This phase 2, multi-institutional clinical trial assesses whether patients with resected primary gastrointestinal stromal tumor receiving 5 years of adjuvant imatinib mesylate therapy have improved recurrence-free survival and overall survival compared with patients receiving a shorter duration of therapy in previous studies.
Key Points
Question
What is the tolerability and efficacy of 5 years of adjuvant imatinib mesylate therapy in patients with resected primary gastrointestinal stromal tumor?
Findings
In this phase 2 study of 91 adults, no patient with resected imatinib-sensitive primary gastrointestinal stromal tumor who received 5 years of adjuvant therapy had disease recur while receiving imatinib. However, rates of early discontinuation of imatinib were high (49%).
Meaning
Longer-duration adjuvant imatinib may be efficacious in appropriately selected patients with resected primary gastrointestinal stromal tumor, but adherence to the planned course of therapy may be challenging despite its benefits.
Abstract
Importance
Three years of adjuvant imatinib mesylate therapy is associated with reduced recurrence rates and improved overall survival in patients with high-risk primary gastrointestinal stromal tumor (GIST) compared with patients who receive 1 year of treatment. The impact of a longer duration of therapy is unknown.
Objective
To determine whether adjuvant treatment for primary GIST with imatinib for 5 years is tolerable and efficacious.
Design, Setting, and Participants
This prospective, single-arm, phase 2 clinical trial (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib [PERSIST-5]) included adult patients with primary GIST (expressing KIT) at 21 US institutions who underwent a macroscopically complete resection and were at intermediate or high risk of recurrence, defined as primary GIST at any site measuring 2 cm or larger with 5 or more mitoses per 50 high-power field or nongastric primary GIST measuring 5 cm or larger. Data were collected from August 5, 2009, through December 20, 2016.
Interventions
Imatinib, 400 mg once daily, orally for 5 years or until discontinuation of therapy because of progression or intolerance.
Main Outcomes and Measures
The primary end point was recurrence-free survival (RFS). The secondary end point was overall survival.
Results
Of the 91 patients enrolled, 48 (53%) were men with a median age of 60 years (range, 30-90 years). Median tumor size was 6.5 cm (range, 2.3-30.0 cm). Median treatment duration was 55.1 months (range, 0.5-60.6 months); 46 patients (51%) completed 5 years of imatinib therapy. Estimated 5-year RFS was 90% (95% CI, 80%-95%), and overall survival was 95% (95% CI, 86%-99%). Recurrence was noted in 7 patients: 1 had disease recur while receiving imatinib (PDGFRA D842V mutation) and died; 6 had disease recur after discontinuation of imatinib therapy. Two additional deaths were unrelated to treatment or tumor progression. Forty-five patients (49%) stopped treatment early because of patient choice (10 [21%]), adverse events (15 [16%]), or other (11 [12%]). All 91 patients experienced at least 1 adverse event, and 17 (19%) experienced grade 3 or 4 adverse events.
Conclusions and Relevance
In this first adjuvant trial, to our knowledge, of patients with resected primary GIST who received 5 years of imatinib therapy, no patient with imatinib-sensitive mutations had disease recur during therapy. For patients in whom disease recurred, recurrence was within 2 years of discontinuation of imatinib therapy. Approximately half of the patients discontinued treatment early, most commonly because of patient choice, thus emphasizing the importance of close clinical monitoring to continue imatinib treatment for patients at appropriate risk.
Trial Registration
ClinicalTrials.gov identifier: NCT00867113
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract, with an incidence of 3000 to 5000 cases per year in the United States.1,2 Approximately 80% of GIST harbor activating mutations in the KIT proto-oncogene (164920), encoding the KIT receptor tyrosine kinase; 3% to 5% have mutations in PDGFRA (173490), encoding platelet-derived growth factor receptor α.3 Historically, patients with localized, primary GIST undergoing macroscopically complete R0/R1 resections (R0, macroscopically complete with negative microscopic margins; R1, macroscopically complete with positive microscopic margins) in the pre-imatinib era had high rates of recurrence and a 5-year disease-specific survival of 54%.4 Several risk-stratification models have been developed to predict the risk of recurrence after R0/R1 resection.5,6,7,8,9
Three phase 3 trials have evaluated the utility of adjuvant imatinib mesylate for 1, 2, and 3 years in patients with R0/R1 resections.10,11,12,13 These studies demonstrated that adjuvant imatinib treatment improved recurrence-free survival (RFS) compared with control (placebo in the 1-year American College of Surgeons Oncology Group [ACOSOG] Z9001 study,10 observation in the 2-year European Organization for Research and Treatment of Cancer [EORTC] 62024 study,12 and 1 year of adjuvant imatinib therapy in the 3-year Scandinavian Sarcoma Group [SSG] XVIII/Arbeitsgemeinschaft Internistische Onkologie [AIO] study13). Furthermore, SSG XVIII/AIO reported improvement in overall survival (OS) with 3 years of adjuvant imatinib therapy. Disease eventually recurred in a similar proportion of patients after adjuvant therapy was discontinued, with the slope of the RFS curve after treatment cessation in the experimental arms resembling those for the control arms.
Thus, adjuvant imatinib therapy of any duration appears to delay recurrence of GIST and, in a subset of patients, may improve survival. However, it is unclear whether extending exposure to imatinib beyond 3 years will further delay recurrence by shifting the RFS curve or biologically impacting the disease to prevent recurrences and decrease the slope of the RFS curve for high-risk patients. The phase 2 multi-institutional Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib (PERSIST-5) trial was conducted to determine the long-term efficacy, safety, and tolerability of 5 years of adjuvant imatinib treatment in patients with intermediate or high risk of recurrence after resection of primary GIST (the trial protocol is available in Supplement 1).
Methods
Patients
Eligible patients were adults (aged ≥18 years) with primary GIST (based on central pathology review) expressing KIT (CD117, confirmed by immunohistochemistry) who underwent a macroscopically complete resection within 12 weeks before the initiation of imatinib therapy. All patients had Eastern Cooperative Oncology Group performance status of 0 (able to perform activities without restriction) or 1 (restricted in physically strenuous activity but able to perform light work) and adequate hepatic, renal, and bone marrow function. All patients had intermediate or high risk of recurrence, defined as primary GIST at any site, 2 cm or larger with 5 or more mitoses per 50 high-power field or nongastric primary GIST measuring 5 cm or larger.7 The study was conducted following the approval of local institutional review boards (eMethods in Supplement 2) and in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki 2008.14 Eligible patients who elected to participate in the study provided written informed consent from after undergoing resection of their primary GIST and their data were deidentified.
Patients were excluded if they had metastatic and/or recurrent GIST, received previous treatment for GIST except for previous adjuvant imatinib therapy lasting 8 weeks or less, received previous treatment with other investigational agents within 28 days of the study treatment, had New York Heart Association class III or IV disease, had severe and/or uncontrolled concurrent disease, had a known diagnosis of HIV infection, or received concurrent warfarin treatment.
Study Design
The PERSIST-5 trial was a phase 2, single-arm, nonrandomized, open-label trial conducted at 21 institutions in the United States. All enrolled patients received imatinib, 400 mg once daily. Dose adjustments were permitted for intolerance of the medication. No dose reductions were performed for grade 1 or 2 hematologic or grade 1 nonhematologic toxic effects.
The first patient was enrolled on August 5, 2009, and the final patient completed 5 years of treatment on May 18, 2016. Patients were followed up every 2 weeks for the first month after registration, every 4 months for the first 3 years, and every 6 months for years 4 and 5. The original plan for a 2-year follow-up was cancelled by the sponsor (Novartis Pharmaceuticals Corporation).
Assessments
Imatinib dosage and compliance, Eastern Cooperative Oncology Group performance status, and the presence of adverse events (AEs) were assessed at each visit. Surveillance imaging with abdominal/pelvic computed tomography with oral and intravenous contrast enhancement or magnetic resonance imaging with intravenous contrast enhancement (for patients with an allergy to computed tomography contrast material) was obtained at baseline and at each protocol visit (±14 days) after the first month of receiving treatment. Each type of radiographic imaging was performed with section thickness of 10 mm or less. For each patient, the same technique and method of assessment were generally used for each surveillance scan, and laboratory data were collected at designated time points throughout the study.
Imatinib pharmacokinetic assessment was initially planned on day 29 and at months 4, 12, 24, 36, and 48; this assessment was discontinued after August 31, 2011, by protocol amendment. Pharmacokinetics were analyzed considering data on day 29 and at months 4 and 12. Plasma imatinib concentration was determined using a validated liquid chromatography–tandem mass spectrometry assay (TDM Pharmaceutical Research, LLC). Quality of life (QoL) was assessed using the Functional Assessment of Cancer Therapy–General15 questionnaire completed by the patient at baseline and at each visit after the first month while enrolled in the study. Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.03.16 Treatment-emergent AEs were defined as all new or worsening events collected from the date of the first dose until 30 days after the last dose of imatinib. Presence of KIT and PDGFRA mutations in primary tumors was determined as previously described.17 Data were collected from August 5, 2009, through December 20, 2016.
Statistical Analysis
The primary end point of this study was RFS, defined as the time from the date of the first dose of imatinib to the date of the first documented disease recurrence (by radiographic imaging) or death (from any cause). Pathologic confirmation of recurrence was not required. Patients who were alive and without recurrence were censored at the last assessment. Secondary end points were OS, safety, and tolerability. Overall survival was defined as the time from the date of the first dose of imatinib to the date of death (from any cause). Exploratory end points included QoL assessment, pharmacokinetics, and the association between mutation status and outcomes. The RFS and OS were calculated using the Kaplan-Meier method. Cox proportional hazards model analysis was performed to assess the associations of appropriate covariates. Pharmacokinetic trough concentrations were summarized by descriptive statistics (mean, SD, and coefficient of variation). The Functional Assessment of Cancer Therapy–General QoL index total score (sum of social, physical, functional, and emotional subscales) was summarized by visit; change from baseline scores was summarized. Statistical significance was based on a 2-sided test with P ≤ .05. Statistical analysis was provided by United BioSource Corporation.
Sample size was based on the assumption that 5-year RFS would be at least 80%. To test the null hypothesis of 65% RFS at 5 years (observed in ACOSOG Z9001)10 against the alternative hypothesis of 80% RFS with a 1-sided significance level of 5% (exponential maximum likelihood estimation test) and 80% power,18 51 evaluable patients (20 events) were required. To allow for a 40% discontinuation rate, 85 patients were required (planned enrollment). Even though the actual discontinuation rate was higher than anticipated, enrolling 91 patients maintained at least 80% power, given the higher 90% RFS against the null hypothesis of 65% RFS.
Results
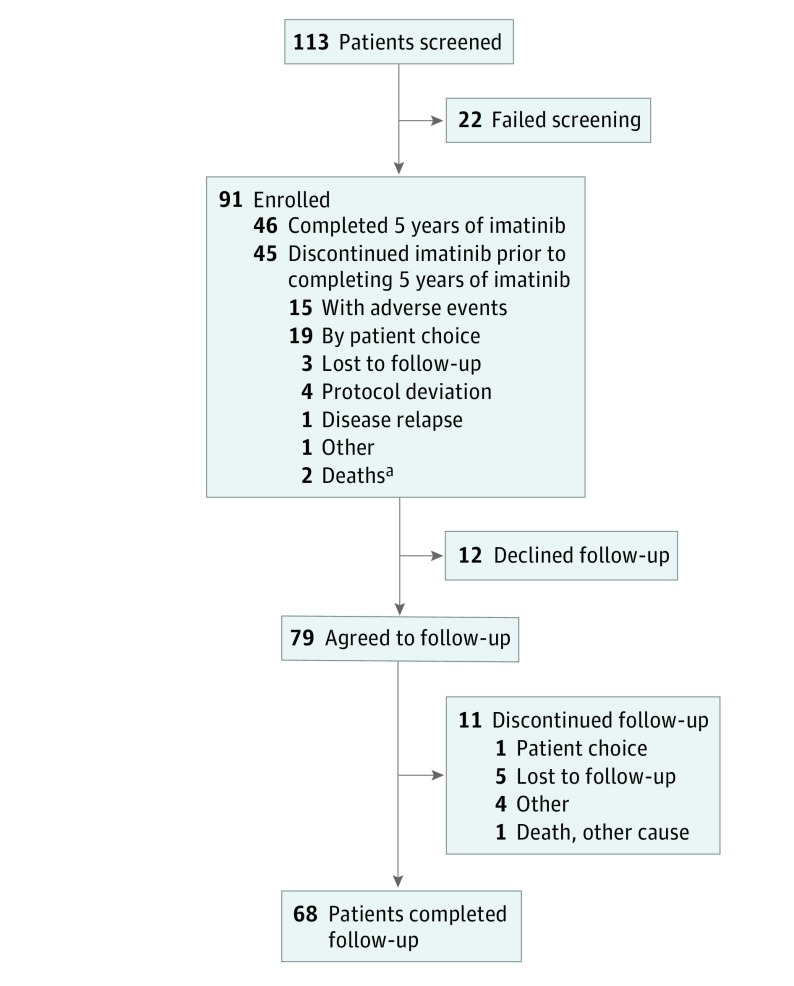
Of 113 patients screened for the study, 91 (48 [53%] male and 43 [47%] female; median age 60 years [range, 30-90 years]) were enrolled (22 failed screening) (Figure 1) and received at least 1 dose of imatinib. Patient demographics, tumor features, and treatment details are summarized in eTable 1 in Supplement 2. Risk of recurrence was intermediate in 24 patients (26%) and high in 67 (74%) based on the Miettinen and Lasota classification.19 Median time from diagnosis to the first dose of imatinib was 10.3 weeks (range, 3.1-23.9 weeks) and from surgery to the first dose of imatinib was 9.6 weeks (range, 3.1-12.3 weeks). Six patients (7%) received previous adjuvant imatinib therapy for a median of 21.5 days (range, 9-29 days).
Figure 1. Trial Flow Diagram.
aOne death occurred while the patient had interrupted imatinib treatment for an unrelated medical issue.
Of 85 patients who agreed to biomarker assessment, KIT or PDGFRA mutations were identified at baseline in 73 patients (86%) (eTable 2 in Supplement 2).
Efficacy
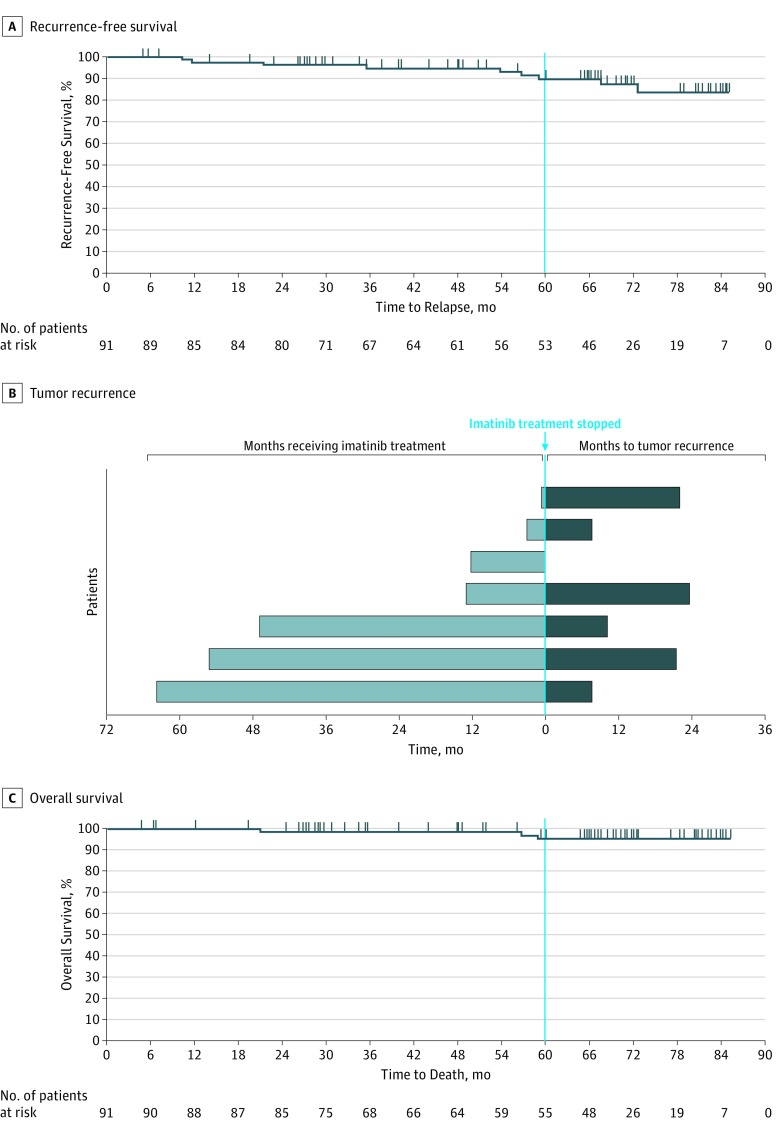
Forty-six patients completed the study treatment, including 19 who continued imatinib therapy after study completion. Of 91 patients who enrolled, 79 (87%) agreed to follow-up, and 68 (75%) completed follow-up after treatment. With median treatment duration of 55.1 months (range, 0.5-60.6 months) and median follow-up after treatment of 19.6 months (range, 4.6-32.1 months), the 5-year RFS was 90% (95% CI, 80%-95%) (Figure 2A). Of the 91 patients, 7 (8%) developed recurrent disease, including 1 patient whose disease recurred while receiving imatinib 11.5 months into therapy and 6 patients whose disease recurred from 7.4 to 23.1 months after discontinuing imatinib therapy (Figure 2B and eTable 3 in Supplement 2). The patient who had recurrent disease while receiving imatinib had a PDGFRA D842V mutation and was the only patient in this study who died of progressive disease. The 6 patients whose disease recurred had discontinued imatinib treatment before recurrence because of patient choice (n = 3), AEs (n = 2), or completion of 5 years of treatment (n = 1). Five of the 6 patients had imatinib-insensitive mutations (PDGFRA exon 18/D842V), KIT exon 9 mutations, or no KIT or PDGFRA mutations at baseline. Age, sex, baseline Eastern Cooperative Oncology Group performance status, and location of primary lesion were not associated with RFS.
Figure 2. Efficacy of Imatinib Therapy.
A, Recurrence-free survival at 5 years (60 months) was 90% (95% CI, 80%-95%). + Indicates censored. B, Disease in 7 patients recurred. One patient’s disease recurred while receiving imatinib mesylate therapy and the patient discontinued imatinib therapy at the time of recurrence. C, Overall survival at 5 years was 95% (95% CI, 86%-99%). + Indicates censored.
The 5-year OS was 95% (95% CI, 86%-99%) (Figure 2C). Of the 3 patients who died, only the patient with a PDGFRA mutation whose disease recurred while receiving imatinib died of progressive disease. The other 2 patients died of causes unrelated to the treatment or the tumor (eTable 3 in Supplement 2); 1 patient was still receiving imatinib therapy at the time of death.
Pharmacokinetics
Pharmacokinetic data were available only for the first 12 months of treatment since few patients agreed to have trough levels of imatinib measured subsequently. For 60 patients with available pharmacokinetic data, mean (SD) imatinib trough levels were 1006.2 (685.0) ng/mL (range, 248-4798 ng/mL) (eFigure in Supplement 2). Levels remained constant during the first 12 months of therapy and were 33% higher in women at 4 months of treatment and overall for the first 12 months. Pharmacokinetic data were too limited to draw any association with drug toxicity.
Quality of Life
Median QoL scores remained stable between baseline (96.4; range, 46.0-108.0) and 60 months (97.5; range, 47.0-108.0) for patients who completed surveys and never decreased more than 3 points from baseline. Thus, QoL remained stable at 60 months throughout treatment for 40 patients who continued follow-up and completed surveys. Surveys were not completed by patients after early discontinuation of treatment, and no associations between QoL and early discontinuation could be made.
Early Treatment Discontinuation and Safety
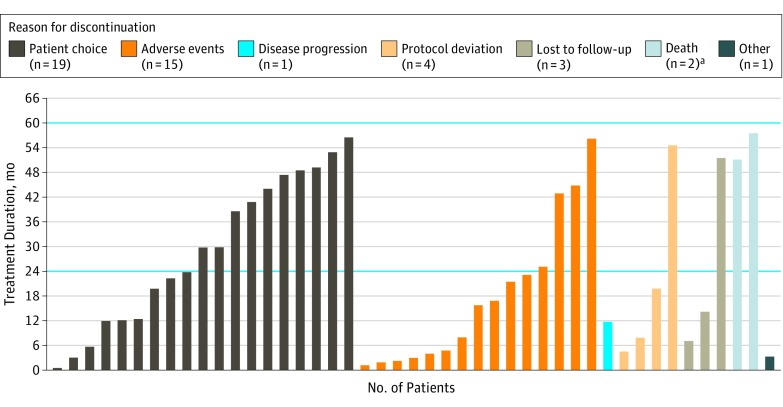
Imatinib therapy was discontinued before completion of 5 years in 45 of 91 patients (49%) (Figure 3), of whom 27 (60%) were women. Reasons for early discontinuation were patient choice (19 [21%]), AEs (15 [17%]), protocol deviation (4 [4%]), loss to follow-up (3 [3%]), death (2 [2%]), disease recurrence (1 [1%]), and other (1 [1%]). Most treatment discontinuations occurred during the first 2 years of treatment. However, discontinuation of treatment because of patient choice occurred at a relatively steady rate throughout the duration of the treatment. Dose reductions were required in 36 of 91 patients (40%) for AEs (26 [29%]), laboratory abnormalities (9 [10%]), scheduling or administrative issues (7 [8%]), protocol-mandated (2 [2%]), and dosing error (1[1%]).
Figure 3. Timing and Reasons for Early Discontinuation of Imatinib Therapy.
aBoth deaths were unrelated to tumor recurrence or imatinib mesylate treatment.
All patients experienced at least 1 AE. The most common AEs (any grade) associated with study treatment were nausea (56 of 91 [62%]), diarrhea (45 [50%]), fatigue (34 [37%]), periorbital edema (30 [33%]), and muscle spasms (29 [32%]) (eTable 4 in Supplement 2). Grade 3 or 4 AEs associated with study treatment were identified in 17 of 91 patients (19%). The most common AEs (any grade) regardless of whether there was an association with the study drug were nausea (65 of 91 [71%]), diarrhea (57 [63%]), fatigue (45 [50%]), muscle spasms (37 [41%]), vomiting (35 [39%]), and periorbital edema (31 [34%]) (eTable 5 in Supplement 2). Grade 3 or 4 AEs regardless of whether associated with the study drug were identified in 45 of 91 patients (50%).
Discussion
This is the first study, to our knowledge, to report results from 5 years of adjuvant imatinib therapy. Long-term imatinib treatment was effective in preventing recurrence of GIST during the course of treatment for patients with mutations sensitive to this drug. The 5-year estimated RFS was 90%, and the OS rate was 95%. Of the 7 patients who developed recurrent GIST, disease recurred in 6 patients after discontinuation of imatinib treatment. Furthermore, only 1 of 57 patients with an imatinib-sensitive, KIT exon 11 mutation had a recurrence, and this only occurred after discontinuation of imatinib treatment. Survival rates were comparable with those from previous adjuvant trials (eTable 6 in Supplement 2). The EORTC 62024 trial,12 which included intermediate- and high-risk patients, reported 5-year RFS of 69% in the 2-year arm and 63% in the placebo arm. The SSG XVIII/AIO trial,13 which included only high-risk patients, reported 5-year RFS of 66% in the 3-year arm and 48% in the 1-year arm. In the EORTC 62024 study,12 5-year OS was 100% among the intermediate-risk patients and 99% among the high-risk patients,12 and in the SSG XVIII/AIO study,13 the 5-year OS was 92% among the immediate-risk patients and 82% among the high-risk patients.
A second important finding from this study was that 49% of patients discontinued treatment early despite the availability of the SSG XVIII/AIO study13 reported in 2012 while PERSIST-5 (current study) was under way. The discontinuation rate was higher than in the 4 previously reported multi-institutional adjuvant trials (ACOSOG Z9000 1-year trial [17% discontinuation rate], ACOSOG Z9001 1-year trial [27%], EORTC 62024 2-year trial [25%], and SSG XVIII/AIO 3-year trial [26%] and 1-year trial [13%]).10,12,13,20 Much of this difference can be attributed to a large percentage of patients (21%) who discontinued therapy because of patient choice (compared with 0%-10% in the other trials).10,12,13,20 The percentages of patients who discontinued imatinib treatment before the completion of the trial for other reasons (ie, AEs or disease progression) were similar to those in the previous trials. To minimize follow-up burden on patients, the recommended interval for surveillance imaging was changed for years 4 and 5 from every 4 months to every 6 months. This was consistent with the surveillance practice at many institutions.
Data reflecting QoL were stable for those who completed surveys, but we did not capture data as the study progressed for patients who stopped treatment early. The data should be interpreted with caution because of a potential for bias. None of the other adjuvant imatinib trials reported QoL data. Therefore, it was difficult to know what could have been done to reduce this dropout rate. However, the high discontinuation rate suggests that critical elements of adjuvant therapy for this disease were effective symptom management and strong commitment from clinicians to retain patients on therapy. Although this is a relatively well-tolerated drug with proven survival efficacy in the adjuvant setting, retaining patients on therapy after a curative operation is challenging. Women had higher imatinib trough levels, suggesting that lower doses could be considered. Lower doses could reduce discontinuation rates because 60% of patients who discontinued therapy early were women. However, we could not identify any association between early discontinuation of imatinib therapy and imatinib trough levels because of limited available pharmacokinetic data.
Because this was a single-arm study, we could not determine whether 5 years of adjuvant imatinib therapy provided any survival benefit compared with 3 years of adjuvant therapy. Nevertheless, the RFS rates are comparable with those in previous studies, confirming that imatinib is effective in reducing the risk of recurrence for patients with sensitive mutations (as opposed to the insensitive PDGFRA exon 18 D842V) while still receiving imatinib therapy. Long-term follow-up results (median, 7.5 years) of the 3-year adjuvant SSG XVIII/AIO study confirmed that the initial benefit of RFS and OS was sustained.21 Long-term follow-up in the present study will determine whether recurrence rates after discontinuation of imatinib therapy are similar to those from other studies. Furthermore, a randomized trial of 3-year treatment vs 5-year treatment with imatinib (SSG XXII, NCT 02413736) is under way. The ACOSOG Z9000 trial demonstrated that recurrences were detected 4 years after completion of adjuvant therapy; thus, ongoing radiographic surveillance is recommended.20 Because many recurrences were observed within 2 years of treatment discontinuation, surveillance after discontinuation may need to be more frequent (for instance, every 3 months during the first 2 years after stopping imatinib therapy) even if surveillance intervals were longer toward the end of the treatment period.
In patients with metastatic GIST treated with tyrosine kinase inhibitor therapy, secondary resistance may develop because of a proliferation of tumor cells harboring preexisting tyrosine kinase inhibitor–resistant mutations. With the only recurrence of GIST on adjuvant therapy occurring in a patient with an insensitive mutation (PDGFRA exon 18 D842V), we cannot comment on whether such a long duration of therapy results in acquired resistance in the adjuvant setting. Because only 3 targeted therapy agents are currently approved for advanced disease (imatinib, sunitinib malate, and regorafenib), it seems reasonable to consider at least delaying, if not preventing recurrence, with the use of adjuvant therapy in patients with primary localized GIST who are at the greatest risk of recurrence.
Overall, imatinib was well tolerated, and the AEs reported were consistent with the overall imatinib safety profile. All patients in this trial and nearly all of the patients in the ACOSOG Z9001 (95%) and SSG XVIII/AIO (99%) trials experienced at least 1 AE, although most AEs were not severe (grades 3-4).10,11
Limitations
A weakness in our study is that 26% of patients had intermediate-risk GIST, a subset of patients for whom the benefit of adjuvant therapy is debated. The SSG XVIII/AIO study excluded intermediate-risk patients.13 European Society of Medical Oncology guidelines currently recommend adjuvant imatinib therapy for patients with a significant risk of relapse, with “room for shared decision-making when the risk is intermediate.”22(piii22) The US National Comprehensive Cancer Network guidelines for GIST recommend adjuvant imatinib therapy for patients with significant risk of recurrence, defined as intermediate risk or high risk.23 The National Comprehensive Cancer Network does not parse which intermediate-risk patients should be considered, but it does reference the SSG XVIII/AIO and ACOSOG Z9001 trials. Furthermore, when we designed this study, we did not exclude patients with mutations now appreciated as imatinib insensitive, such as PDGFR D842V-, RAF-, PI3K-, NF1-mutant, or succinate dehydrogenase–deficient GIST, because data on the association between mutation type and efficacy from the previous trials were not available until 2014 (SSG XVIII/AIO) and 2017 (ACOSOG Z9001).22,24,25 If this trial was designed today, we would exclude patients with PDGFRA exon 18 D842V mutations; this mutation was seen in the single patient in our study whose disease recurred while receiving imatinib and who died of progressive disease. Finally, we did not alter the 400-mg/d dosage for patients with KIT exon 9 mutations who, in the setting of metastatic disease, benefit from an 800-mg/d dosage. When this trial was designed, the MetaGIST data26 demonstrating the benefit of the higher imatinib dose were not published, and therefore, the dosage was uniform across patients with all mutations.24 It remains unclear whether patients with KIT exon 9 or PDGFRA mutations would benefit from higher adjuvant imatinib doses or from adjuvant therapy at all. We cannot determine whether patients with GIST mutations insensitive to the standard dose of imatinib had discontinued the therapy early because of presumed lack of efficacy.
Conclusion
In our trial, 5 years of adjuvant imatinib therapy was safe and effective at controlling recurrence rates in patients with imatinib-sensitive mutations while receiving therapy following resection of primary GIST. Because of the high early discontinuation rate with prolonged therapy, clinician support of patients receiving imatinib may improve compliance with their adjuvant regimen. A randomized trial of 3-year therapy vs 5-year therapy of imatinib that is currently under way will help determine whether a longer duration of therapy is superior.
Clinical Trial Protocol.
eMethods: List of Independent Ethics Committees or Institutional Review Boards by Study Center
eFigure. Pharmacokinetic Analysis.
eTable 1: Baseline Characteristics
eTable 2: Mutations
eTable 3: Events
eTable 4: The Most Common Adverse Events Suspected to be Related to Study Drug Reported in 10% or More of the Patients
eTable 5: The Most Common Adverse Events Regardless of Relationship to Study Drug Reported in 10% or More of the Patients
eTable 6: Adjuvant Imatinib Trials
References
- 1.Pisters PW, Blanke CD, von Mehren M, et al. ; reGISTry Steering Committee . A USA registry of gastrointestinal stromal tumor patients: changes in practice over time and differences between community and academic practices. Ann Oncol. 2011;22(11):-. doi: 10.1093/annonc/mdq773 [DOI] [PubMed] [Google Scholar]
- 2.Rubin JL, Sanon M, Taylor DC, Coombs J, Bollu V, Sirulnik L. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int J Gen Med. 2011;4:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369(9574):1731-1741. doi: 10.1016/S0140-6736(07)60780-6 [DOI] [PubMed] [Google Scholar]
- 4.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51-58. doi: 10.1097/00000658-200001000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459-465. doi: 10.1053/hupa.2002.123545 [DOI] [PubMed] [Google Scholar]
- 6.Gold JS, Gönen M, Gutiérrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10(11):1045-1052. doi: 10.1016/S1470-2045(09)70242-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70-83. doi: 10.1053/j.semdp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411-1419. doi: 10.1016/j.humpath.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265-274. doi: 10.1016/S1470-2045(11)70299-6 [DOI] [PubMed] [Google Scholar]
- 10.Dematteo RP, Ballman KV, Antonescu CR, et al. ; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team . Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104. doi: 10.1016/S0140-6736(09)60500-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272. doi: 10.1001/jama.2012.347 [DOI] [PubMed] [Google Scholar]
- 12.Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma group intergroup randomized trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33(36):4276-4283. doi: 10.1200/JCO.2015.62.4304 [DOI] [PubMed] [Google Scholar]
- 13.Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J Clin Oncol. 2016;34(3):244-250. doi: 10.1200/JCO.2015.62.9170 [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.Functional Assessment of Cancer Therapy–General http://www.facit.org/FACITOrg/Questionnaires. Accessed September 7, 2018.
- 16.Common Terminology Criteria for Adverse Events https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.html. Accessed September 7, 2018.
- 17.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342-4349. doi: 10.1200/JCO.2003.04.190 [DOI] [PubMed] [Google Scholar]
- 18.Lawless JF. Statistical Models and Methods for Lifetime Data. 2nd ed Hoboken, New Jersey: John Wiley & Sons; 2003. [Google Scholar]
- 19.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-1478. [DOI] [PubMed] [Google Scholar]
- 20.DeMatteo RP, Ballman KV, Antonescu CR, et al. ; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team for the Alliance for Clinical Trials in Oncology . Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258(3):422-429. doi: 10.1097/SLA.0b013e3182a15eb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3(11):655-664. doi: 10.1016/S1470-2045(02)00899-9 [DOI] [PubMed] [Google Scholar]
- 22.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii21-iii26. doi: 10.1093/annonc/mdu255 [DOI] [PubMed] [Google Scholar]
- 23.von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(6):758-786. doi: 10.6004/jnccn.2016.0078 [DOI] [PubMed] [Google Scholar]
- 24.Corless CL, Ballman KV, Antonescu C, et al. ; American College of Surgeons Oncology Group . Relation of tumor pathologic and molecular features to outcome after surgical resection of localized primary gastrointestinal stromal tumor (GIST): results of the intergroup phase III trial ACOSOG Z9001. J Clin Oncol. 2010;28(15)(suppl):10006. doi: 10.1200/jco.2010.28.15_suppl.10006 [DOI] [Google Scholar]
- 25.Joensuu H, Eriksson M, Hall KS, et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer. 2014;120(15):2325-2333. doi: 10.1002/cncr.28669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28(7):1247-1253. doi: 10.1200/JCO.2009.24.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Trial Protocol.
eMethods: List of Independent Ethics Committees or Institutional Review Boards by Study Center
eFigure. Pharmacokinetic Analysis.
eTable 1: Baseline Characteristics
eTable 2: Mutations
eTable 3: Events
eTable 4: The Most Common Adverse Events Suspected to be Related to Study Drug Reported in 10% or More of the Patients
eTable 5: The Most Common Adverse Events Regardless of Relationship to Study Drug Reported in 10% or More of the Patients
eTable 6: Adjuvant Imatinib Trials