians posted on OncoLink (https://www.OncoLink.org) to determine current practices regarding topical therapy use (eMethods 2 in the Supplement).5 OncoLink is a nonprofit educational website providing free cancer information to patients and clinicians. Patients completed a 24-question survey about skin care and radiotherapy. Clinicians completed a separate 18-question survey. Responses were collected between January 15, 2015, and March 15, 2017. Responders were not compensated for their participation. Differences in responses with respect to baseline characteristics were assessed using χ2 and Mann-Whitney tests, and 2-sided P < .05 was considered significant. The University of Pennsylvania institutional review board approved this study and granted a waiver of written informed consent from participants.
Dosimetric Experiments
To evaluate the dosimetric effect of topical agents, we delivered 200 monitor units at a 100-cm surface-to-skin distance to a 10 × 10–cm field using a TrueBeam linear accelerator (Varian). We measured the dose at the surface and at a depth of 2 cm in a tissue-equivalent phantom with and without application of 2 commonly used topical agents for radiation dermatitis, a petroleum-based ointment (petrolatum, 41% [Aquaphor; Beiersdorf AG]), which is available over the counter, and silver sulfadiazine cream, 1%, which is available by prescription only, using optically stimulated luminescent dosimeters (OSLDs) (NanoDot; Landauer) (eImage in the Supplement). We assessed the association of various photon and electron energy levels (6 megavolts [MV], 15 MV, 6 million electron volts [MeV], and 9 MeV), topical agent thicknesses (moderate or very thick), and beam angles (0°, 15°, 30°, 45°, and 60°) with dose to the skin. Experiments were conducted using a relatively thick (1- to 2-mm) application of each topical agent as well as a thicker layer (≥3 mm). The 1- to 2-mm thickness was based on measurements of the thickness of the petroleum-based ointment used by 20 patients with breast or head and neck cancer with CTCAE grade 2 or higher dermatitis, with no patients applying a thickness greater than 2 mm. Patients were shown an ointment thickness of 3 mm; all denied applying 3 mm or more of the ointment. The 3-mm or greater thickness was therefore chosen to represent an extreme scenario not encountered in the clinic of applying a very thick layer of topical treatments immediately before RT.
Preclinical Experiments
The association of topical agent use with radiation dose to the skin was also evaluated in C57BL/6 mice by using γ-H2AX immunofluorescent staining to detect radiation-induced DNA damage and TUNEL staining to assess radiation-induced apoptotic cell death.
Twenty-four 8-week-old female C57BL/6 mice (The Jackson Laboratory) were housed in the animal facilities of the University of Pennsylvania. All experimental procedures were conducted in accordance with the guidelines and protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Mice were anesthetized using ketamine hydrochloride and xylazine and then the hair of the dorsal thorax/abdomen was shaved (eFigure 1 in the Supplement). A grid pattern consisting of four 0.5 × 0.5–in squares was drawn on each mouse. A moderate (50-mg) application of a petroleum-based ointment (petrolatum, 41% [Aquaphor; Beiersdorf AG]) was applied to the upper right square and a heavy (200-mg) application to the lower left square. The amount of ointment applied was determined to replicate the thickness of the ointments used in the dosimetric studies. The remaining 2 squares had no ointment and served as internal controls. Mice were then focally irradiated using an animal irradiator (XRAD 320 ix; Precision X-ray), with lead shielding to protect the head and distal hindquarters (eMethods 1 in the Supplement). Optically stimulated luminescent dosimeters were used to confirm that the delivered dose matched the prescription dose. Mice were treated with either 2 Gy ×1 or 15 Gy ×1, with 3 mice in each treatment group (to convert gray to rad, multiply by 100). Another group of 3 mice served as unirradiated controls. Skin tissue was harvested from each of the four 0.5 × 0.5–in quadrants, embedded in optimal cutting temperature molds, and stored at −80°C for further processing (eFigure 1 in the Supplement). Experiments were repeated with tissue harvested at different intervals after RT. Sections were cut and stained for γ-H2AX to detect radiation-induced double-stranded DNA breaks and with TUNEL assay to detect apoptotic cells (eMethods 1 in the Supplement).6,7,8,9 Quantification of γ-H2AX and TUNEL-positive cells in immunofluorescent images was performed using ImageJ software (National Institutes of Health). Quantification of γ-H2AX–positive foci per cell was performed for the 2 Gy ×1 experiments, and quantification of γ-H2AX–positive cells per 10× field was performed for the 15 Gy ×1 experiments.
Results
Survey Results
Two hundred forty-one respondents completed the survey, 133 patients and 108 clinicians. Among the 133 patients, the median (range) age was 60 (18-86) years, 117 (87.9%) were female, and 114 (85.7%) were white (Table). Of these, 111 (83.4%) reported that they were advised to avoid applying topical agents just before RT, 98 (73.7%) were advised to avoid applying topical agents for several hours before RT, and 72 (54.1%) were advised to wipe off any residual topical agents before RT. Among the 124 patients (93.2%) for whom topical therapy was recommended, 105 (84.7%) were advised to avoid applying topical agents right before RT, 93 (75.0%) were advised to avoid topical agents for several hours before RT, and 68 (54.8%) were told to wipe off any residual topicals before RT. Eighty-three of the total 133 patients (62.4%) reported “moderate” or “severe” skin erythema during RT. Of those 83, 74 (89.1%) were advised to avoid applying topical agents just before RT, 65 (78.3%) were told to avoid topical application for several hours before RT, and 44 (53.0%) were instructed to remove excess topical agents before RT. Most respondents had breast cancer. There was no difference in patient response regarding topical agent recommendations based on cancer diagnosis, sex, educational level, treatment setting (academic vs private practice), primary clinician managing their dermatitis (physician vs nurse), time since completion of RT, and degree of skin peeling or erythema.
Table. Survey Results From 133 Patients About Topical Therapy Use During Radiotherapy.
| Variable | No. (%)a |
|---|---|
| Age, median (range) y | 60 (18-86) |
| Sex, No. (%) | |
| Male | 15 (11.3) |
| Female | 117 (87.9) |
| Other | 1 (0.7) |
| Race/ethnicity, No. (%) | |
| White | 114 (85.7) |
| Black | 7 (5.3) |
| Latino | 5 (3.7) |
| Asian | 3 (2.2) |
| Mixed race | 3 (2.2) |
| Educational level | |
| High school diploma | 17 (12.8) |
| Some college | 27 (20.3) |
| College degree | 58 (43.6) |
| Graduate school | 31 (23.3) |
| Diagnosis | |
| Breast cancer | 87 (65.4) |
| Head and neck cancer | 11 (8.3) |
| Lung cancer | 6 (4.5) |
| Multiple diagnoses | 12 (9.0) |
| Otherb | 17 (12.8) |
| Radiation treatment center | |
| Academic center | 63 (47.3) |
| Community practice | 70 (52.6) |
| Time since RT | |
| Currently receiving treatment | 19 (14.3) |
| RT completed in past 6 mo | 39 (29.3) |
| RT completed in past 7-24 mo | 21 (15.8) |
| RT completed >24 mo ago | 56 (42.1) |
| Did clinician recommend topical agents? | |
| Yes | 124 (93.2) |
| No | 9 (6.8) |
| Main clinician managing RT dermatitis | |
| Physician | 59 (44) |
| Nurse | 74 (56) |
| Did the patient report skin peeling? | |
| Yes | 60 (44.4) |
| No | 73 (54.9) |
| Did the patient report skin erythema? | |
| None | 0 |
| Mild | 50 (37.6) |
| Moderate | 39 (29.3) |
| Severe | 44 (33.1) |
| Advised to avoid use of topical agents just before RT? | |
| Yes | 111 (83.4) |
| No | 23 (17.3) |
| Advised to avoid use of topical agents for several hours before RT? | |
| Yes | 98 (73.7) |
| No | 35 (26.3) |
| Advised to wipe off topical agents before RT? | |
| Yes | 72 (54.1) |
| No | 61 (45.9) |
Abbreviation: RT, radiotherapy.
Totals may not equal 100 because of rounding.
Includes anal cancer, esophageal cancer, endometrial cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, melanoma, nonmelanomatous skin cancer, penile cancer, prostate cancer, rectal cancer, sarcoma, and testicular cancer.
Ninety-two patients with breast cancer completed the survey, with 87 (94.6%) of these patients having breast cancer as their only diagnosis. Results for these 87 patients were similar to those for the entire cohort. Seventy-four patients (85.0%) were advised to avoid applying topical agents just before RT, 66 (75.8%) were advised to avoid topical agents for several hours before RT, and 49 (56.3%) were advised to wipe off residual topical agents before RT. Fifty patients (57.5%) reported moderate or severe skin erythema and 37 (42.5%) reported skin peeling. There was no difference in patient response based on educational level, treatment setting (academic vs private practice), primary clinician (physician vs nurse), time since completion of RT, or degree of skin peeling or erythema.
One hundred five of 108 clinicians (97.2%) reported that they are directly involved in managing radiation dermatitis and were included in the analysis. Of these, 52 (49.5%) were physicians and 53 (50.5%) were nurses. Ninety-six (91.4%) reported that they advise their patients to avoid applying topical agents just before or within a few hours of RT. Reasons cited were bolus effect by 81 (84.3%) and routine clinical practice by 57 (59.3%). Ninety-eight clinicians (93.3%) advised their patients to avoid applying metal-containing creams just before or several hours before RT. Reasons cited included concern about electron scatter from metals by 69 (70.4%), bolus effect by 59 (60.0%), and routine clinical practice by 50 (51.0%). There were no significant differences in responses based on academic vs private practice or physicians vs nurses.
Dosimetric Results
Measurements showed no difference in dose at the surface or 2-cm depth with or without a relatively thick 1- to 2-mm application of either topical agent when using en face 6- or 15-MV photons. Similarly, there was no association with increased surface dose with 1- to 2-mm applications of either topical agent at various beam angles except for a 6% increase at an incident angle of 60° observed only with the silver sulfadiazine cream, 1% (1.20 Gy with silver sulfadiazine cream vs 1.13 Gy without topical agents). When a thicker (≥3-mm) layer of either topical was applied, a bolus effect was observed with increased skin dose. For en face 6-MV beams, the surface dose was 1.05 Gy with a thick layer of the petroleum-based ointment and 1.02 Gy for silver sulfadiazine cream compared with 0.88 Gy without topicals. For en face 15-MV beams and a layer of cream 3 mm or greater, the surface doses were 0.70 Gy for petroleum-based ointment, 0.60 Gy for silver sulfadiazine cream, and 0.52 Gy without topicals.
Even with a thick (≥3-mm) layer of either topical agent, there was only a 2% to 5% increase in the surface dose with en face 6- or 9-MeV electrons. With en face 6-MeV electrons, the surface doses were 1.57 Gy for a thick layer of petroleum-based ointment, 1.52 Gy for silver sulfadiazine, and 1.49 Gy without topicals. With en face 9-MeV electrons, the surface doses were 1.63 Gy for a thick layer of petroleum-based ointment, 1.64 Gy for silver sulfadiazine, and 1.59 Gy without topicals. No differences in dose were observed at 2-cm depth with or without topical agents regardless of the topical agent type, applied thickness of the topical agent, or beam energy.
Preclinical Results
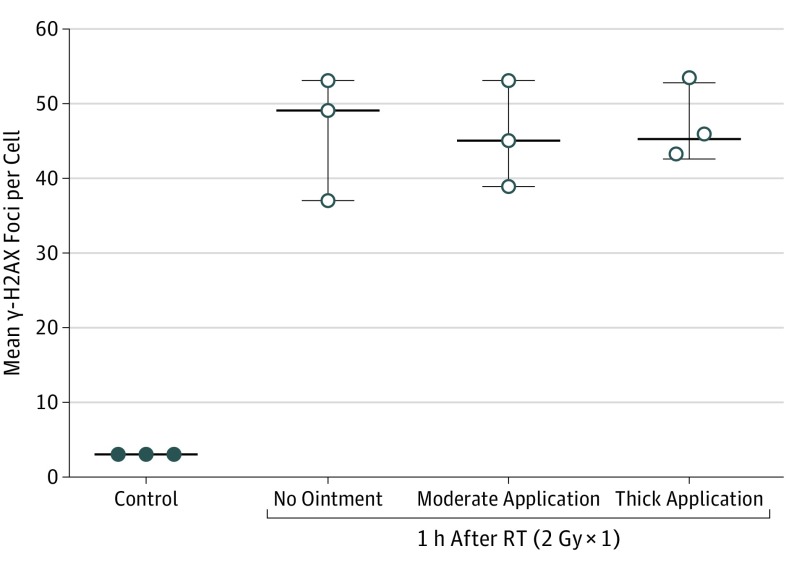
Irradiated skin in C57BL/6 mice showed no difference in γ-H2AX–positive foci (or intensity) with or without petroleum-based ointment when 2 Gy ×1 or 15 Gy ×1 were administered. Thickness of the ointment application also did not alter the γ-H2AX staining. eFigure 2 in the Supplement shows representative images from the γ-H2AX staining from frozen sections from the unirradiated control mice (n = 3) compared with skin sections from mice irradiated to 2 Gy (n = 3) with or without petroleum-based ointment of varying thickness (50 mg vs 200 mg). Each irradiated mouse served as its own control. There was a significant increase in mean (SEM) γ-H2AX–positive foci in the skin of irradiated mice vs unirradiated mice: 46.3 (4.8) vs 3 (0.03), P < .001 (Figure 1). There was no difference in the quantification of γ-H2AX–positive foci with or without ointment of varying thickness when 2 Gy of radiation was administered. Quantification of γ-H2AX was 46.8 (3.1) for the thick application of ointment, 45.7 (4.1) for the moderate application of ointment, and 46.3 (4.8) for irradiated controls without any ointment (P = .67) (Figure 1). No difference in levels of apoptosis was shown by the TUNEL assay for the mice irradiated to 2 Gy in the presence or absence of creams of varying thickness.
Figure 1. Quantification of Phosphorylated Histone (γ-H2AX) Staining of Skin From Unirradiated Mice (Controls) Compared With Skin From Mice Irradiated With 2 Gy ×1 in the Presence or Absence of Skin Ointment of Varying Thickness.
Quantification of γ-H2AX staining in frozen sections of skin from unirradiated mice (control) compared with skin sections from mice irradiated with 2 Gy in the presence or absence of petrolatum-based ointment (petrolatum, 41% [Aquaphor; Beiersdorf AG]) of varying thickness, including application of 50 mg of ointment (moderate application) and application of 200 mg of ointment (thick application). Data are expressed as mean; whiskers indicate SEM (n = 3). RT indicates radiotherapy. To convert gray to rad, multiply by 100.
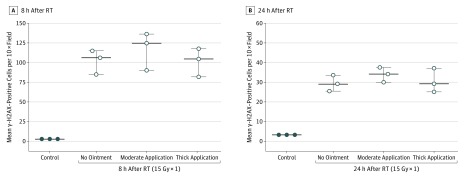
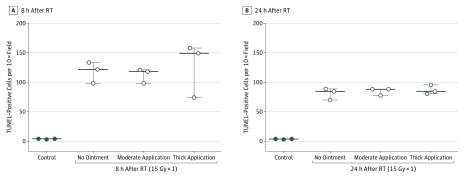
To determine if these findings would be replicated in the setting of a much higher biologically equivalent dose (eg, hypofractionated treatment or conventionally fractionated treatment delivered across many days), we repeated the experiment irradiating mice (n = 3) to 15 Gy ×1 in the presence or absence of petroleum-based ointment of varying thickness (50 mg vs 200 mg) and found that there was no significant difference in mean (SEM) for γ-H2AX–positive stained cells at either 8 hours or 24 hours after RT (Figure 2 and eFigure 3 in the Supplement). Quantification of γ-H2AX staining at 8 hours was 116.7 (13.8) for the thicker application of ointment, 101 (10.5) for the moderate application of ointment, and 102 (8.9) without ointment (P = .2). At 24 hours, γ-H2AX staining was 33.8 (2.2), 30.3 (3.5), and 29.3 (2.3), respectively (P = .56). There was no difference in positively stained TUNEL cells at 8 and 24 hours after RT (Figure 3 and eFigure 4 in the Supplement). At 8 hours after RT, quantification of TUNEL staining was 127 (26.6) for the thicker application of ointment, 112 (7) for the moderate application of ointment, and 118 (10.3) without ointment (P = .89). After 24 hours, quantification of TUNEL staining was 86.7 (4.3), 85 (3.5), and 80.8 (5.5), respectively (P = .72).
Figure 2. Quantification of Phosphorylated Histone (γ-H2AX) Staining of Skin From Unirradiated Mice (Controls) Compared With Skin From Mice Irradiated With 15 Gy ×1 in the Presence or Absence of Skin Ointment of Varying Thickness.
Quantification of γ-H2AX staining in frozen sections of skin from unirradiated mice (control) compared with skin sections from mice irradiated with 15 Gy ×1 in the presence or absence of petroleum-based ointment (petrolatum, 41% [Aquaphor; Beiersdorf AG]) of varying thickness, including application of 50 mg of ointment (moderate application) and application of 200 mg of ointment (thick application). Quantification of γ-H2AX–positive cells per 10× field at 8 hours (A) and 24 hours (B) after 15 Gy of RT in immunofluorescent images is presented. Data are expressed as mean; whiskers indicate SEM (n = 3). RT indicates radiotherapy. To convert gray to rad, multiply by 100.
Figure 3. Quantification of Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining in Frozen Sections of Skin From Control Mice Compared With Skin Sections From Mice Irradiated to 15 Gy ×1 in the Presence or Absence of Skin Ointment of Varying Thickness.
Quantification of positively stained TUNEL apoptotic cells in frozen sections of skin from unirradiated mice (control) compared with skin sections from mice irradiated to 15 Gy ×1 in the presence or absence of petroleum-based ointment (petrolatum, 41% [Aquaphor; Beiersdorf AG]) of varying thickness at 8 hours (A) and 24 hours (B) after 15 Gy ×1, including application of 50 mg of ointment (moderate application) and application of 200 mg of ointment (thick application). Data are expressed as mean; whiskers indicate SEM (n = 3). RT indicates radiotherapy. To convert gray to rad, multiply by 100.
Discussion
There are limited data on the dosimetric effect of topical agents on the skin surface dose of patients receiving RT. The literature has focused on the effect of antiperspirants for patients undergoing breast RT, with 2 dosimetric studies showing no difference in surface dose with antiperspirants5,10 and several clinical trials showing no increased toxic effects with their use.11,12 To our knowledge, the present study is the first dosimetric assessment of whether commonly used creams and ointments alter surface dose. It is somewhat surprising that there have been no studies assessing dose alterations resulting from cream or ointment use because this is an issue that affects not only patients with breast cancer but also most patients undergoing radiotherapy. The results of this analysis therefore have the potential to influence care for patients undergoing RT across a range of disease sites.
Our survey results indicate that patients are still routinely advised to avoid applying topical agents just prior to RT, although modern radiation equipment and techniques are associated with greater skin sparing than the cobalt-60 and orthovoltage machines used in the 1950s through 1980s when the recommendation to avoid applying topical agents before RT became widespread practice.
Results of our dosimetric analysis suggest that thin or moderately thick applications of topical agents, even if applied immediately before RT, only minimally alter the dose to the skin regardless of beam energy or beam angles. Very thick applications of a topical agent just before RT may have a bolus effect with increased surface dose and should be avoided. However, the very thick application used in this study was designed to represent an extreme situation that is very unlikely to occur in the clinic. Because a 0.5-cm tissue-equivalent bolus is frequently applied to the skin to deliberately increase the surface dose for therapeutic purposes,13 it should not be surprising that a cream thickness of 3 mm or greater would result in a modest increase in surface dose.
Our study also found no increase in the surface dose for the metallic vs nonmetallic topical agents. Theoretically, metallic creams might be expected to increase the skin dose owing to electron scatter from the metallic atoms, but our study found no such increase in dose, perhaps because of the very low metallic content in silver sulfadiazine. The dosimetric results were so similar between the petroleum-based ointment and silver sulfadiazine cream that we did not think it necessary to perform preclinical studies on silver sulfadiazine, which is less commonly used clinically.
Studies in mouse models to evaluate the association of topical agent use on skin dose by using γ-H2AX and TUNEL staining similarly demonstrated no significant difference in radiation-induced DNA damage or apoptosis in the skin with or without petroleum-based ointment after 2 Gy ×1. We also tested the use of a much higher dose (15 Gy ×1) to simulate the biological outcomes after a course of fractionated radiotherapy or a large hypofractionated treatment as is used for stereotactic body radiotherapy. Immunofluorescent analysis of the mice treated to 15 Gy showed no difference in the surface dose in the presence or absence of ointments of varying thickness whether assessed using γ-H2AX or TUNEL staining.
The preclinical results recapitulated the results observed in the dosimetric studies: there was no increase in the surface dose with or without a moderately thick application of ointment. This is noteworthy because the mice were irradiated with kilovoltage RT, which has less skin-sparing than the megavoltage beams used in modern clinical practice and in our dosimetry studies. The preclinical studies did not confirm the findings that very thick applications of ointment alter the surface dose. We suspect that OSLD measurements are more accurate as an assessment of surface dose than γ-H2AX and TUNEL assays, but slight differences in surface dose may not be clinically meaningful.
The results of this multidisciplinary study suggest that the current practice of advising patients to avoid applying topical agents for several hours before RT does not rest on sound science, with the possible exception of patients who apply very copious amounts of topical agents immediately before treatment. We suggest that patients be advised that applying thin or moderate amounts of a topical agent, even right before RT, is acceptable and unlikely to increase toxic effects to the skin. Allowing patients to apply topical agents regardless of the timing with respect to RT will simplify patient instructions and reduce patient confusion and anxiety because many patients are concerned that having any residual topicals on their skin at the time of RT will increase toxic effects to the skin. In our experience, these patients often underuse emollients or exacerbate their dermatitis by mechanical irritation of the skin to remove residual topicals before RT. Of the 133 patients surveyed, 72 (54.1%) were told to wipe away excess topical agents before RT; this practice appears to be relatively common and likely to cause discomfort for many patients. Allowing patients to apply topical agents as needed without restrictions on the timing of application is likely to improve patient quality of life.
Limitations
The study has several limitations. The size of the survey population (n = 241) is relatively modest; however, we believe the survey results are reasonably representative of current practice. More than 100 clinicians, who presumably offer their advice to many patients, participated, and the responses of patients and clinicians with respect to avoiding topical agents before RT were virtually identical. The study does not report on toxic effects to the skin in the presence or absence of topical agents during a course of RT. Instead, we report the surrogate end points of surface dose assessed using phantoms and dosimeters and the degree of radiation-induced DNA double-strand breaks and cellular apoptosis in the skin of mice irradiated in the presence and absence of topical agents. Although a clinical trial would be the criterion standard, such a trial would rely on more subjective end points and may not be able to detect a small but potentially meaningful difference in toxic effects. Like the randomized clinical trials assessing antiperspirant use during breast RT, a trial of skin creams would be limited by inherent difficulties in assessing skin toxic effects by using the overly broad CTCAE system.5 Grade 2 dermatitis is so broadly defined that it could mask potentially meaningful differences by grouping together asymptomatic patients with moderate erythema and patients experiencing significant pain and pruritus from severe erythema and focal moist desquamation. This grading system is acknowledged to be a major limitation in trials assessing dermatitis.14,15 We believe that the quantitative dosimetric and preclinical assessments are compelling enough that a clinical trial may not be necessary to inform practice. Other limitations include limits in OSLD accuracy in measuring surface dose. The study also does not directly quantify the effect of electron scatter caused by silver in silver sulfadiazine, although the effect is likely very modest given the dosimetric results.
Conclusions
To our knowledge, this is the first dosimetric and preclinical assessment of the association of topical therapy use with radiation dose to the skin. Our findings suggest that thin or moderately applied topical agents, even if applied immediately before RT, have minimal ability to alter the skin dose regardless of the beam energy or beam incidence. Applying very thick amounts of a topical agent just before RT increased the surface dose and should be avoided.
eMethods 1. Dosimetric and Preclinical Radiation Technique and Staining
eImage. Dosimetric Experimental Setup
eFigure 1. Schematic Presentation of the Experimental Design
eFigure 2. γ-H2AX Staining in Frozen Sections of Skin From Control Mice (Untreated) Compared With Skin Sections From Irradiated With 2Gy Mice +/− Application of Aquaphor Cream, at 1h Post RT
eFigure 3. γ-H2AX Staining in Frozen Sections of Skin From Mice Irradiated to 15Gy in the Presence or Absence of Aquaphor Cream of Varying Thickness, Including Application of 50mg of Aquaphor (Lite Cream) and Application of 200mg of Aquaphor (Thick Cream)
eFigure 4. TUNEL Staining in Frozen Sections of Skin From Control Mice Compared With Skin Sections From Mice Irradiated to 15Gy in the Presence or Absence of Aquaphor Cream of Varying Thickness
eMethods 2. Patient and Provider Survey Forms
References
- 1.Salvo N, Barnes E, van Draanen J, et al. . Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17(4):94-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelan TJ, Olivotto IA, Parulekar WR, et al. ; MA.20 Study Investigators . Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316. doi: 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magrini SM, Buglione M, Corvò R, et al. . Cetuximab and radiotherapy versus cisplatin and radiotherapy for locally advanced head and neck cancer: a randomized phase II trial. J Clin Oncol. 2016;34(5):427-435. doi: 10.1200/JCO.2015.63.1671 [DOI] [PubMed] [Google Scholar]
- 4.Kachnic LA, Winter K, Myerson RJ, et al. . RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86(1):27-33. doi: 10.1016/j.ijrobp.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann BC, Zeng C, Freedman GM, et al. . Avoiding antiperspirants during breast radiation therapy: myth or sound advice? Radiother Oncol. 2017;124(2):204-207. doi: 10.1016/j.radonc.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 6.Baumann BC, Benci JL, Santoiemma PP, et al. . An integrated method for reproducible and accurate image-guided stereotactic cranial irradiation of brain tumors using the small animal radiation research platform. Transl Oncol. 2012;5(4):230-237. doi: 10.1593/tlo.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann BC, Kao GD, Mahmud A, et al. . Enhancing the efficacy of drug-loaded nanocarriers against brain tumors by targeted radiation therapy. Oncotarget. 2013;4(1):64-79. doi: 10.18632/oncotarget.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verginadis II, Kanade R, Bell B, Koduri S, Ben-Josef E, Koumenis C. A novel mouse model to study image-guided, radiation-induced intestinal injury and preclinical screening of radioprotectors. Cancer Res. 2017;77(4):908-917. doi: 10.1158/0008-5472.CAN-16-2724 [DOI] [PubMed] [Google Scholar]
- 9.Tang MM, Mah LJ, Vasireddy RS, et al. . Quantitation of gammaH2AX foci in tissue samples. J Vis Exp. 2010;(40):2063. doi: 10.3791/2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burch SE, Parker SA, Vann AM, Arazie JC. Measurement of 6-MV X-ray surface dose when topical agents are applied prior to external beam irradiation. Int J Radiat Oncol Biol Phys. 1997;38(2):447-451. doi: 10.1016/S0360-3016(97)00095-3 [DOI] [PubMed] [Google Scholar]
- 11.Watson LC, Gies D, Thompson E, Thomas B. Randomized control trial: evaluating aluminum-based antiperspirant use, axilla skin toxicity, and reported quality of life in women receiving external beam radiotherapy for treatment of stage 0, I, and II breast cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):e29-e34. doi: 10.1016/j.ijrobp.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 12.Lewis L, Carson S, Bydder S, Athifa M, Williams AM, Bremner A. Evaluating the effects of aluminum-containing and non-aluminum containing deodorants on axillary skin toxicity during radiation therapy for breast cancer: a 3-armed randomized controlled trial. Int J Radiat Oncol Biol Phys. 2014;90(4):765-771. doi: 10.1016/j.ijrobp.2014.06.054 [DOI] [PubMed] [Google Scholar]
- 13.Gunderson LL, Tepper JE. Clinical Radiation Oncology. 4th ed Philadelphia, PA: Elsevier Saunders; 2015. [Google Scholar]
- 14.Langendijk JA, Oosting SF. Grading system and management guidelines for dermatitis induced by head and neck radiotherapy plus cetuximab: clinical validation required. Ann Oncol. 2011;22(10):2157-2159. doi: 10.1093/annonc/mdr410 [DOI] [PubMed] [Google Scholar]
- 15.Freedman GM. Radiation complications and their management In: Bland KI, Copeland EM, eds. The Breast: Comprehensive Management of Benign and Malignant Diseases. 4th ed Philadelphia, PA: Saunders; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Dosimetric and Preclinical Radiation Technique and Staining
eImage. Dosimetric Experimental Setup
eFigure 1. Schematic Presentation of the Experimental Design
eFigure 2. γ-H2AX Staining in Frozen Sections of Skin From Control Mice (Untreated) Compared With Skin Sections From Irradiated With 2Gy Mice +/− Application of Aquaphor Cream, at 1h Post RT
eFigure 3. γ-H2AX Staining in Frozen Sections of Skin From Mice Irradiated to 15Gy in the Presence or Absence of Aquaphor Cream of Varying Thickness, Including Application of 50mg of Aquaphor (Lite Cream) and Application of 200mg of Aquaphor (Thick Cream)
eFigure 4. TUNEL Staining in Frozen Sections of Skin From Control Mice Compared With Skin Sections From Mice Irradiated to 15Gy in the Presence or Absence of Aquaphor Cream of Varying Thickness
eMethods 2. Patient and Provider Survey Forms