Abstract
Nitrous oxide (N2O) is a powerful greenhouse gas; however, limited information is currently available on the microbiomes involved in its sink and source in seagrass meadow sediments. Using laboratory incubations, a quantitative PCR (qPCR) analysis of N2O reductase (nosZ) and ammonia monooxygenase subunit A (amoA) genes, and a metagenome analysis based on the nosZ gene, we investigated the abundance of N2O-reducing microorganisms and ammonia-oxidizing prokaryotes as well as the community compositions of N2O-reducing microorganisms in in situ and cultivated sediments in the non-eelgrass and eelgrass zones of Lake Akkeshi, Japan. Laboratory incubations showed that N2O was reduced by eelgrass sediments and emitted by non-eelgrass sediments. qPCR analyses revealed that the abundance of nosZ gene clade II in both sediments before and after the incubation as higher in the eelgrass zone than in the non-eelgrass zone. In contrast, the abundance of ammonia-oxidizing archaeal amoA genes increased after incubations in the non-eelgrass zone only. Metagenome analyses of nosZ genes revealed that the lineages Dechloromonas-Magnetospirillum-Thiocapsa and Bacteroidetes (Flavobacteriia) within nosZ gene clade II were the main populations in the N2O-reducing microbiome in the in situ sediments of eelgrass zones. Sulfur-oxidizing Gammaproteobacteria within nosZ gene clade II dominated in the lineage Dechloromonas-Magnetospirillum-Thiocapsa. Alphaproteobacteria within nosZ gene clade I were predominant in both zones. The proportions of Epsilonproteobacteria within nosZ gene clade II increased after incubations in the eelgrass zone microcosm supplemented with N2O only. Collectively, these results suggest that the N2O-reducing microbiome in eelgrass meadows is largely responsible for coastal N2O mitigation.
Keywords: nitrous oxide-reducing microbiome, nosZ, amoA, eelgrass sediments, sulfur-oxidizing Gammaproteobacteria, Flavobacteriia
Nitrous oxide (N2O) is a powerful, long-lived greenhouse gas (45) and causes the depletion of the stratospheric ozone layer (54). In natural ecosystems, N2O is predominantly produced through the microbial processes of denitrification, nitrification, and nitrifier-denitrification (27, 72, 83). Among these mechanisms, coastal and estuarial N2O sources are assumed to be mainly due to sediment denitrification (46, 47). N2O emissions from an open ocean have recently been attributed to nitrification by ammonia-oxidizing archaea (AOA) (40, 61). However, limited information is currently available on the relationship between N2O emissions and nitrifiers in seagrass sediments. Moreover, the consumption of N2O was previously reported in sediments of eelgrass (Zostera marina) meadows (47). Considerable sediment N2O uptake has recently been reported in pristine shallow coastal ecosystems (20, 41), and rapid N2O reduction has been discovered in the suboxic ocean (4).
Denitrification is the sequential reaction of nitrate to the gaseous products N2O and/or nitrogen gas (N2) via nitrite (NO2−) and nitric oxide (NO) (NO3−→NO2−→NO→N2O→N2). Z-type N2O reductase (NosZ) is a key enzyme that catalyzes the reduction of N2O to N2 during denitrification under sufficiently low molecular oxygen conditions (11, 34). Therefore, the step of N2O reduction by the enzyme NosZ is a major process that influences N2O flux to the atmosphere (72, 84). Many prokaryotes, including more than 60 genera of bacteria, have the ability to denitrify heterotrophically (65). Autotrophic denitrifiers are also able to utilize nitrate (NO3−) and/or NO2− as the electron acceptor and reduce N2O to N2 using the enzyme NosZ (64). Some autotrophic denitrifying sulfur oxidizers have the nosZ gene on the whole genome, such as Thiobacillus denitrificans and Sulfuritalea hydrogenivorans within Betaproteobacteria (5, 33), Sedimenticola thiotaurini within Gammaproteobacteria (19), and Sulfurimonas denitrificans within Epsilonproteobacteria (67). Chemolithoautotrophic denitrifiers within Gammaproteobacteria and Epsilonproteobacteria play an important role in the nitrogen cycle in the oxygen minimum zone in the ocean (37). nosZ genes have recently been classified into two phylogenetically distinct NosZ clades: the first encodes typical NosZ proteins, now designated as nosZ gene clade I, while the other encodes atypical NosZ proteins, now designated as nosZ gene clade II, including the denitrifying members of Gammaproteobacteria, Epsilonproteobacteria, and the phylum Bacteroidetes as well as the non-denitrifying microorganisms of genera such as Anaeromyxobacter, Dyadobacter, and Ignavibacterium (29, 60). PCR and metagenomic analyses based on the nosZ gene revealed that nosZ gene clade II is more abundant and widespread than nosZ gene clade I in several environments such as soil (29, 30, 43, 51), wastewater treatment plants (29), marine water (70), and marine sediments (3, 56, 79). Furthermore, previous studies demonstrated that non-denitrifying N2O-reducing bacteria within nosZ gene clade II played an important role in the consumption of N2O within soil (14, 52, 81). However, limited information is currently available on the distribution and community structure of microbiomes with the capacity to reduce N2O in eelgrass sediments.
Seagrass meadows provide the stabilization of sediment, a habitat for ecologically diverse and productive ecosystems that reduce the exposure of humans, fishes, and invertebrates to bacterial pathogens in coastal environments (16, 38). Sediments inhabited by seagrasses are generally anoxic due to sulfide produced by sulfate-reducing bacteria that utilize sulfate as an electron acceptor for the mineralization of organic matter accumulated by seagrasses (9). Molecular ecological studies based on 16S rDNA previously revealed the predominance of sulfur-oxidizing bacteria (SOB) within Gammaproteobacteria and/or Epsilonproteobacteria together with sulfate-reducing bacteria in seagrass sediments (10, 12, 17, 73). Some SOB within Gammaproteobacteria and/or Epsilonproteobacteria possess the nosZ gene (22, 28). Since high denitrification rates have been reported within the surface of seagrass sediments (8), we hypothesized that SOB possessing the nosZ gene may be responsible for the N2O sink in seagrass sediments alongside their role in sulfide detoxification for seagrasses growing in sulfidic sediments (24, 75).
To test this hypothesis, we characterized the microbiomes responsible for N2O reduction in eelgrass meadow sediments using the high-throughput sequencing of the nosZ gene and quantitative PCR (qPCR) analyses of nosZ and ammonia monooxygenase subunit A (amoA) genes. Since PCR primers for nosZ gene clade II have limitations for investigating the diversities of the genera Anaeromyxobacter and Sulfurimonas and the phylum Bacteroidetes due to PCR bias (29), we examined the community structures of the N2O-reducing microbiome using shotgun metagenomic analyses based on nosZ gene clades I and II. The community structures of microbiomes in sediments were compared between non-eelgrass and eelgrass zones. Laboratory incubations of sediment microcosms were also conducted for sediments from both zones to elucidate the relationship between the community structures of the N2O-reducing microbiome and N2O sink. Since few studies have investigated the influence of nitrogen fertilizers on the coastal areas, such as the sharp increase in (NH4)2SO4 due to increased precipitation, we examined the influence of ammonium on nitrification and N2O production in non-eelgrass and eelgrass zone sediments.
Materials and Methods
Study area and sampling
The study area was Lake Akkeshi, a brackish lake located in Hokkaido, Japan. Most of the lake (31.8 km2) is covered with the eelgrass Zostera marina (80). Sediment core samples were obtained at two different positions, a non-eelgrass zone (43°03′54″N, 144°51′36″E) (n=1) (Fig. S1A and C) and an eelgrass zone (43°03′18″N, 144°53′24″E) (n=1) (Fig. S1B and D), by a diver using a plastic corer (8 cm in diameter and 50 cm in length) on 28 July, 2015. A sample of the surface water was also obtained in a 1-L sterilized plastic bottle at the two different zones described above. The temperature, pH, dissolved oxygen (DO), concentrations of NO3− and sulfate (SO42−) in surface water, and water depth in the non-eelgrass zone were 18.2°C, pH 7.6, DO 6.2 mg L−1, 4.1 μM and 17.3 mM in surface water, and 0.5 m, respectively. The eelgrass zone had a temperature of 19.6°C, pH of 7.5, DO of 6.5 mg L−1, 3.5 μM NO3−, 19.3 mM SO42−, and water depth of 1.0 m. A 0.0–4.0-cm layer of sediment (Fig. S1E) was sliced from the core using a stainless steel corer (7.0 cm in diameter and 4.0 cm in length). While the color of the sediment obtained from the non-eelgrass zone was dark brown, that from the eelgrass zone was black and sulfidic (Fig. S1F and G). The sediments collected were placed into sterilized 50-mL plastic tubes. The concentrations of NO3− and SO42− in the pore water of sediments were 8.1 μM and 20.2 mM in the non-eelgrass zone and 5.2 μM and 20.9 mM in the eelgrass zone, respectively. Samples were transferred to the laboratory in an ice-cooled box within 3 d. Sediments were centrifuged in the sterile 50-mL plastic tubes (5,800×g, 4°C, 10 min) and then mixed well after the supernatant had been discarded. Sediments for the incubation test were kept on ice until incubation experiments. Sediments for DNA extraction were stored at −80°C. One liter of seawater from the two different zones was filtered using Nalgene Rapid-Flow Filters (pore size, 0.2 μm; Thermo Fisher Scientific, Waltham, MA, USA).
Cultivation of sediment microcosms and N2O analysis
Sediment incubation experiments (three biological replicates) were performed for the non-eelgrass zone and eelgrass zone. Fifty-milliliter serum bottles (GL Sciences, Tokyo, Japan) containing 5 g of sediments and 15 mL of filter-sterilized seawater (Fig. S1F and G) were closed with black butyl rubber stoppers (Nichiden-Rika Glass, Kobe, Japan), capped with an aluminum crimp seal (GL Sciences), and then incubated at 20°C for 7 d in the dark. The bottles were shaken by hand for a few second once a day during the 7-d incubation (except on day 6 of the incubation). Three treatments were prepared for two different zones: one as a control (not amended) designated with the sample names N1 for the non-eelgrass zone and E1 for the eelgrass zone, one treated with 3.2 mM NH4Cl seawater (N2 for the non-eelgrass zone and E2 for the eelgrass zone) to enhance the activity of ammonia oxidizers, and one spiked with 0.3 mL of 99.5% N2O (N3 for the non-eelgrass zone and E3 for the eelgrass zone) in order to confirm differences in net N2O absorption between the non-eelgrass and eelgrass zones. Each treatment was conducted in triplicate, giving a total of nine bottles in each zone. The gas in the headspace of the bottle after day 7 of the incubation was withdrawn via the closed butyl rubber stopper using a Pressure-Lok precision analytical syringe (VICI Precision Sampling, Baton Rouge, LA, USA) for the N2O analysis. N2O concentrations were measured with a gas chromatograph equipped with an electron capture detector (GC-14B; Shimadzu, Kyoto, Japan). Sediment and seawater in serum bottles after day 8 of the incubation were centrifuged in the sterile 50-mL plastic tubes (5,800×g, 4°C, 10 min) and then stored at −80°C after the supernatant had been discarded.
Nucleic acid extraction
Each wet sediment (~0.5 g) before (in situ) and after the incubation was added to a plastic tube containing lysis solutions and beads in ISOIL for the Beads Beating Kit (Nippon Gene, Toyama, Japan), mixed vigorously for 45 s, and then incubated at 65°C for 1 h. Nucleic acids were extracted according to the manufacturer’s protocol, and extracted nucleic acids in 20 μL of Tris-EDTA (TE) buffer were then stored at −20°C. Twenty-four DNA samples were extracted from the incubated sediments (N1, N2, N3, E1, E2, and E3) and in situ sediments were designated with the sample names Ni for the non-eelgrass zone and Ei for the eelgrass zone.
qPCR of nosZ and amoA genes
Regarding the quantification of nosZ and amoA gene copies, each DNA solution extracted from sediments before (in situ) and after the incubation was quantified by real-time PCR in a CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA) using the SYBR Premix Ex Taq (Tli RNase H Plus) kit (TaKaRa Bio, Kusatsu, Japan). Twenty-three of the DNA samples were used for qPCR: duplicate DNA samples from N3 due to the loss of samples, and triplicate DNA samples from the remaining sediment samples. The following detection primer sets were used: nosZ2F and nosZ2R for nosZ gene clade I (26), modified nosZ-II-Fn (5′-CTN GGN CCN YTK CAY AC-3′) and nosZ-II-Rn (5′-GCN GAR CAR AAN TCB GTR C-3′) for nosZ gene clade II (29), GenAOAF and GenAOAR for the AOA amoA gene (44), and amoA-1F and amoA-2R for the beta-proteobacterial ammonia-oxidizing bacteria (AOB) amoA gene (57). Standard curves were generated from a plasmid containing each of the following cloned genes: the nosZ clade I gene fragment of Pseudomonas stutzeri NBRC14165 amplified with the PCR primers nosZ_1F (15) and nosZ2R for nosZ gene clade I (26), the nosZ clade II gene fragment of the environmental clone G3H008 amplified with the PCR primers nosZ-II-Fn and nosZ-II-Rn (Fig. S2), the AOA amoA gene fragment of Nitrosopumilus sp. NM25 amplified with the PCR primers CrenAMO_F and CrenMO_R (23), and the AOB amoA gene fragment of Nitrosomonas stercoris KYUHI-ST amplified with the PCR primers amoA-1F and amoA-2R. Each reaction was performed in a volume of 25 μL containing 2 μL of diluted DNA solution (one fiftieth of template DNA), 0.2 μM of each primer (1.0 μM of each primer only for nosZ gene clade I), and 12.5 μL of SYBR Premix Ex Taq (Tli RNase H Plus). Cycling conditions were as follows: for nosZ clade I (26), an initial denaturation step at 95°C for 3 min, followed by 6 cycles at 95°C for 15 s, 65°C for 30 s, and 72°C for 30 s, and then 40 cycles at 95°C for 15 s, 65°C for 15 s, 72°C for 30 s, and 80°C for 15 s. Fluorescence intensity was measured at 80°C. Regarding nosZ clade II (29), cycling conditions were an initial denaturation step at 95°C for 3 min, followed by 40 cycles at 95°C for 10 s, 60°C for 30 s, 72°C for 30 s, and 80°C for 30 s. Fluorescence intensity was measured at 80°C. Cycling conditions for AOA amoA (44) were an initial denaturation step at 95°C for 3 min, followed by 40 cycles at 95°C for 10 s, 55°C for 30 s, 72°C for 30 s, and 80°C for 1 s. Fluorescence intensity was measured at 80°C. Regarding AOB amoA (2), cycling conditions were an initial denaturation step at 95°C for 3 min, followed by 40 cycles at 95°C for 10 s, 57°C for 30 s, 72°C for 30 s, and 78°C for 1 s. Fluorescence intensity was measured at 78°C. After each run, the amplicon was visualized on an agarose gel to confirm the specific product bands of the expected size. Efficiencies for nosZ clade I, clade II, AOA amoA, and AOB amoA amplification were estimated at 62, 62, 108, and 82%, with R2 of 0.963, 0.999, 0.980, and 0.998, respectively.
Metagenomic library construction and sequencing of nosZ genes
The library for the shotgun metagenomic analysis for nosZ genes was created with the tagmentation-based Nextera DNA Library Prep Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol, and samples were then stored at −20°C. Twenty-one DNA samples were used for tagmentation: a single DNA sample from N1 due to the loss of samples, duplicate DNA samples from N3 due to the loss of samples, and triplicate DNA samples from the remaining sediment samples. The DNA quality of each library was verified by 2200 TapeStation (Agilent Technologies, Santa Clara, CA, USA), and quantified with the QubiT dsDNA HS assay kit and QubiT fluorometer (Life Technologies, Carlsbad, CA, USA). The sequencing of composite DNA samples was performed using the MiSeq V2 reagent kit (2×150 bp) on MiSeq (Illumina).
The initial quality filtering of paired-end reads was performed with MiSeq software version 2.5.0.5 (Illumina) to remove some reads with base calls below the threshold (Q30) and the trim sequences of tag and adapter. All paired-end Illumina reads were imported into CLC Genomic Workbench version 8.5.1 (CLCBio; QIAGEN, Aarhus, Denmark), and some reads that were shorter than 90 nucleotides were discarded from the libraries, resulting in 38,029,139 read numbers (Table S1). Due to the limitation of computer memory, 30% of the N1, N2, and N3 reads were used in subsequent analyses. To detect nosZ reads in the metagenomes derived from each sediment sample, publicly available NosZ amino acid references were downloaded from FunGene (http://fungene.cme.msu.edu) (18) of the Ribosomal Database Project (RDP) and the National Center for Biotechnology Information (NCBI), and then imported into the CLC Genomic Workbench. NosZ-encoding reads were identified by blastx (1) against NosZ amino acid references with an e-value threshold of 10−15. To exclude the non-NosZ amino acid sequences of uncultured bacteria with an e-value of more than 10−15 from the references, the amino acids of uncultured bacteria with an e-value of more than 10−15 in the NosZ amino acid references were aligned with archetype amino acid sequences using the CLUSTAL W program in MEGA version 7 (35). The phylogenetic tree was constructed by the maximum-likelihood method in MEGA7, resulting in 1,986 of nosZ gene reads (Table S1). Similarly, the numbers of nitric oxide reductase subunit B (norB) and amoA gene reads in the metagenomes were investigated as described in the supplementary information. Operational taxonomic units (OTUs) were defined as sequence groups based on the lineages constructed from the phylogenetic tree in order to compare nosZ-based diversity with a rarefaction analysis using Analytic Rarefaction 1.3 (https://strata.uga.edu/software/index.html).
Statistical analysis
A one-way analysis of variance (ANOVA) was used to analyze the significance of differences in N2O concentrations and the abundance of nosZ and amoA genes before and after the incubation. Pearson’s product moment correlation (PPMC) analysis was performed to assess the relationship between the increased abundance of amoA genes and elevated concentrations of N2O in the headspace of bottles after the 7-d incubation. ANOVA and PPMC analyses were performed with SPSS Statistics version 20 (IBM, Armonk, NY, USA).
Nucleotide sequence accession number
All metagenomics reported in the present study were deposited in the DNA Data Bank Japan (DDBJ) Sequence Read Archive (DRA) under accession number DRA006867. The corresponding tables between the sample names and deposit IDs of nosZ gene reads on DRA006867 have been submitted to FigShare (http://dx.doi.org/10.6084/m9.figshare.6726428).
Results
N2O emissions and sink
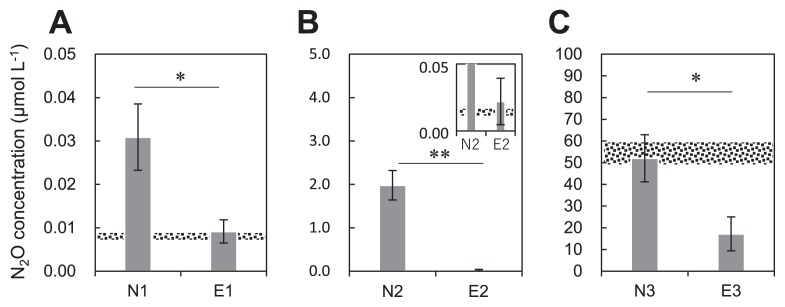
N2O concentrations in the headspace after the incubation were significantly higher in non-eelgrass sediment bottles than in eelgrass sediment bottles (P=0.010) (Fig. 1A), indicating that N2O emissions were higher from non-eelgrass sediments than from eelgrass sediments. Even though ammonium was supplemented to sediments, the N2O concentration in eelgrass sediment bottles (0.022 μmol L−1) was similar to that in eelgrass sediment bottles without ammonium (0.009 μmol L−1) (Fig. 1B). In contrast, the N2O concentration in the headspace of non-eelgrass sediment was 90-fold higher than that in eelgrass sediment, implying low N2O emissions from eelgrass sediments. In addition, when a high concentration of N2O was injected into sediment bottles, N2O concentrations in the headspace after the incubation were significantly lower in eelgrass sediment bottles than in non-eelgrass sediment bottles (P=0.011) (Fig. 1C), suggesting that eelgrass sediments have the capability to absorb more N2O than non-eelgrass sediments.
Fig. 1.
Concentrations of N2O in the headspace of bottles after a 7-d incubation. (A) Sediments incubated without NH4Cl or N2O in the non-eelgrass zone N1 and eelgrass zone E1. (B) Sediments incubated with NH4Cl in the non-eelgrass zone N2 and eelgrass zone E2. (C) Sediments incubated with N2O in the non-eelgrass zone N3 and eelgrass zone E3. Error bars indicate the standard deviation (n=3 biologically independent samples). * shows a significant difference (*, P<0.05; **, P<0.005). The gravel zone (at approximately 0.007 μmol L−1, and approximately 55 μmol L−1) shows the initial concentrations of N2O in the headspace of bottles.
qPCR of nosZ and amoA genes
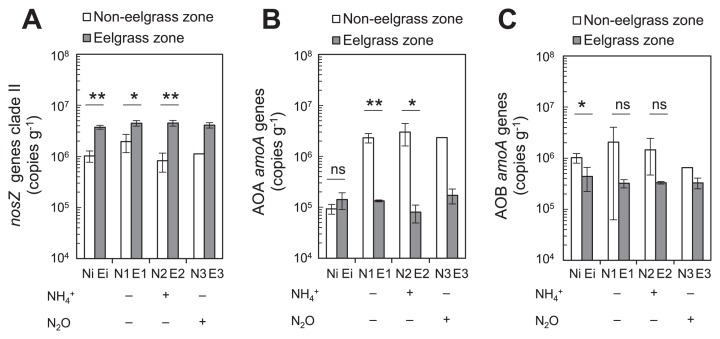
The abundance of nosZ gene clade II in in situ sediments was significantly higher in eelgrass zone sediment Ei than in non-eelgrass zone sediment Ni (P=0.000) (Fig. 2A). Similarly, after the incubation, the abundance of nosZ gene clade II was higher in all types of bottles with eelgrass zone sediments E1, E2, and E3 than in all bottles with non-eelgrass zone sediments N1, N2, and N3. Although the target lengths of PCR products for nosZ gene clade I were detected by qPCR for all samples, the abundance of nosZ gene clade I was not elucidated because the fluorescent intensities of amplicons for the non-targeted region were as strong as those of the target PCR products after qPCR.
Fig. 2.
Abundance of nosZ gene clade II (A), AOA amoA genes (B), and AOB amoA genes (C) in in situ sediments and bottles after day 7 of an incubation of non-eelgrass and eelgrass zones. Error bars indicate the standard deviation (n=3 biologically independent samples). Only N3 is shown (n=2). * indicates a significant difference (*, P<0.05; **, P<0.005; ns, not significant).
The abundance of the AOA amoA genes in incubated bottles with non-eelgrass zone sediments N1, N2, and N3 was approximately 10-fold higher than that in the in situ non-eelgrass zone sediment Ni (Fig. 2B). In contrast, no significant changes were observed in the abundance of AOA amoA genes between before and after the incubation among eelgrass sediment samples. In AOB amoA genes, no significant differences were noted between before and after the incubation for both sediment samples (Fig. 2C). The abundance of AOB amoA genes in in situ sediments from the non-eelgrass zone (Fig. 2C, Ni) was significantly higher than that of AOA amoA genes in in situ sediments from the non-eelgrass zone (Fig. 2B, Ni) (P=0.002). However, there was no significant change in the abundance of amoA genes between AOA (Fig. 2B, Ei) and AOB (Fig. 2C, Ei) in the eelgrass zone (P=0.082).
nosZ gene metagenome
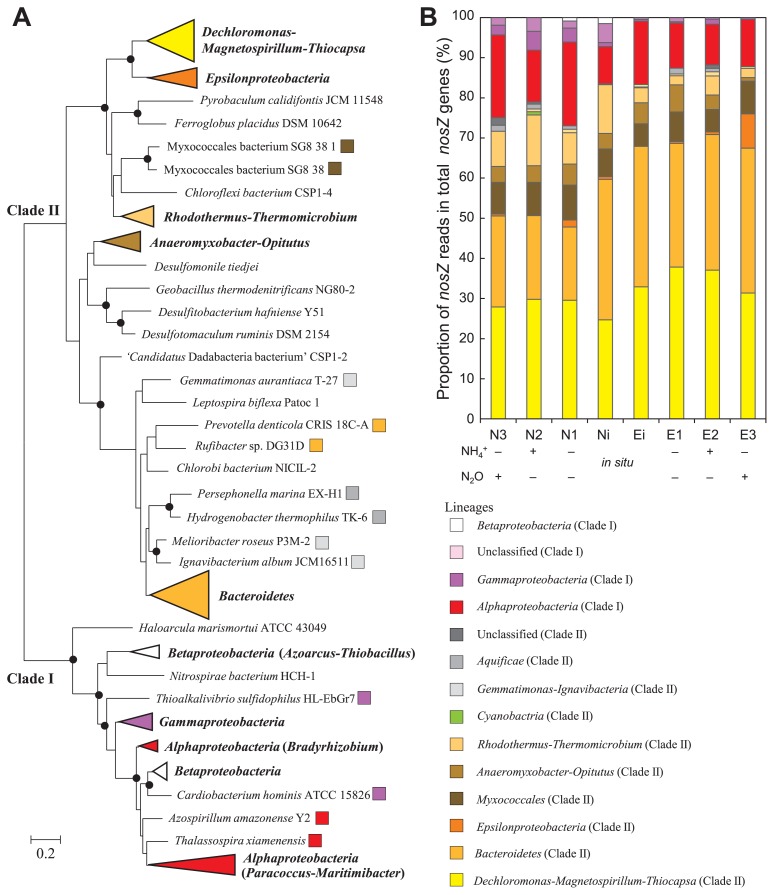
Shotgun metagenomic analyses based on the nosZ gene revealed that nosZ gene reads mainly fell into the lineages Dechloromonas-Magnetospirillum-Thiocapsa, Bacteroidetes, Myxococcales, Anaeromyxobacter-Opitutus, Rhodothermus-Thermomicrobium, Epsilonproteobacteria, and Gemmatimonadetes-Ignavibacteria within nosZ gene clade II, and into the lineages Alphaproteobacteria and Gammaproteobacteria within nosZ gene clade I in the non-eelgrass and/or eelgrass zones (Fig. 3A). nosZ genes within Bacteroidetes and Epsilonproteobacteria were detected as predominant members using shotgun metagenomic sequencing (Fig. 3A and B) even though they were not detected by the cloning analysis (Fig. S2). The community structural proportions of nosZ gene clade II were approximately four-fold higher than those of nosZ gene clade I in in situ sediments for both zones (Fig. 3B). This result was consistent with previous findings on nosZ gene clade II (51, 56, 70). Furthermore, the numbers of nosZ gene reads were slightly higher than those of norB gene reads (Table S1).
Fig. 3.
Bootstrapped maximum likelihood tree (A) and average relative abundance of nosZ genes (B). The tree was built from 138 archetype amino acid sequences. Branches with bootstrap support of more than 70% are revealed by closed circles. The scale bar represents an estimated sequence divergence of 20%. Data represent the mean of biologically independent samples (n=3). Only N1 is shown (n=1), and only N3 is shown (n=2).
In the eelgrass zone, the reads of nosZ gene clade II were occupied by the dominant members of the lineages Dechloromonas-Magnetospirillum-Thiocapsa (approximately 30%) and Bacteroidetes (approximately 30%) in in situ and incubated sediments (Fig. 3B, Ei, E1, E2, and E3). In the lineage Dechloromonas-Magnetospirillum-Thiocapsa, the reads of the sulfur-oxidizing gammaproteobacterial nosZ gene dominated (more than approximately 50%) in in situ and incubated sediments in the eelgrass zone (Table 1, Ei, E1, E2, and E3). The majority of sulfur-oxidizing Gammaproteobacteria were related to sulfur- and sulfide-oxidizing symbionts, such as Thiolapillus brandeum isolated from a hydrothermal vent (48), ‘Candidatus Thiodiazotropha endoloripes’ and ‘Candidatus Thiosymbion oneisti’ in seagrass sediments (53), the marine bivalve mollusk Solemya velesiana gill symbiont (58), and endosymbionts of the deep-sea tubeworms Ridgeia piscesae and Tevnia jerichonana (22). Further predominant nosZ gene reads were related to the giant sulfur-oxidizing bacterium ‘Candidatus Thiomargarita nelsonii’ (77) and anaerobic phototrophic nitrite oxidizer Thiocapsa sp. KS1 (25) in the in situ sediment of the eelgrass zone. In addition, the spiking of N2O into serum bottles (Table 1, E3 and N3) induced an increase in unclassified nosZ reads related to the cytochrome c N2O reductase (cNos) of Epsilonproteobacteria, which dominated in the gill chamber epibiosis of the deep-sea shrimp (28).
Table 1.
Average relative taxonomic distribution of nosZ gene reads within the Dechloromonas-Magnetospirillum-Thiocapsa lineage at the genus level.
| Genus of the lowest E-value (Accession numbers) | Sulfur-oxidizing bacteria | %a | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N3 | N2 | N1 | Ni | Ei | E1 | E2 | E3 | ||
| Thiolapillus brandeum (WP_041065396) | Sb | 9 | 5 | 15 | 22 | 15 | 10 | 6 | 10 |
| Candidatus Thiodiazotropha spp. (WP_069019464, WP_069128191) | S | 9 | 16 | 6 | 8 | 14 | 10 | 12 | 13 |
| Solemya velesiana gill symbiont (OOZ42363) | S | 9 | 12 | 15 | 2 | 10 | 8 | 6 | 4 |
| Candidatus Thiosymbion oneisti (WP_089723768) | S | 9 | 5 | 15 | 14 | 8 | 9 | 15 | 4 |
| Candidatus Thiomargarita spp. (OAD21140, KHD07088) | S | 2 | 8 | 9 | 1 | 7 | 1 | 1 | 0 |
| Gammaproteobacteria bacterium LUC14_002_19_P1 (OQX30387) | S | 9 | 8 | 6 | 4 | 5 | 2 | 0 | 4 |
| Thiocapsa spp. (EGV19470, CRI65417) | S | 0 | 2 | 0 | 5 | 4 | 3 | 4 | 4 |
| Gammaproteobacteria bacterium LUC003_P10 (OQX42463) | S | 9 | 6 | 6 | 1 | 4 | 4 | 6 | 10 |
| endosymbiont of Ridgeia piscesae (WP_060528325, KRT60036) | S | 2 | 3 | 0 | 0 | 3 | 6 | 2 | 0 |
| Sulfuricella denitrificans (WP_009206857) | S | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 0 |
| Thioploca ingrica (BAP57181) | S | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 |
| endosymbiont of Tevnia jerichonana (vent Tica) (EGW55450) | S | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 |
| sulfur-oxidizing symbionts (WP_005958984) | S | 4 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Sulfuritalea sp. (AIC84793) | S | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| marine sediment metagenome (GAG54355) | ndc | 2 | 5 | 0 | 10 | 4 | 10 | 8 | 19 |
| Sedimenticola spp. (WP_029133254, WP_046859760) | nd | 4 | 5 | 15 | 9 | 5 | 7 | 7 | 5 |
| Gammaproteobacteria bacterium RIFOXYD12_FULL_61_37 (OGT89854) | nd | 3 | 6 | 0 | 8 | 5 | 9 | 5 | 1 |
| Magnetospira sp. (CCQ73153) | nd | 0 | 2 | 0 | 2 | 4 | 0 | 0 | 2 |
| Magnetospirillum spp. (CAM74903, CDK98645, BAE51890, KIM00076, OAN53142, OAN47899) | nd | 3 | 1 | 0 | 2 | 2 | 0 | 4 | 0 |
| Gammaproteobacteria bacterium HGW-Gammaproteobacteria-1 (PKM46105) | nd | 0 | 1 | 3 | 1 | 2 | 4 | 0 | 0 |
| Thauera phenylacetica (ENO96869) | nd | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 |
| Candidatus Accumulibacter phosphatis (ACV36679) | nd | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Dechlorosoma aromatica (AAZ46320) | nd | 4 | 2 | 0 | 4 | 0 | 0 | 1 | 1 |
| Maritimibacter sp. (WP_085526419) | nd | 0 | 0 | 3 | 0 | 0 | 0 | 8 | 2 |
| unclassified bacterium | nd | 23 | 11 | 6 | 7 | 6 | 5 | 10 | 19 |
Data (%) represent the mean of biologically independent samples (n=3). Only N1 is shown (n=1), and only N3 is shown (n=2).
S represents the sulfur-oxidizing bacterium.
nd represents not determined.
In both zones, the lineage Bacteroidetes was dominated by nosZ reads related to marine Flavobacteriia (Lutibacter, Seonamhaeicola, Maribacter, Arenibacter, Aquimarina, Cellulophaga, Bizionia, and Muricauda), such as Lutibacter profundi (78), Flavobacteriales bacterium ALC-1 (7), and Flavobacteriaceae bacterium NORP142 (74) (Table 2). In N2O-supplemented sediment E3 in the eelgrass zone, in which the proportion of Epsilonproteobacteria increased (Fig. 3B), epsilonproteobacterial reads were related to S. gotlandica GD1 (36), Sulfurospirillum multivorans DSM 12446 (62), and Arcobacter spp. (13, 71). In the non-eelgrass and eelgrass zones, approximately 90% of alphaproteobacterial reads within nosZ gene clade I fell into the lineage Azospirillum-Thalassospira-Maritimibacter-Paracoccus (Fig. 3A); however, most alphaproteobacterial reads were related to uncultured bacteria. A rarefaction analysis showed that there was no significant difference in diversity among samples (Fig. S3).
Table 2.
Average relative taxonomic distribution of nosZ gene reads within the Bacteroidetes lineage at the genus level.
| Genus of the lowest E-value (Accession numbers) | Classb | %a | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N3 | N2 | N1 | Ni | Ei | E1 | E2 | E3 | ||
| unclassifiedFlavobacteriales (EDP71844) | F | 0 | 5 | 0 | 7 | 11 | 6 | 4 | 19 |
| unclassifiedFlavobacteriaceae (PCI11421) | F | 6 | 13 | 5 | 14 | 10 | 17 | 5 | 7 |
| Lutibacter spp. (WP_090224353, AMC12244, AFX81533, KUO67356) | F | 7 | 8 | 29 | 20 | 10 | 12 | 14 | 14 |
| Seonamhaeicola spp. (WP_076698534) | F | 0 | 0 | 0 | 0 | 6 | 2 | 4 | 0 |
| Maribacter spp. (WP_079511925, EAR02377, APQ16066) | F | 9 | 5 | 0 | 7 | 6 | 8 | 17 | 4 |
| Arenibacter spp. (WP_072764458, GAU57464) | F | 8 | 1 | 5 | 3 | 5 | 3 | 1 | 0 |
| Gaetbulibacter sp. (WP_099565396) | F | 2 | 7 | 5 | 2 | 5 | 1 | 4 | 9 |
| Aquimarina spp. (EZH71920) | F | 11 | 9 | 0 | 11 | 4 | 3 | 0 | 0 |
| Cellulophaga spp. (WP_047414439, WP_084061063) | F | 5 | 1 | 10 | 3 | 4 | 3 | 7 | 2 |
| Bizionia spp. (EGV44024, OBX22568) | F | 4 | 0 | 0 | 1 | 4 | 1 | 2 | 0 |
| Muricauda spp. (WP_045802108, AEM71845) | F | 8 | 3 | 0 | 2 | 4 | 3 | 2 | 0 |
| Vitellibacter spp. (OAD90232, KXO01187) | F | 3 | 0 | 10 | 2 | 3 | 0 | 1 | 2 |
| Xanthomarina sp. (WP_007651736) | F | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 |
| Tenacibaculum soleae (OCK42558) | F | 0 | 12 | 0 | 0 | 2 | 1 | 2 | 5 |
| Zobellia galactanivorans (CAZ96392) | F | 0 | 0 | 5 | 0 | 2 | 6 | 0 | 2 |
| Algibacter alginicilyticus (WP_054727401) | F | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 2 |
| Myroides spp. (EKB05496, KZE78434, EHO10957, GAQ13346) | F | 8 | 0 | 0 | 2 | 1 | 1 | 0 | 2 |
| Aequorivita sublithincola (AFL79671) | F | 2 | 0 | 0 | 0 | 1 | 0 | 4 | 0 |
| Flavobacterium enshiense (ESU23366) | F | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Mangrovimonas yunxiaonensis (KFB01940) | F | 0 | 3 | 0 | 2 | 1 | 5 | 0 | 6 |
| Robiginitalea biformata (EAR16321) | F | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 7 |
| Ulvibacter litoralis (WP_093139708) | F | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 |
| Capnocytophaga spp. (CEN36467, EGD34772, WP_095896667) | F | 5 | 0 | 5 | 1 | 1 | 0 | 0 | 3 |
| Psychroflexus gondwanensis (EMY80080) | F | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 7 |
| Gelidibacter algens (OBX25598) | F | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Gramella forsetii (CAL66385) | F | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 |
| Imtechella halotolerans (EID76831) | F | 3 | 1 | 0 | 1 | 0 | 5 | 0 | 0 |
| Owenweeksia hongkongensis (AEV34412) | F | 2 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Salegentibacter mishustinae (KRG30563) | F | 3 | 1 | 0 | 0 | 0 | 2 | 2 | 0 |
| Zhouia amylolytica (ETN94445) | F | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 |
| Fulvivirga imtechensis (ELR70764) | Cy | 6 | 7 | 0 | 1 | 1 | 1 | 2 | 0 |
| Cesiribacter andamanensis (EMR03384) | Cy | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| Rufibacter spp. (AMM52593, AKQ46153) | Cy | 0 | 0 | 0 | 2 | 0 | 0 | 4 | 4 |
| Runella slithyformis (AEI46569) | Cy | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Haliscomenobacter hydrossis (AEE53312) | Sa | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 |
| Phaeodactylibacter xiamenensis (KGE85855) | Sa | 3 | 2 | 0 | 1 | 2 | 1 | 0 | 0 |
| Pedobacter glucosidilyticus (KHJ39550) | Sh | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Chlorobium spp. (KXB98523, KXK48569) | Ch | 2 | 0 | 5 | 0 | 1 | 0 | 1 | 0 |
| unclassified bacterium | nd | 6 | 12 | 19 | 13 | 6 | 8 | 18 | 0 |
Data (%) represent the mean of biologically independent samples (n=3). Only N1 is shown (n=1), and only N3 is shown (n=2).
F, Flavobacteriia; Ch, Chlorobia; Cy, Cytophagia; Sa, Saprospiria; Sh, Sphingobacteriia.
Discussion
qPCR and shotgun metagenomic analyses
The qPCR analysis based on the nosZ gene revealed that the abundance of N2O-reducing microbes within nosZ gene clade II in in situ sediments of the eelgrass zone was 3.7-fold higher than that in the non-eelgrass zone (Fig. 2A). A previous study reported that PCR primers for nosZ gene clade II are limited by PCR bias (29). Although Bacteroidetes and Epsilonproteobacteria were detected in incubated sediment E3 (sample ID: aA8) with N2O in the eelgrass zone as predominant members using shotgun metagenomic sequencing (Fig. 3B), nosZ genes related to Bacteroidetes and Epsilonproteobacteria were not detected in the same sediment E3 (sample ID: aA8) by a PCR-dependent analysis based on the nosZ gene in the present study (Fig. S2). This result suggests an underestimation of the abundance of nosZ gene clade II within Bacteroidetes (Flavobacteriia) and Epsilonproteobacteria. However, a shotgun metagenomic analysis based on the nosZ gene not only detected sulfide-oxidizing Gammaproteobacteria within the lineage Dechloromonas-Magnetospirillum-Thiocapsa, but also successfully identified N2O-reducing microbes within Flavobacteriia and Epsilonproteobacteria from incubated sediment E3 of the eelgrass zone (Fig. 3). Therefore, a parallel analysis (qPCR and shotgun metagenomic sequencing) demonstrated that sulfide-oxidizing Gammaproteobacteria and marine Flavobacteriia were the dominant N2O-reducing microbes in in situ sediments of the eelgrass zone. However, a new qPCR primer set needs to be designed to accurately evaluate the enumeration of assemblages. In addition to the contribution of N2O-reducing microbes, since the numbers of nosZ reads detected were higher than those of norB reads in both in situ sediments (Table S1), the net N2O emission in in situ sediments also appears to have been suppressed by the lower abundance of microbes possessing nitric oxide reductase, which produces N2O.
Sulfide scavengers for N2O reduction in sulfidic sediments
Laboratory incubation tests (Fig. 1) revealed N2O absorption by eelgrass sediments, which were black and sulfidic due to the vigorous sulfate-reducing activity of SRB in eelgrass zone (Fig. S1E). The continuous supply of hydrogen sulfide (H2S) from the bottom sediment and dissolved dioxygen (O2) from surface water appeared to be responsible for the growth of SOB within surface sediments in the eelgrass zone. The abundance of nosZ gene clade II in in situ sediments in the eelgrass zone was approximately four-fold higher than that in in situ non-eelgrass zone sediments (Fig. 2A). However, previous studies reported that sulfide inhibits the activity of N2O-reducing microbes (42, 50, 68). Furthermore, the reduction of N2O by Anaeromyxobacter dehalogenans was inhibited in laboratory culture medium amended with 0.2 mM sulfide (50). Sulfide concentrations may be reduced by SOB activity in seagrass sediments (24, 75). Therefore, SOB may act as sulfide scavengers, reducing sulfide stress for the NosZ enzyme activity of N2O-reducing microbes. In addition, the formation of FeS and FeS2 appeared to contribute to decreasing sulfide concentrations in eelgrass sediments.
Production of N2O by nitrifiers
The production of N2O is attributed to nitrification and denitrification in marine sediments at low oxygen concentrations (31). In the non-eelgrass zone, laboratory incubation tests indicated net N2O emission from incubated sediments (Fig. 1A and B). The abundance of AOB was higher than that of AOA in in situ sediments of the non-eelgrass zone (Fig. 2B and C). This result supports recent findings indicating that AOB outnumbered AOA in estuary sediments influenced by human activity (39, 76, 82). However, the abundance of AOA markedly increased after the incubation for non-eelgrass sediments only (Fig. 2B). In the non-eelgrass zone, the increased concentration of N2O in the headspace of bottles and elevated copy numbers of AOA amoA genes after the incubation in both samples without ammonium and supplemented with ammonium (Fig. S4) showed positive correlations (r=0.922, P<0.01 for sediments incubated without NH4Cl; r=0.774, P=0.07 for sediments incubated with NH4Cl). AOA may grow under microaerobic conditions coupled with the production of N2O (55). Furthermore, dioxygen was presumed to be quickly depleted in the serum bottles. Therefore, N2O production by AOA and AOB appeared to contribute to N2O emissions in the non-eelgrass zone in addition to N2O production by denitrification. In contrast, net N2O emissions from sediments after the incubation were lower in the eelgrass zone than in the non-eelgrass zone (Fig. 1A and B). Furthermore, no significant increase in AOA or AOB amoA genes occurred after the incubation in eelgrass sediment bottles (Fig. 2B and C). Therefore, the production of N2O by nitrification appeared to be inhibited in incubated bottles from the eelgrass zone.
Heterotrophic and autotrophic N2O reduction
nosZ genes within Flavobacteriia were detected from in situ sediments in the non-eelgrass and eelgrass zones (Fig. 3B). Flavobacteriia are one of the most abundant populations in aquatic environments (32) including seagrass sediments (12, 17, 73). They are proficient at degrading high-molecular-weight organic matter (32). Recent metagenomic analyses using next-generation sequencers demonstrated that some Flavobacteriia possess nosZ genes, suggesting their capacity to reduce N2O (7, 74, 78). Heterotrophic N2O reduction is preferable to gain energy, such as N2O reduction using acetate as the electron donor: 2 N2O+1.5 C2H3O2−→2 N2+HCO3−+1.5 H+ (Δ G°=−600 kJ per reaction) (37). N2O reduction may be one of the preferential reactions to yield energy for Flavobacteriia in the absence of dioxygen as an electron acceptor in sulfidic sediments.
Sulfur-oxidizing N2O-reducing microbes within nosZ gene clade II were one of the highest populations in sulfidic sediments of the eelgrass zone (Fig. 3B and Table 1). Autotrophic denitrification coupled with sulfide oxidation, 2 NO3−+5 HS−+7 H+→N2+5 S°+6 H2O (Δ G°=−1,260 kJ per reaction), is a favorable reaction for chemolithotrophs (37). The sulfur-oxidizing gammaproteobacterium T. brandeum (48) and sulfur-oxidizing epsilonproteobacterium S. gotlandica (36) have the ability to grow autotrophically with nitrate as an electron acceptor. Since nosZ genes related to T. brandeum and S. gotlandica were predominantly detected from sediments in the eelgrass zone in the present study, autotrophic denitrification by SOB also appears to be responsible for the N2O sink in sediments in the eelgrass zone. The highest rate of complete denitrification was reported at 40 m within the peak of H2S in the marine oxygen minimum zone off Peru, suggesting that autotrophic SOB reduced N2O with H2S in the oxygen minimum zone (63).
A previous study reported that the N2O consumption rates of the heterotrophic N2O reducers, P. stutzeri, Shewanella loihica, Dechloromonas aromatica, and A. dehalogenans, were 4.16, 0.446, 0.461, and 0.0171 μmol min−1 mg biomass−1, respectively (81). Similarly, the N2O consumption rate of the autotrophic N2O reducer Thiohalorhabdus denitrificans was 180–300 nmol min−1 mg protein−1 (69). Although no significant differences have been reported in N2O reduction rates between heterotrophic and autotrophic N2O reducers, the substrate affinity of clade II nosZ N2O reducers for N2O is generally higher than that of clade I nosZ N2O reducers (6, 59, 81). The Km value of soil Flavobacteriia sp. for N2O was 0.5 μM (6). Thus, the predominance of clade II nosZ N2O reducers in eelgrass sediments appears to be due to differences in affinity for N2O between clade I and clade II nosZ N2O reducers. Since the surface of eelgrass zone sediments was covered with dead leaves (Fig. S1D and E), the heterotrophic denitrifier Flavobacteriia may play an important role in the N2O sink with organic matter as an electron donor. However, soil Flavobacteriia sp. have been shown to produce N2O as oxygen tension increases (6). The Flavobacteriia nosZ phylotype was detected as the predominant member at the N2O peak within marine oxygen-deficient zones in the Eastern Tropical North Pacific (ETNP) (21). Further studies are needed to clarify whether heterotrophic and autotrophic N2O reducers contribute to the N2O sink in in situ eelgrass sediments.
Conclusions
A shotgun metagenomic analysis based on the nosZ gene coupled with quantitative and physiological experiments suggested an N2O sink due to the N2O-reducing microbiome in sediments of the eelgrass zone. Seagrass meadows are widely distributed along coastal environments worldwide (66). Therefore, N2O-reducing microbiomes in seagrass meadows play an important role in the global nitrogen cycle, and have the potential to mitigate N2O from coastal environments worldwide. Future studies on the vertical distribution of N2O-reducing microbiomes coupled with the vertical dynamics of dissolved N2O, sulfide (HS−), NO3−, O2, ferrous (Fe2+), the oxidation-reduction potential, and stable isotopic composition of NO3− (49) in sediments, and also on sulfur-oxidizing symbionts involved in N2O reduction are needed in order to provide a comprehensive understanding of the role of seagrass sediment microbiomes as an N2O sink. Since rapid precipitation is expected to increase, the effects of NO3− outflow by nitrogen fertilizers on the production of N2O in eelgrass zone sediments warrant further study.
Supplementary Information
Acknowledgements
We are grateful to the crew of the Etopirika for their technical expertise. We wish to thank Daisuke Imaizumi, Asuka Yamada, Ken Jinguji, Rino Tomizawa, Yuki Miyamoto, Midori Morikawa, Kengo Sudo, Sanae Taguchi, Naoya Ishikawa, Takumi Nomura, and Hayato Kokubu for their support with the chemical and microbiological analyses, Kazuhiro Umezawa for his support with the field work, the staff of the General Research Institute for their support with sequencing at Nihon University, and Shujiro Okuda for his discussions and technical advice on bioinformatics. We thank the three anonymous reviewers for their helpful comments. This work was supported in part by grants from the Joint Research Program of the Institute of Low Temperature Science, Hokkaido University to T.N.
References
- 1.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando Y., Nakagawa T., Takahashi R., Yoshihara K., Tokuyama T. Seasonal changes in abundance of ammonia-oxidizing archaea and ammonia-oxidizing bacteria and their nitrification in sand of an eelgrass zone. Microbes Environ. 2009;24:21–27. doi: 10.1264/jsme2.me08536. [DOI] [PubMed] [Google Scholar]
- 3.Arfken A., Song B., Bowman J.S., Piehler M. Denitrification potential of the eastern oyster microbiome using a 16S rRNA gene based metabolic inference approach. PLoS One. 2017;12:e0185071. doi: 10.1371/journal.pone.0185071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babbin A.R., Bianchi D., Jayakumar A., Ward B.B. Rapid nitrous oxide cycling in the suboxic ocean. Science. 2015;348:1127–1129. doi: 10.1126/science.aaa8380. [DOI] [PubMed] [Google Scholar]
- 5.Beller H.R., Letain T.E., Chakicherla A., Kane S.R., Legler T.C., Coleman M.A. Whole-genome transcriptional analysis of chemolithoautotrophic thiosulfate oxidation by Thiobacillus denitrificans under aerobic versus denitrifying conditions. J Bacteriol. 2006;188:7005–7015. doi: 10.1128/JB.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betlach M.R., Tiedje J.M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981;42:1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonilla-Rosso G., Eguiarte L., Souza V. Functional and taxonomic diversity of the nitrogen cycling guild in the Sargasso Sea metagenomes. Theory, Methods and Applications. In: Marco D., editor. Metagenomics of the Microbial Nitrogen Cycle Caister. Academic Press; Norfolk: 2014. pp. 153–173. [Google Scholar]
- 8.Caffrey J.M., Kemp W.M. Nitrogen cycling in sediments with estuarine populations of Potamogeton perfoliatus and Zostera marina. Mar Ecol Prog Ser. 1990;66:147–160. [Google Scholar]
- 9.Calleja M.L., Marbà N., Duarte C.M. The relationship between seagrass (Posidonia oceanica) decline and sulfide porewater concentration in carbonate sediments. Estuarine, Coastal Shelf Sci. 2007;73:583–588. [Google Scholar]
- 10.Cifuentes A., Antón J., Benlloch S., Donnelly A., Herbert R.A., Rodríguez-Valera F. Prokaryotic diversity in Zostera noltii-colonized marine sediments. Appl Environ Microbiol. 2000;66:1715–1719. doi: 10.1128/aem.66.4.1715-1719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyle C.L., Zumft W.G., Kroneck P.M.H., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985;153:459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 12.Cúcio C., Engelen A.H., Costa R., Muyzer G. Rhizosphere microbiomes of european seagrasses are selected by the plant, but are not species specific. Front Microbiol. 2016;7:440. doi: 10.3389/fmicb.2016.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diéguez A.L., Balboa S., Magnesen T., Romalde J.L. Arcobacter lekithochrous sp. nov., isolated from a molluscan hatchery. Int J Syst Evol Microbiol. 2017;67:1327–1332. doi: 10.1099/ijsem.0.001809. [DOI] [PubMed] [Google Scholar]
- 14.Domeignoz-Horta L.A., Putz M., Spor A., Bru D., Breuil M.C., Hallin S., Philippot L. Non-denitrifying nitrous oxide-reducing bacteria–an effective N2O sink in soil. Soil Biol Biochem. 2016;103:376–379. [Google Scholar]
- 15.Duan Y.-F., Kong X.-W., Schramm A., Labouriau R., Eriksen J., Petersen S.O. Microbial N transformations and N2O emission after simulated grassland cultivation: effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) Appl Environ Microbiol. 2017;83:e02019–16. doi: 10.1128/AEM.02019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy J.E., Reynolds P.L., Boström C., et al. Biodiversity mediates top-down control in eelgrass ecosystems: a global comparative-experimental approach. Ecol Lett. 2015;18:696–705. doi: 10.1111/ele.12448. [DOI] [PubMed] [Google Scholar]
- 17.Fahimipour A.K., Kardish M.R., Lang J.M., Green J.L., Eisen J.A., Stachowicz J.J. Global-scale structure of the eelgrass microbiome. Appl Environ Microbiol. 2017;83:e03391–16. doi: 10.1128/AEM.03391-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fish J.A., Chai B., Wang Q., Sun Y., Brown C.T., Tiedje J.M., Cole J.R. FunGene: the functional gene pipeline and repository. Front Microbiol. 2013;4:291. doi: 10.3389/fmicb.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flood B.E., Jones D.S., Bailey J.V. Complete genome sequence of Sedimenticola thiotaurini strain SIPG1, a polyphosphate-and polyhydroxyalkanoate-accumulating sulfur-oxidizing gammaproteobacterium isolated from salt marsh sediments. Genome Announc. 2015;3:e00671–15. doi: 10.1128/genomeA.00671-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster S.Q., Fulweiler R.W. Sediment nitrous oxide fluxes are dominated by uptake in a temperate estuary. Front Mar Sci. 2016;3:40. [Google Scholar]
- 21.Fuchsman C.A., Devol A.H., Saunders J.K., McKay C., Rocap G. Niche partitioning of the N cycling microbial community of an offshore oxygen deficient zone. Front Microbiol. 2017;8:2384. doi: 10.3389/fmicb.2017.02384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardebrecht A., Markert S., Sievert S.M., et al. Physiological homogeneity among the endosymbionts of Riftia pachyptila and Tevnia jerichonana revealed by proteogenomics. ISME J. 2012;6:766–776. doi: 10.1038/ismej.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallam S.J., Mincer T.J., Schleper C., Preston C.M., Roberts K., Richardson P.M., DeLong E.F. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLoS Biol. 2006;4:520–536. doi: 10.1371/journal.pbio.0040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasler-Sheetal H., Holmer M. Sulfide intrusion and detoxification in the seagrass Zostera marina. PLoS One. 2015;10:e0129136. doi: 10.1371/journal.pone.0129136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemp J., Lücker S., Schott J., Pace L.A., Johnson J.E., Schink B., Daims H., Fischer W.W. Genomics of a phototrophic nitrite oxidizer: insights into the evolution of photosynthesis and nitrification. ISME J. 2016;10:2669–2678. doi: 10.1038/ismej.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry S., Bru D., Stres B., Hallet S., Philippot L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol. 2006;72:5181–5189. doi: 10.1128/AEM.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbert R.A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev. 1999;23:563–590. doi: 10.1111/j.1574-6976.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 28.Jan C., Petersen J.M., Werner J., et al. The gill chamber epibiosis of deep-sea shrimp Rimicaris exoculata: an in-depth metagenomic investigation and discovery of Zetaproteobacteria. Environ Microbiol. 2014;16:2723–2738. doi: 10.1111/1462-2920.12406. [DOI] [PubMed] [Google Scholar]
- 29.Jones C.M., Graf D.R., Bru D., Philippot L., Hallin S. The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J. 2013;7:417–426. doi: 10.1038/ismej.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones C.M., Spor A., Brennan F.P., Breuil M.-C., Bru D., Lemanceau P., Griffiths B., Hallin S., Philippot L. Recently identified microbial guild mediates soil N2O sink capacity. Nat Clim Change. 2014;4:801–805. [Google Scholar]
- 31.Jørgensen K.S., Jensen H.B., Sørensen J. Nitrous oxide production from nitrification and denitrification in marine sediment at low oxygen concentrations. Can J Microbiol. 1984;30:1073–1078. [Google Scholar]
- 32.Kirchman D.L. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol Ecol. 2002;39:91–100. doi: 10.1111/j.1574-6941.2002.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 33.Kojima H., Fukui M. Sulfuritalea hydrogenivorans gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake. Int J Syst Evol Microbiol. 2011;61:1651–1655. doi: 10.1099/ijs.0.024968-0. [DOI] [PubMed] [Google Scholar]
- 34.Körner H., Zumft W.G. Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Paeudomonas stutzeri. Appl Environ Microbiol. 1989;55:1670–1676. doi: 10.1128/aem.55.7.1670-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labrenz M., Grote J., Mammitzsch K., Boschker H.T.S., Laue M., Jost G., Glaubitz S., Jürgens K. Sulfurimonas gotlandica sp. nov., a chemoautotrophic and psychrotolerant epsilonproteobacterium isolated from a pelagic redoxcline, and an emended description of the genus Sulfurimonas. Int J Syst Evol Microbiol. 2013;63:4141–4148. doi: 10.1099/ijs.0.048827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam P., Kuypers M.M.M. Microbial nitrogen cycling processes in oxygen minimum zone. Ann Rev Mar Sci. 2011;3:317–345. doi: 10.1146/annurev-marine-120709-142814. [DOI] [PubMed] [Google Scholar]
- 38.Lamb J.B., van de Water J.A.J.M., Bourne D.G., Altier C., Hein M.Y., Fiorenza E.A., Abu N., Jompa J., Harvell C.D. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science. 2017;355:731–733. doi: 10.1126/science.aal1956. [DOI] [PubMed] [Google Scholar]
- 39.Li J., Nedwell D.B., Beddow J., Dumbrell A.J., McKew B.A., Thorpe E.L., Whitby C. amoA Gene abundances and nitrification potential rates suggest that benthic ammonia-oxidizing bacteria and not archaea dominate N cycling in the Colne Estuary, United Kingdom. Appl Environ Microbiol. 2015;81:159–165. doi: 10.1128/AEM.02654-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Löscher C.R., Kock A., Könneke M., LaRoche J., Bange H.W., Schmitz R.A. Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences. 2012;9:2419–2429. [Google Scholar]
- 41.Maher D.T., Sippo J.Z., Tait D.R., Holloway C., Santos I.R. Pristine mangrove creek waters are a sink of nitrous oxide. Sci Rep. 2016;6:25701. doi: 10.1038/srep25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manconi I., van der Maas P., Lens P. Effect of copper dosing on sulfide inhibited reduction of nitric and nitrous oxide. Nitric Oxide. 2006;15:400–407. doi: 10.1016/j.niox.2006.04.262. [DOI] [PubMed] [Google Scholar]
- 43.Masuda Y., Itoh H., Shiratori Y., Isobe K., Otsuka S., Senoo K. Predominant but previously-overlooked prokaryotic drivers of reductive nitrogen transformation in paddy soils, revealed by metatranscriptomics. Microbes Environ. 2017;32:180–183. doi: 10.1264/jsme2.ME16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meinhardt K.A., Bertagnolli A., Pannu M.W., Strand S.E., Brown S.L., Stahl D.A. Evaluation of revised polymerase chain reaction primers for more inclusive quantification of ammonia-oxidizing archaea and bacteria. Environ Microbiol Rep. 2015;7:354–363. doi: 10.1111/1758-2229.12259. [DOI] [PubMed] [Google Scholar]
- 45.Montzka S.A., Dlugokencky E.J., Butler J.H. Non-CO2 greenhouse gases and climate change. Nature. 2011;476:43–50. doi: 10.1038/nature10322. [DOI] [PubMed] [Google Scholar]
- 46.Murray R.H., Erler D.V., Eyre B.D. Nitrous oxide fluxes in estuarine environments: response to global change. Glob Chang Biol. 2015;21:3219–3245. doi: 10.1111/gcb.12923. [DOI] [PubMed] [Google Scholar]
- 47.Nishio T., Koike I., Hattori A. Estimates of denitrification and nitrification in coastal and estuarine sediments. Appl Environ Microbiol. 1983;45:444–450. doi: 10.1128/aem.45.2.444-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunoura T., Takaki Y., Kazama H., Kakuta J., Shimamura S., Makita H., Hirai M., Miyazaki M., Takai K. Physiological and genomic features of a novel sulfur-oxidizing gammaproteobacterium belonging to a previously uncultivated symbiotic lineage isolated from a hydrothermal vent. PLoS One. 2014;9:e104959. doi: 10.1371/journal.pone.0104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunoura T., Nishizawa M., Hirai M., et al. Microbial diversity in sediments from the bottom of the Challenger Deep, the Mariana Trench. Microbes Environ. 2018;33:186–194. doi: 10.1264/jsme2.ME17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onley J.R., Ahsan S., Sanford R.A., Löffler F.E. Denitrification by Anaeromyxobacter dehalogenans, a common soil bacterium lacking the nitrite reductase genes nirS and nirK. Appl Environ Microbiol. 2018;84:e01985–17. doi: 10.1128/AEM.01985-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orellana L.H., Rodriguez-R L.M., Higgins S., Chee-Sanford J.C., Sanford R.A., Ritalahti K.M., Konstantinidis K.T. Detecting nitrous oxide reductase (nosZ) genes in soil metagenomes: method development and implications for the nitrogen cycle. mBio. 2014;5:e01193–14. doi: 10.1128/mBio.01193-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park D., Kim H., Yoon S. Nitrous oxide reduction by an obligate aerobic bacterium, Gemmatimonas aurantiaca strain T-27. Appl Environ Microbiol. 2017;83:e00502–17. doi: 10.1128/AEM.00502-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen J.M., Kemper A., Gruber-Vodicka H., et al. Chemosynthetic symbionts of marine invertebrate animals are capable of nitrogen fixation. Nat Microbiol. 2016;2:16195. doi: 10.1038/nmicrobiol.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portmann R.W., Daniel J.S., Ravishankara A.R. Stratospheric ozone depletion due to nitrous oxide: influences of other gases. Phil Trans R Soc B. 2012;367:1256–1264. doi: 10.1098/rstb.2011.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin W., Meinhardt K.A., Moffett J.W., Devol A.H., Armbrust E.V., Ingalls A.E., Stahl D.A. Influence of oxygen availability on the activities of ammonia-oxidizing archaea. Environ Microbiol Rep. 2017;9:250–256. doi: 10.1111/1758-2229.12525. [DOI] [PubMed] [Google Scholar]
- 56.Rasigraf O., Schmitt J., Jetten M.S.M., Lüke C. Metagenomic potential for and diversity of N–cycle driving microorganisms in the Bothnian Sea sediment. MicrobiologyOpen. 2017;6:e00475. doi: 10.1002/mbo3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rotthauwe J.-H., Witzel K.-P., Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol. 1997;63:4704–4712. doi: 10.1128/aem.63.12.4704-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell S.L., Corbett-Detig R.B., Cavanaugh C.M. Mixed transmission modes and dynamic genome evolution in an obligate animal-bacterial symbiosis. ISME J. 2017;11:1359–1371. doi: 10.1038/ismej.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sameshima-Saito R., Chiba K., Hirayama J., Itakura M., Mitsui H., Eda S., Minamisawa K. Symbiotic Bradyrhizobium japonicum reduces N2O surrounding the soybean root system via nitrous oxide reductase. Appl Environ Microbiol. 2006;72:2526–2532. doi: 10.1128/AEM.72.4.2526-2532.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanford R.A., Wagner D.D., Wu Q., et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc Natl Acad Sci USA. 2012;109:19709–19714. doi: 10.1073/pnas.1211238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santoro A.E., Buchwald C., McIlvin M.R., Casciotti K.L. Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science. 2011;333:1282–1285. doi: 10.1126/science.1208239. [DOI] [PubMed] [Google Scholar]
- 62.Scholz-Muramatsu H., Neumann A., Meßmer M., Moore E., Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 63.Schunck H., Lavik G., Desai D.K., et al. Giant hydrogen sulfide plume in the oxygen minimum zone off Peru supports chemolithoautotrophy. PLoS One. 2013;8:e68661. doi: 10.1371/journal.pone.0068661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao M.-F., Zhang T., Fang H. Sulfur-deriving autotrophic denitrification: diversity, biochemistry and engineering applications. Appl Microbiol Biotechnol. 2010;88:1027–1024. doi: 10.1007/s00253-010-2847-1. [DOI] [PubMed] [Google Scholar]
- 65.Shapleigh J.P. The denitrifying prokaryotes. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes: A Handbook on the Biology of Bacteria. 3rd ed. Vol. 2. Springer; New York: 2006. pp. 769–792. (Ecophysiology and Biochemistry). [Google Scholar]
- 66.Short F., Carruthers T., Dennison W., Waycott M. Global seagrass distribution and diversity: A bioregional model. J Exp Mar Biol Ecol. 2007;350:3–20. [Google Scholar]
- 67.Sievert S.M., Scott K.M., Klotz M.G., et al. Genome of the epsilonproteobacterial chemolithoautotroph Sulfurimonas denitrificans. Appl Environ Microbiol. 2008;74:1145–1156. doi: 10.1128/AEM.01844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sørensen J., Tiedje J.M., Firestone R.B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980;39:105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorokin D.Y., Tourova T.P., Galinski E.A., Muyzer G., Kuenen J.G. Thiohalorhabdus denitrificans gen. nov., sp. nov., an extremely halophilic, sulfur-oxidizing, deep-lineage gammaproteobacterium from hypersaline habitats. Int J Syst Evol Microbiol. 2008;58:2890–2897. doi: 10.1099/ijs.0.2008/000166-0. [DOI] [PubMed] [Google Scholar]
- 70.Sun X., Jayakumar A., Ward B.B. Community composition of nitrous oxide consuming bacteria in the oxygen minimum zone of the eastern tropical south pacific. Front Microbiol. 2017;8:1183. doi: 10.3389/fmicb.2017.01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka R., Cleenwerck I., Mizutani Y., Iehata S., Bossier P., Vandamme P. Arcobacter haliotis sp. nov., isolated from abalone species Haliotis gigantea. Int J Syst Evol Microbiol. 2017;67:3050–3056. doi: 10.1099/ijsem.0.002080. [DOI] [PubMed] [Google Scholar]
- 72.Thomson A.J., Giannopoulos G., Pretty J., Baggs E.M., Richardson D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos Trans R Soc, B. 2012;367:1157–1168. doi: 10.1098/rstb.2011.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trevathan-Tackett S.M., Seymour J.R., Nielsen D.A., et al. Sediment anoxia limits microbial-driven seagrass carbon remineralization under warming conditions. FEMS Microbiol Ecol. 2017;93:fix033. doi: 10.1093/femsec/fix033. [DOI] [PubMed] [Google Scholar]
- 74.Tully B.J., Wheat C.G., Glazer B.T., Huber J.A. A dynamic microbial community with high functional redundancy inhabits the cold, oxic subseafloor aquifer. ISME J. 2018;12:1–16. doi: 10.1038/ismej.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van der Heide T., Govers L.L., de Fouw J., et al. A three-stage symbiosis forms the foundation of seagrass ecosystems. Science. 2012;336:1432–1434. doi: 10.1126/science.1219973. [DOI] [PubMed] [Google Scholar]
- 76.Wankel S.D., Mosier A.C., Hansel C.M., Paytan A., Francis C.A. Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough estuary, California. Appl Environ Microbiol. 2011;77:269–280. doi: 10.1128/AEM.01318-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winkel M., Salmon-Carvalho V., Woyke T., Richter M., Schulz-Vogt H.N., Flood B.E., Bailey J.V., Muβmann M. Single-cell sequencing of Thiomargarita reveals genomic flexibility for adaptation to dynamic redox conditions. Front Microbiol. 2016;7:964. doi: 10.3389/fmicb.2016.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wissuwa J., Bauer S.L.M., Steen I.H., Stokke R. Complete genome sequence of Lutibacter profundi LP1T isolated from an Arctic deep-sea hydrothermal vent system. Stand Genomic Sci. 2017;12:5. doi: 10.1186/s40793-016-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wittorf L., Bonilla-Rosso G., Jones C.M., Bäckman O., Hulth S., Hallin S. Habitat partitioning of marine benthic denitrifier communities in response to oxygen availability. Environ Microbiol Rep. 2016;8:486–492. doi: 10.1111/1758-2229.12393. [DOI] [PubMed] [Google Scholar]
- 80.Yamada K., Takahashi K., Vallet C., Taguchi S., Toda T. Distribution, life history, and production of three species of Neomysis in Akkeshi-ko estuary, northern Japan. Mar Biol (Heidelberg, Ger) 2007;150:905–917. [Google Scholar]
- 81.Yoon S., Nissen S., Park D., Sanford R.A., Löffler F.E. Nitrous oxide reduction kinetics distinguish bacteria harboring clade I nosZ from those harboring clade II nosZ. Appl Environ Microbiol. 2016;82:3793–3800. doi: 10.1128/AEM.00409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Y., Hou L., Newell S., Liu M., Zhou J., Zhao H., You L., Cheng X. Community dynamics and activity of ammonia-oxidizing prokaryotes in intertidal sediments of the Yangtze Estuary. Appl Environ Microbiol. 2014;80:408–419. doi: 10.1128/AEM.03035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu X., Burger M., Doane T.A., Horwath W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci USA. 2013;110:6328–6333. doi: 10.1073/pnas.1219993110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zumft W.G., Kroneck P.M.H. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by Bacteria and Archaea. Adv Microb Physiol. 2007;52:107–227. doi: 10.1016/S0065-2911(06)52003-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.