Key Points
Questions
Is a vaccination approach with PVX-410 multipeptide vaccine safe and immunogenic with or without lenalidomide in human leukocyte antigen A2–positive patients with moderate- to high-risk smoldering myeloma?
Findings
The PVX-410 vaccine with or without lenalidomide was well tolerated. Immune responses were seen, as indicated by an increase in percentage of tetramer-positive cells and interferon γ–positive cells in the CD3+CD8+ cell population, which was further enhanced in combination with lenalidomide.
Meaning
These results demonstrate immune responses to a vaccination strategy in a patient population with smoldering myeloma; further evaluation of PVX-410 and lenalidomide among patients with smoldering myeloma who are at a risk of progression is warranted.
This nonrandomized clinical trial examines the safety, tolerability, immunogenicity, and anti–multiple myeloma activity of the PVX-410 multipeptide vaccine with or without lenalidomide.
Abstract
Importance
Increasing evidence suggests the significance of the role of the immune system in the progression of smoldering multiple myeloma (SMM) to symptomatic multiple myeloma (MM). Boosting the immune system via vaccination in the earlier, asymptomatic SMM stage may provide a novel strategy to prevent or slow progression to active MM.
Objective
To determine the safety, tolerability, immunogenicity, and anti-MM activity of the PVX-410 multipeptide vaccine with or without lenalidomide.
Design, Setting, and Participants
This 3-cohort phase 1/2a multicenter dose-escalation study accrued 22 adults (≥18 years) with SMM with normal organ/marrow function who were human leukocyte antigen A2-positive and at moderate or high risk of progression to MM.
Interventions
Patients received 6 doses of PVX-410 emulsified in Montanide ISA 720 VG, 0.4 mg total (0.1 mg/peptide) (n = 3) or 0.8 mg total (0.2 mg/peptide) (n = 9), biweekly via subcutaneous injection. In the combination cohort (n = 10), patients also received three 21-day cycles of lenalidomide, 25 mg, orally daily every 28 days. All patients received 0.5 mL (1 mg) poly-ICLC (2 mg/mL) via intramuscular injection with each PVX-410 dose.
Main Outcomes and Measures
Adverse events (AEs) were evaluated using the Common Terminology Criteria for Adverse Events, version 4.03. PVX-410–specific T lymphocytes by flow cytometry to assess tetramer and interferon (IFN)-γ response. Disease response was assessed by investigators using the International Myeloma Working Group (IMWG) and modified European Group for Bone Marrow Transplantation (EBMT) criteria.
Results
Overall, 14 (64%) patients were men and the median age at enrollment was 56 years in the monotherapy and 57 years in the combination cohorts (overall range, 39-82 years). Six of 12 patients in the monotherapy and 9 of 10 in the combination cohorts were at moderate risk. The PVX-410 vaccine was well tolerated. The most common AEs were mild-to-moderate injection site reactions and constitutional symptoms. Of note, PVX-410 was immunogenic as monotherapy (10 of 11 patients) and in combination with lenalidomide (9 of 9 patients), as demonstrated by an increase in percentage of tetramer-positive cells and IFN-γ cells in the CD3+CD8+ cell population. The combination resulted in greater mean fold increases in proportions of CD3+CD8+ T cells that were tetramer-positive and IFN–γ-positive, statistically significant for IFN–γ-positive cells after 2 and 4 vaccinations. An increase and persistence of vaccine-specific effector memory cells was noted. In total, 7 of 12 patients in the PVX-410–alone cohort had stable disease with 2 of 3 (low-dose cohort) and 1 of 9 of the target-dose cohort progressing (median TTP, 36 weeks), whereas 5 of 12 patients in the combination cohort showed, clinical response, with 1 patient progressing (median TTP not reached).
Conclusions and Relevance
Overall, these results suggest that the vaccine is safe and immunogenic in this patient population and support continued study of PVX-410 in SMM.
Trial Registration
ClinicalTrials.gov identifier: NCT01718899
Introduction
The precise triggers of progression to symptomatic multiple myeloma (MM) from the asymptomatic state of smoldering MM (SMM) are unknown; however, it has been suggested that the immune system plays a role in controlling malignant growth.1,2,3,4 It is hypothesized that boosting the immune system via vaccination in the asymptomatic stage of disease may prevent or slow progression to active MM.
The PVX-410 vaccine (OncoPep, Inc.) is a human leukocyte antigen A2-restricted multipeptide cancer vaccine being developed for patients with SMM. The PVX-410 vaccine is composed of 4, 9-mer chemically synthesized peptides from unique regions of 3 MM-associated antigens, X-box binding protein 1 (XBP1), syndecan-1 (CD138), and CS1.5 The 4 peptides were selected based on in vitro data,6 which showed that individually, each peptide stimulated antigen-specific cytotoxic T lymphocytes (CTLs), demonstrating MM-specific T-lymphocyte responses, including cell proliferation, interferon-γ (IFN-γ) secretion, and cytotoxic activity in response to MM tumor cells.7 Thus, CTLs generated by PVX-410 can evoke a spectrum of tumor-specific immune responses that may prove beneficial as an immunotherapy through targeting multiple tumor-associated antigens on MM cells.8
The PVX-410 vaccine was investigated in combination with the immunomodulatory compound lenalidomide to maximize antitumor activity. It was hypothesized that coadministration of lenalidomide would enhance the T–cell-mediated immune response induced by PVX-410.9
Methods
Trial Design
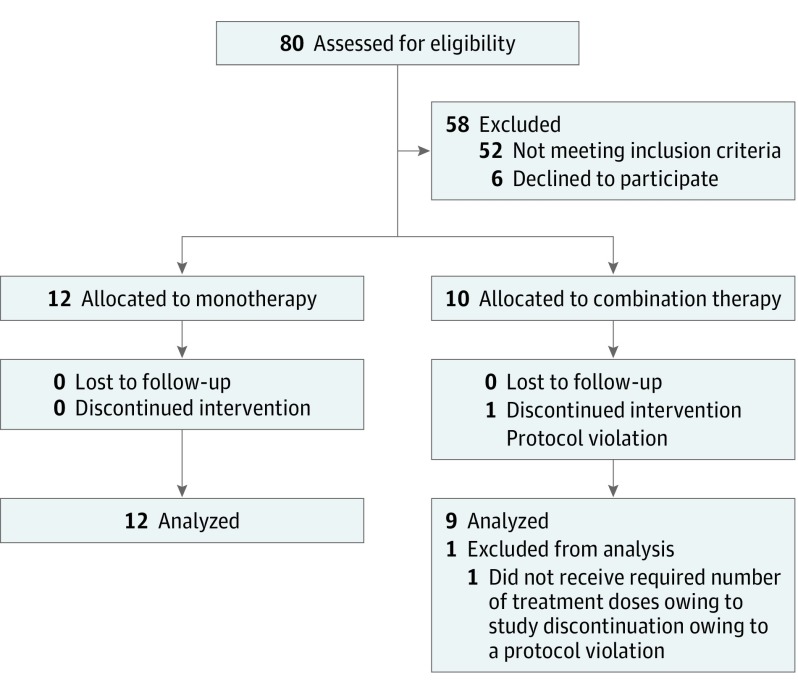
The trial protocol is available in Supplement 1. In this phase 1/2a dose-escalation study, patients were assigned based on timing of enrollment to 1 of 3 treatment cohorts: (1) low-dose cohort (PVX-410 0.4 mg), (2) target-dose cohort (PVX-410 0.8 mg), or (3) combination cohort (PVX-410 0.8 mg plus 3 cycles of lenalidomide, 25 mg orally) (Figure 1) (eFigure 1 in Supplement 2).
Figure 1. CONSORT Flow Diagram.
Eighty patients were assessed for eligibility, 12 patients were included in the final analysis in monotherapy, and 9 patients in combination therapy.
The study was conducted from November 2012 through September 2016. The protocol received institutional review board approval, and all patients provided written informed consent to participate. They were not compensated.
Eligibility
Adults 18 years or older with SMM with normal organ/marrow function who were human leukocyte antigen A2-positive and at moderate (2 risk factors) or high risk (3 risk factors) of progression to MM, were eligible.
Treatment
Patients received a total of 6 PVX-410 doses via subcutaneous injection every 2 weeks and then were assessed at months 1, 2, 3, 6, 9 and 12 posttreatment. Each PVX-410 dose was accompanied by intramuscular injection of an adjuvant, polyinosinic-polycytidylic acid-poly-L-lysine (poly-ICLC; Hiltonol; Oncovir, Inc), 1 mg.
Safety
Safety was assessed by documentation of adverse events (AEs), including serious adverse events (SAEs), vaccination site examinations, clinical laboratory testing, and physical examination findings.
Immunogenicity
A flow cytometry (FACS)-based assay was used to detect antigen-specific T-cell responses to the PVX-410 vaccine using peripheral blood mononuclear cells collected from patients at weeks 0 (baseline), 4, and 8, and at 1 and 3 months posttreatment. A positive immune response was defined as a minimum increase from baseline of 1.5-fold for percentage of IFN–γ-positive cells and a 2-fold increase from baseline for percentage of tetramer-positive cells in at least 1 time point.
Antitumor Activity
Disease response was evaluated by the investigators (N.S.R., A.K.N., and P.G.R.) using the IMWG Criteria10 and the modified EBMT Criteria.11
Statistics
Descriptive statistics including means and standard deviations were generated. For intergroup comparisons (low-dose monotherapy [n = 3] was not included in comparison) 2-sample t tests were used. P ≤ .05 was considered significant. Time-to-event analyses (ie, time to progression, progression-free survival, and duration of response) used standard survival analysis techniques such as Kaplan-Meier life test methods.
Results
Patient Disposition
A total of 22 patients were enrolled, 3 in the low-dose cohort; 9 in the target-dose cohort; and 10 in the combination cohort.
Twenty-one (95%) patients completed study treatment. One patient in the combination cohort discontinued treatment permanently; in error by the study center, a protocol deviation; patient was excluded from the evaluable population.
Patient Characteristics
Overall, 14 (64%) patients were men. Median age at enrollment was 56 years in the monotherapy and 57 years in the combination cohorts (range, 39-82 years). Six of 12 patients in the monotherapy and 9 of 10 in the combination cohorts were moderate risk.
Safety and Tolerability
The PVX-410 vaccine was safe and well tolerated. Vaccine-related treatment-emergent AEs (TEAEs) included injection-site pain (monotherapy, 7 [58%]; combination, 10 [100%]); injection-site erythema (monotherapy, 3 [25%]; combination, 8 [80%]); and injection-site discomfort (monotherapy, 2 [17%]; combination, 3 [30%]), induration (monotherapy, 2 [17%]; combination, 3 [30%]), irritation (monotherapy, 2 [17%]; combination, 4 [40%]), and cutaneous eruption (monotherapy, 2 [17%]; combination, 2 [20%]) as well as chills, fatigue, myalgia, and pyrexia. All TEAEs with monotherapy were grade 1 or 2 in intensity.
Overall, the TEAE profile of PVX-410 in combination with lenalidomide was similar to that seen with PVX-410 alone, although individual local and systemic TEAEs occurred at a higher incidence with the combination as summarized in eTable 1 in Supplement 2. No patient experienced a PVX-410–related SAE or discontinued PVX-410 because of a TEAE.
Immune Response
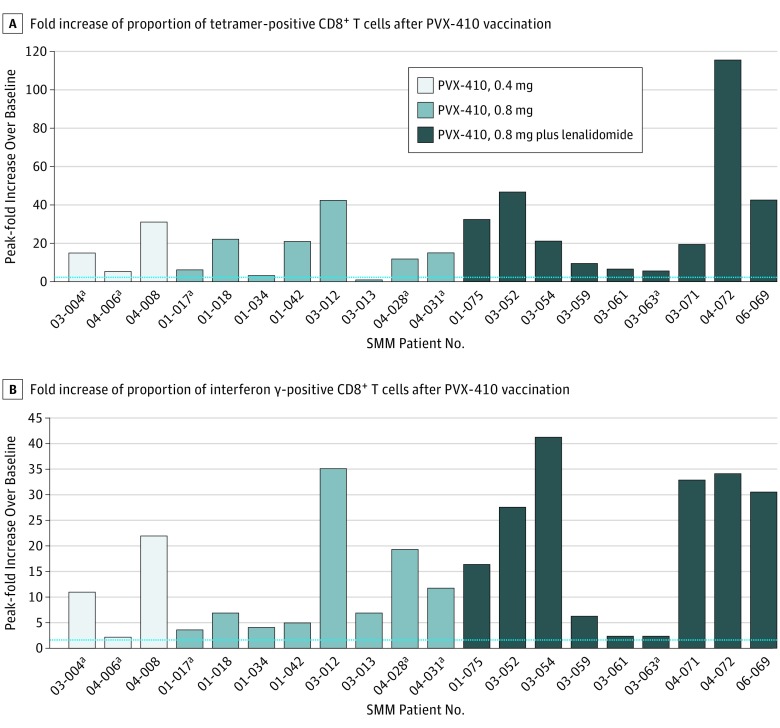
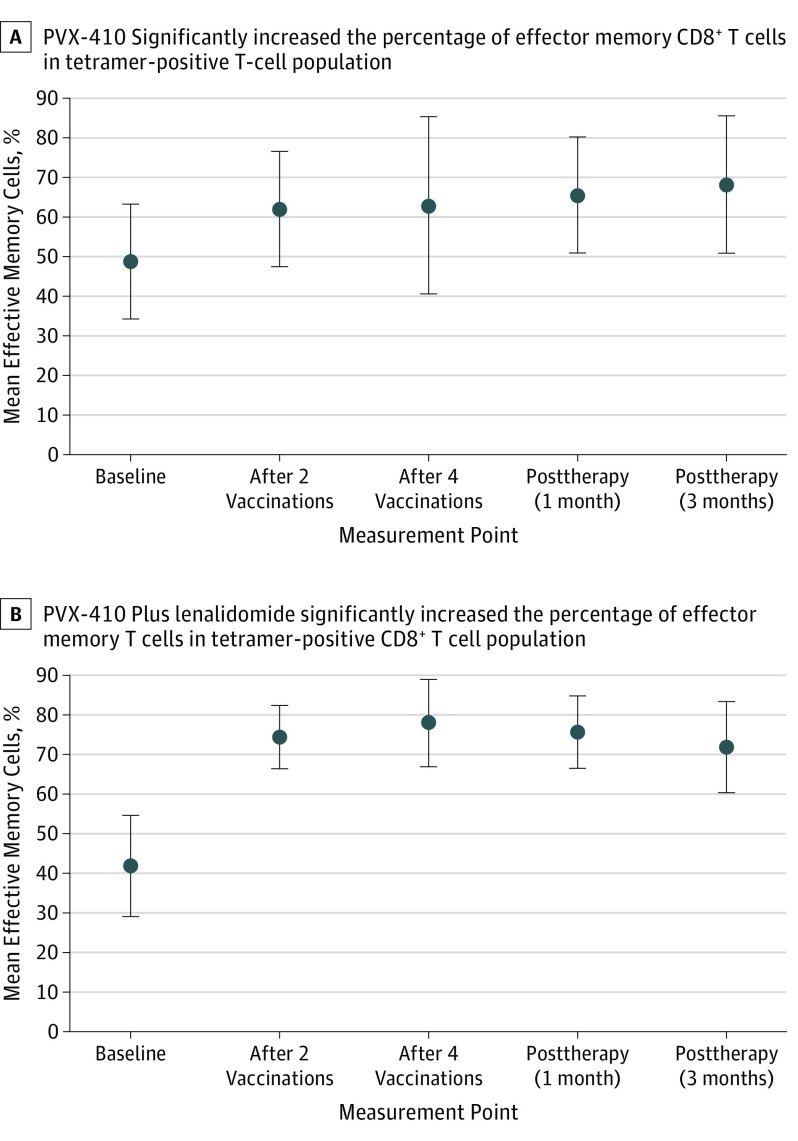
Nineteen of 20 (95%) evaluable patients achieved an immune response to PVX-410 (Figure 2, A and B). The addition of lenalidomide increased the magnitude of immune response, particularly during study treatment, according to post hoc analyses comparing evaluable patients receiving target-dose PVX-410 alone (n = 8) vs combination therapy (n = 9). There was an increase in PVX-410–specific tetramer-positive cells following stimulation with the PVX-410 peptide cocktail; no increase was seen in human immunodeficiency virus (HIV)-specific tetramer-positive cells (eFigure 2 in Supplement 2), which served as a control. Likewise, there was an increase from baseline in IFN-γ–positive T cells following stimulation with the PVX-410, but not with the HIV peptide. Greater mean fold increases over baseline in proportions of CD3+ and CD8+ peripheral blood mononuclear cells that were PVX-410 peptide tetramer-positive, IFN-γ–positive, interleukin 2-positive, or tumor necrosis factor α-positive were seen with the combination vs monotherapy. Combination therapy also resulted in higher percentages at all 4 posttreatment measurement times of tetramer-positive, CD3+, and CD8+ peripheral blood mononuclear cells exhibiting an effector memory cell phenotype (Figure 3). The differences in immune responses in the 2 cohorts were statistically significant at week 2 for IFN-γ, interleukin 2, tumor necrosis factor α, and effector memory cell variables, and at week 4 for interleukin 2, and tumor necrosis factor α.
Figure 2. Fold Increases.
A, PVX-410, alone or with lenalidomide, was associated with vaccine-specific immune responses in patients with smoldering multiple myeloma (SMM), demonstrated by postvaccination peak fold increases over baseline in proportion of tetramer-positive T cells. B, PVX-410, alone or with lenalidomide, was associated with vaccine-specific immune responses in patients with SMM, demonstrated by postvaccination peak fold increases over baseline in proportion of interferon-γ–positive T cells. Horizontal dashed line indicates the cutoff for positive response. Len indicates lenalidomide; vac, vaccination.
aPatients with progressive disease.
Figure 3. Effector Memory Cells Percentage Increase After PVX-410 Vaccination.
PVX-410 alone or with lenalidomide on average was associated with increased proportions of gated tetramer-positive, CD3+, and CD8+ T cells displaying an effector memory cell phenotype (CD45RO+ and CCR7-), suggesting potential durability of immune response. Error bars indicate the standard deviations.
Clinical Response
As expected, objective clinical responses to the relatively short courses of study treatment were modest. All patients receiving PVX-410 alone (n = 12) had stable disease (SD) as their best clinical response, with 5 of 12 patients progressing within the 12-month follow-up period. In the combination therapy cohort (n = 9), the best clinical response was partial response (PR) in 1 patient and minimal response and SD in 4 each.
There appeared to be a relationship between clinical response and the magnitude of immune response, with responders having a significantly greater immune response compared with those who progressed. Specifically, patients showing a greater than 10-fold response over baseline at more than 2 time points were more likely to demonstrate a clinical response or SD at the last study visit (4 of 4 responders, 6 of 10 SD, 1 of 6 progressors). The relationship of immune response and clinical response is summarized in eTable 2 in Supplement 2.
Discussion
This phase 1/2a nonrandomized clinical trial is the first vaccine study to our knowledge to evaluate treatment for patients with SMM at moderate or high risk of progression to MM. Findings suggest that PVX-410 multipeptide vaccine is safe and well tolerated whether given as monotherapy or combined with lenalidomide. The PVX-410 vaccine appears to consistently achieve specific, durable immune responses, which may be enhanced during treatment with lenalidomide. Further study of the vaccine is warranted, including in combination with other agents and for longer durations.12 The percentage of patients experiencing a myeloma-defining event may be correlated with immune responses in SMM using this vaccination approach.
Limitations
Absence of longer duration of follow-up to assess ongoing clinical responses remains the major limitation for the current study.
Conclusions
Our findings demonstrate that PVX-410 either alone or in combination with lenalidomide is safe and immunogenic and warrants further investigation in patients with SMM.
Trial Protocol
eFigure 1. Treatment Regimen Schematic
eFigure 2. Representative Patient CD3+CD8+T Cell Response
eTable 1. Most frequent reported treatment-emergent adverse events
eTable 2. Relationship of immune response to clinical response
References
- 1.Fairfield H, Falank C, Avery L, Reagan MR. Multiple myeloma in the marrow: pathogenesis and treatments. Ann N Y Acad Sci. 2016;1364:-. doi: 10.1111/nyas.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shain KH, Dalton WS, Tao J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene. 2015;34(36):4673-4682. doi: 10.1038/onc.2014.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romano A, Conticello C, Cavalli M, et al. Immunological dysregulation in multiple myeloma microenvironment. Biomed Res Int. 2014;2014:198539. doi: 10.1155/2014/198539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. 2012;2012:157496. doi: 10.1155/2012/157496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae J, Samur M, Munshi A, et al. Heteroclitic XBP1 peptides evoke tumor-specific memory cytotoxic T lymphocytes against breast cancer, colon cancer, and pancreatic cancer cells. Oncoimmunology. 2014;3(12):e970914. doi: 10.4161/21624011.2014.970914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae J, Smith R, Daley J, et al. Myeloma-specific multiple peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma and other plasma cell disorders. Clin Cancer Res. 2012;18(17):4850-4860. doi: 10.1158/1078-0432.CCR-11-2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae J, Prabhala R, Voskertchian A, et al. A multiepitope of XBP1, CD138 and CS1 peptides induces myeloma-specific cytotoxic T lymphocytes in T cells of smoldering myeloma patients. Leukemia. 2015;29(1):218-229. doi: 10.1038/leu.2014.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae J, Hideshima T, Zhang GL, et al. Identification and characterization of HLA-A24-specific XBP1, CD138 (Syndecan-1) and CS1 (SLAMF7) peptides inducing antigens-specific memory cytotoxic T lymphocytes targeting multiple myeloma. Leukemia. 2018;32(3):752-764. doi: 10.1038/leu.2017.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luptakova K, Rosenblatt J, Glotzbecker B, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39-49. doi: 10.1007/s00262-012-1308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Harousseau JL, Durie B, et al. ; International Myeloma Workshop Consensus Panel 1 . Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691-4695. doi: 10.1182/blood-2010-10-299487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bladé J, Samson D, Reece D, et al. ; Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant . Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115-1123. doi: 10.1046/j.1365-2141.1998.00930.x [DOI] [PubMed] [Google Scholar]
- 12.Bae J, Hideshima T, Tai Y-T, et al. Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors. [published online February 22, 2018]. Leukemia. 2018. doi: 10.1038/s41375-018-0062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Treatment Regimen Schematic
eFigure 2. Representative Patient CD3+CD8+T Cell Response
eTable 1. Most frequent reported treatment-emergent adverse events
eTable 2. Relationship of immune response to clinical response