A standard multiplex PCR offers comprehensive testing for respiratory viruses. However, it has traditionally been performed in a referral laboratory with a lengthy turnaround time, which can reduce patient flow through the hospital.
KEYWORDS: influenza, molecular diagnostic, rapid PCR, respiratory viruses
ABSTRACT
A standard multiplex PCR offers comprehensive testing for respiratory viruses. However, it has traditionally been performed in a referral laboratory with a lengthy turnaround time, which can reduce patient flow through the hospital. We aimed to determine whether the introduction of a rapid PCR, but with limited targets (Cepheid Xpert Flu/RSV XC), was associated with improved outcomes for adults hospitalized with respiratory illness. A controlled quasi-experimental study was conducted across three hospitals in New South Wales, Australia. Intervention groups received standard multiplex PCR during the preimplementation, July to December 2016 (n = 953), and rapid PCR during the postimplementation, July to December 2017 (n = 1,209). Control groups (preimplementation, n = 937, and postimplementation, n = 1,102) were randomly selected from adults hospitalized with respiratory illness during the same periods. The outcomes were hospital length of stay (LOS) and microbiology test utilization (blood culture, urine culture, sputum culture, and respiratory bacterial and virus serologies). The introduction of rapid PCR was associated with a nonsignificant 8.9-h reduction in median LOS (95% confidence interval [CI], −21.5 h to 3.7 h; P = 0.17) for all patients and a significant 21.5-h reduction in median LOS (95% CI, −36.8 h to −6.2 h; P < 0.01) among patients with positive test results in an adjusted difference-in-differences analysis. For patients receiving test results before disposition, rapid PCR use was associated with a significant reduction in LOS, irrespective of test results. Compared with standard PCR testing, rapid PCR use was significantly associated with fewer blood culture (adjusted odds ratio [aOR], 0.67; 95% CI, 0.5 to 0.82; P < 0.001), sputum culture (aOR, 0.56; 95% CI, 0.47 to 0.68, P < 0.001), bacterial serology (aOR, 0.44; 95% CI, 0.35 to 0.55, P < 0.001) and viral serology (aOR, 0.42; 95% CI, 0.33 to 0.53, P < 0.001) tests, but not with fewer urine culture tests (aOR, 0.94; 95% CI, 0.78 to 1.12, P = 0.48). Rapid PCR testing of adults hospitalized with respiratory illnesses can deliver benefits to patients and reduce resource utilization. Future research should consider a formal economic analysis and assess its potential impacts on clinical decision making.
INTRODUCTION
Influenza and other respiratory viruses, such as a respiratory syncytial virus (RSV), rhinovirus, coronavirus, human metapneumovirus, and parainfluenza viruses, cause significant morbidity and mortality in Australia and worldwide (1–3). Acute respiratory viral infections are estimated to cause 5.8 million deaths worldwide (4), and each year, up to 650, 000 deaths are related to respiratory diseases from seasonal influenza (5). In Australia, influenza-like illness is a common presentation among working-age adults and is responsible for decreased productivity and lost working days (3).
The laboratory diagnosis of respiratory viruses has evolved considerably in recent years with the development of novel molecular methods (6). Molecular methods use nucleic acid amplification techniques, such as reverse transcription PCR (PCR), and are considered effective diagnostic tests to confirm respiratory viral infections (7). A standard multiplex PCR test can detect several respiratory viruses with very high sensitivity and specificity in a single test (8). While it offers comprehensive testing for respiratory viruses, multiplex PCR requires specialized laboratory facilities and expertise (9), and it has traditionally been performed in a referral laboratory with a lengthy test turnaround time (TAT), which can reduce patient flow through the hospital.
New rapid PCR-based tests have recently been developed, including the Cepheid Xpert assays (Cepheid, Sunnyvale, CA) (10). Rapid viral diagnosis at hospital-based laboratories can improve the timeliness of care in hospital and has the potential for reducing resource utilization (11). The use of rapid PCR was introduced across hospitals in New South Wales (NSW), Australia, in July 2017 (N. Wabe, L. Li, R. Lindeman, R. Yimsung, M.R. Dahm, S. McLennan, K. Clezy, J.I Westbrook, and A. Georgiou, submitted for publication). It is an easy-to-use diagnostic test for accurate detection and differentiation of influenza A/B and RSV. Existing international evidence has shown an association between the implementation of rapid viral diagnosis and reductions in hospital length of stay (LOS) (12) and other laboratory test ordering practices (13–15). However, the impact of the introduction of a rapid PCR has not yet been investigated in Australia.
In this study, we compared outcomes of patients tested for influenza A/B and RSV using a rapid PCR (Cepheid Xpert Flu/RSV XC) at the local hospital laboratory during the first six months following its introduction (July to December 2017), with patients tested at a referral laboratory using a standard multiplex PCR (Allplex respiratory panels; Seegene) during the corresponding period in 2016. The objective was to determine whether the introduction of a rapid PCR testing for influenza A/B and RSV for adults hospitalized with respiratory illness was associated with a reduction in hospital LOS and a change in microbiology test utilization compared with those associated with standard multiplex PCR testing.
MATERIALS AND METHODS
Study setting.
The study was conducted across three major tertiary teaching hospitals in New South Wales, Australia, each offering a comprehensive range of inpatient and community services, hospital A (>65,000 admissions per year), hospital B (>50,000 admissions per year), and hospital C (>28,000 admissions per year). Ethics approval was granted by the Human Research Ethics Committee of the South Eastern Sydney Local Health District (approval HREC/16/POWH/412).
Study design and population.
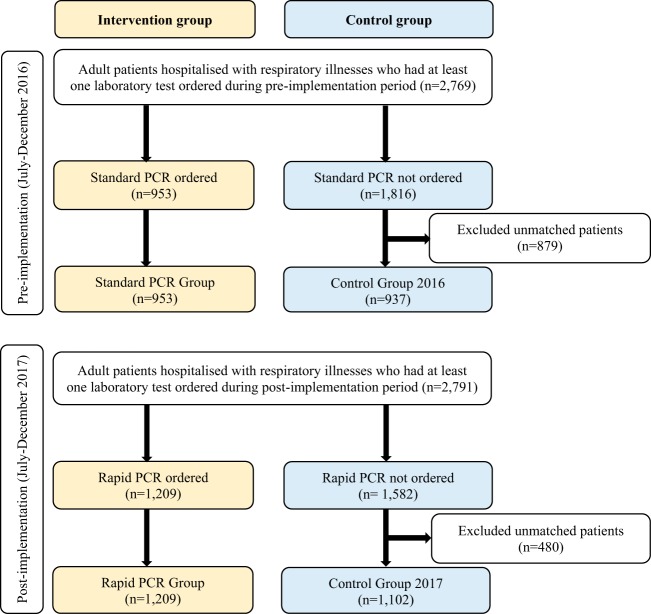
We conducted a controlled, nonrandomized, quasi-experimental study. A patient selection flow chart is presented in Fig. 1. The study periods were between July and December 2016 (preimplementation) and between July and December 2017 (postimplementation). The inclusion criteria were patients (aged >18 years) admitted with a respiratory illness [International Classification of Diseases version 10 Australian Modification (ICD-10-AM), J00-J99] during the study period and having at least one laboratory test result.
FIG 1.
Patient selection flow chart.
The intervention groups included patients tested for respiratory viruses using the standard multiplex PCR during the preimplementation (standard PCR group) and patients tested for influenza or RSV using the rapid PCR during the postimplementation (rapid PCR group). The standard PCR used in this study consisted of Allplex respiratory panels 1, 2, and 3 (Seegene Inc., Seoul, Republic of Korea) (16). It was available as a referral test at a large referral laboratory located at hospital B. All three hospitals sent samples to this laboratory to conduct a standard multiplex PCR. Standard multiplex PCR testing was generally batched. During the influenza season, it was performed 1 to 2 times a day (per demand) on weekdays and once a day on weekends. Outside the influenza season, the test was performed once every day. The multiplex PCR assay was offered as three panels that can detect up to 16 viruses with influenza A subtyping. Panel 1 includes influenza A (subtypes H1, H1pdm09, and H3), influenza B, and RSV (types A and B); panel 2 includes adenovirus, metapneumovirus, enterovirus, and parainfluenza virus (types 1, 2, 3, and 4); and panel 3 includes bocavirus (types 1, 2, 3, and 4), coronavirus (types OC43, 229E, and NL63), and rhinovirus (types A, B, and C) (16). In this study, all patients in the standard PCR group received panel 1 and, depending on whether a specific request for other viruses was made, panels 2 and or 3 were also ordered.
The rapid PCR used in this study was a Cepheid Xpert Flu/RSV XC assay (Cepheid, Sunnyvale, CA). The Cepheid Xpert Flu/RSV XC assay demonstrated a high sensitivity and specificity for detection of influenza A, influenza B, and RSV (17). However, unlike the standard multiplex PCR, Cepheid Xpert Flu/RSV XC cannot discriminate among influenza A virus subtypes. It is a hospital laboratory-based test. Hospitals A and B had an onsite laboratory to perform the rapid testing. Hospital C sent samples to hospital A because of its proximity to this hospital. During the influenza season, rapid PCR testing was available 24 h a day, 7 days a week, and outside the influenza season, the test was available on demand with approval from the pathologist or registrar on duty.
The control groups were selected from the population that fulfilled the above inclusion criteria but were not tested with standard PCR or rapid PCR. They were selected randomly from the population after matching during the respective study periods on study hospital, age quartile, mode of separation from the hospital, and gender. The purpose of matching was to balance the distribution of baseline characteristics across groups. The diagnosis of influenza among patients in the control groups was based on clinical assessment. Relevant demographic, clinical characteristics, and laboratory test data were obtained by linking the admitted patient and laboratory information system data sets. Detailed information on the linkage process has been described elsewhere (18).
Outcome measures.
The primary outcome was hospital LOS. Hospital LOS was calculated by subtracting the admission date/time from the hospital separation date/time. As secondary outcomes, we compared utilization of commonly ordered microbiology tests, including blood culture; urine microscopy, culture, and sensitivity (urine MC&S); sputum culture; respiratory bacterial serology (e.g., Mycoplasma pneumoniae IgM Antibody [Ab], Bordetella pertussis toxin IgA Ab and Pneumocystis jirovecii immunofluorescence assay [IF], and Hemophilus influenzae B Ab), and respiratory virus serology (e.g., Parainfluenza Ab complement fixation test [CFT], Adenovirus CFT and RSV Ab CFT).
Statistical analysis.
Descriptive statistics, including medians with interquartile ranges (IQR), were calculated. Demographic and clinical characteristics of the groups were compared using Pearson’s chi-square test for categorical variables and Mann-Whitney tests for continuous variables.
The impact of a rapid PCR on hospital LOS was assessed using a median regression. As LOS data were highly positively skewed, commonly used approaches such as ordinary least-squares regression, which models the conditional mean of the outcome variable, were not appropriate (19). Median regression is robust to extreme values and therefore well suited for modeling LOS (20). A difference-in-differences (DID) analysis was conducted to estimate the effect of a rapid PCR on the LOS by comparing the median change over time in the LOS for the intervention group, compared to the median change over time for the control group.
Comparison of other microbiology test utilization (e.g., blood culture, yes/no) between intervention and control groups was performed using binary logistic regression. Analyses were adjusted for relevant demographic and clinical characteristics.
For both primary and secondary outcomes, a subgroup analysis was conducted by test results. For the primary outcome, a further subgroup analysis was conducted by limiting the analysis to patients whose test results were returned during their stay in the hospital (i.e., excluding patients discharged before test results became available). All P values were 2-tailed, and a P value of <0.05 was considered statistically significant. Analyses were conducted using Stata version 15 (StataCorp LP, College Station, TX).
RESULTS
Demographic and clinical characteristics.
The intervention group included 2,162 patients (953 preimplementation [standard PCR group] and 1,209 postimplementation [rapid PCR group]). The control group consisted of 2,039 patients (937 preimplementation [control group 2016] and 1,102 postimplementation [control group 2017]) (Fig. 1). All patients in the standard PCR group received panel 1 with or without other panels; 64.1% (n = 611) received panel 1 only; 32.2% (n = 307) received all 3 panels; 2.3% (n = 22) received panels 1 and 3; and 1.4% (n = 13) received panels 1 and 2. Postimplementation, 20.2% (n = 244) of patients in the rapid PCR group were ordered at least one standard multiplex PCR panel in addition to the rapid PCR itself. Of the 244 patients, 40.9% (n = 100) received all 3 panels; 43.4% (n = 106) received panels 2 and 3; 12.3% (n = 30) received panel 1; and 3.3% (n = 8) received panels 1 and 3 (n = 3), panels 1 and 2 (n = 2), panel 2 (n = 2), or panel 3 (n = 1).
The distributions of patient demographic and clinical characteristics in the intervention and control groups were similar in terms of gender, age, study hospital, weekday of admission, and mode of separation from the hospital. However, the distributions of the source of referral, intensive care admission status, and principal diagnosis were different across groups (Table 1).
TABLE 1.
Comparison of demographic and clinical characteristics of intervention and control groups
| Variable | Data for: |
|||||
|---|---|---|---|---|---|---|
| Intervention group |
Control group |
Intervention vs control groups (P value)e |
||||
| Standard PCR (n = 953) | Rapid PCR (n = 1,209) | 2016 (n = 937) | 2017 (n = 1,102) | Standard PCR vs control 2016 | Rapid PCR vs control 2017 | |
| Female, n (%) | 480 (50.4) | 619 (51.2) | 473 (50.5) | 565 (51.3) | 0.96 | 0.97 |
| Age (yrs), median (IQR) | 75 (62–84) | 77 (65–86) | 75 (62–84) | 76 (64–84) | 0.97 | 0.11 |
| Hospital, n (%) | 0.94 | 0.58 | ||||
| A | 434 (45.5) | 672 (55.6) | 431 (46.0) | 589 (53.5) | ||
| B | 340 (35.7) | 205 (17.0) | 327 (34.9) | 198 (18.0) | ||
| C | 179 (18.8) | 332 (27.5) | 179 (19.1) | 315 (28.6) | ||
| Time of day of hospital admission, n (%) | <0.01 | 0.95 | ||||
| 7 a.m.–7 p.m. | 598 (62.8) | 866 (71.6) | 657 (70.1) | 788 (71.5) | ||
| 7 p.m.–7 a.m. | 355 (37.2) | 343 (28.4) | 280 (29.9) | 314 (28.5) | ||
| Day of week of admission, n (%) | 0.99 | 0.24 | ||||
| Weekdays | 700 (73.5) | 920 (76.1) | 688 (73.4) | 815 (74.0) | ||
| Weekends | 253 (26.5) | 289 (23.9) | 249 (26.6) | 287 (26.0) | ||
| Source of referral, n (%) | ||||||
| Emergency department | 926 (97.2) | 1,196 (98.9) | 874 (93.3) | 1,007 (91.4) | <0.01 | <0.01 |
| Othera | 27 (2.8) | 13 (1.1) | 63 (6.7) | 95 (8.6) | ||
| Intensive care admission, n (%) | 110 (11.5) | 113 (11.0) | 41 (4.4) | 52 (4.7) | <0.01 | <0.01 |
| Mode of separation, n (%) | 0.68 | 0.09 | ||||
| Discharged by hospital | 805 (84.5) | 972 (80.4) | 805 (85.9) | 924 (83.9) | ||
| Transferred/discharged at own risk | 110 (11.5) | 171 (14.1) | 98 (10.5) | 125 (11.3) | ||
| Died in hospital | 38 (4.0) | 66 (5.5) | 34 (3.6) | 53 (4.8) | ||
| Charlson comorbidity index, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.01 | 0.13 |
| Principal diagnosis, n (%) | <0.01 | <0.01 | ||||
| Influenza and pneumonia | 527 (55.3) | 758 (62.7) | 276 (29.5) | 358 (32.5) | ||
| Chronic lower respiratory diseases | 229 (24.0) | 258 (21.3) | 293 (31.3) | 307 (27.9) | ||
| Acute upper respiratory infections | 53 (5.6) | 45 (3.7) | 75 (8.0) | 95 (8.6) | ||
| Other acute lower respiratory infectionsb | 70 (7.4) | 51 (4.2) | 64 (6.8) | 62 (5.6) | ||
| Lung diseases due to external agentsc | 36 (3.8) | 43 (3.6) | 97 (10.4) | 91 (8.3) | ||
| Other respiratory diseasesd | 38 (4.0) | 54 (4.5) | 132 (14.1) | 189 (17.2) | ||
Medical practitioner, outpatient, day procedure center, community health.
ICD-10-AM: J20 to J22.
ICD-10-AM: J60 to J70.
ICD-10-AM: J30 to J39; J85 to J84; J85 to J86; J90 to J94, and J95 to J99.
Comparisons were made using Pearson’s chi-square test for categorical variables and the Mann-Whitney test for continuous variables, and the P value shows the distribution of a given variable across groups.
Influenza and pneumonia were the most common principal diagnosis in all groups. However, intervention groups had higher proportions of admissions due to influenza and pneumonia compared to those of the control groups (P < 0.01). Similarly, intensive care unit (ICU) admissions were higher in intervention groups than those in the control groups. Over 80% of patients were subsequently discharged home, and in-hospital death occurred in 3.6 to 7.7% of patients (Table 1).
For all intervention patients admitted to hospital through emergency departments (ED), respiratory virus testing (both standard and rapid PCR) was ordered while patients were in the ED. The tests were ordered a median of 3.8 h and 3.2 h before separation from the ED for standard PCR and rapid PCR groups, respectively. The median ED LOS of the two groups (prior to hospital admission) were roughly comparable, namely, 6.8 h for standard PCR and 7.2 h for the rapid PCR.
Twenty-two percent of patients receiving the standard PCR and 36.4% in the rapid PCR group were positive for at least one respiratory virus. The overall median TAT was significantly shorter for rapid PCR compared to standard multiplex PCR (2.3 h versus 27.4 h, P < 0.01). Significantly more patients were discharged from the hospital before test results became available in the standard PCR group than in the rapid PCR group (18.9% versus 2.2%; P < 0.01) (Table 2). Of these discharged patients, the results of 19.4% (n = 35) in the standard PCR and 11.1% (n = 3) in the rapid PCR groups eventually came back positive for at least one respiratory virus.
TABLE 2.
Test results and timeliness of care among intervention groups
| Variable | Results for: |
|
|---|---|---|
| Standard PCR (n = 953) | Rapid PCR (n = 1,209) | |
| Positive test result to at least one virus, n (%) | 210 (22.0) | 440 (36.4) |
| Virus detected, n (%) | ||
| Influenza A/B | 24 (1.3) | 405 (17.7) |
| Respiratory syncytial virus | 38 (2.0) | 36 (1.6) |
| Rhinovirus | 70 (7.4) | |
| Coronavirus | 36 (3.8) | |
| Human metapneumovirus | 26 (2.7) | |
| Parainfluenza virus | 22 (2.3) | |
| Adenovirus/enterovirus/bocavirusa | 7 (0.4) | |
| Test TAT (h), median (IQR)b | ||
| Hospital A | 29.8 (25.2–40.6) | 1.7 (1.4–2.4) |
| Hospital B | 23.9 (17.4–28.5) | 2.6 (1.9–3.9) |
| Hospital C | 29.2 (24.7–41.4) | 3.7 (2.9–5.8) |
| Overall | 27.4 (23.0–36.8) | 2.3 (1.6–3.7) |
| Time to sample (h) received at the lab from admission, median (IQR) | 5.7 (3.2–17.4) | 3.9 (2.1–7.3) |
| Time to test result from admission, median (IQR) | 40.3 (28.3–52.6) | 7.5 (4.5–16.7) |
| Discharged before a test result became available, n (%) | 180 (18.9) | 27 (2.2) |
Adenovirus = 3, bocavirus = 3, and enterovirus = 1.
TAT, turnaround time; IQR, interquartile range.
Primary outcome.
Table 3 presents the results of median regression for the main and subgroup analyses. In the adjusted analyses, we controlled for multiple factors potentially affecting LOS (Table S1). For the control group, there was no significant difference in the median LOS between controls 2016 and 2017 (75.3 h versus 78.9 h, P = 0.68). For the intervention group, an analysis that included all patients, regardless of test result outcome, showed no significant difference in the median LOS. Similarly, for patients with negative results, there was no significant difference in the median LOS. However, for patients with positive results, the median LOS decreased significantly, by 21.2 h (95% CI, −38.0 to −4.5; P < 0.01) in the within-intervention-groups analysis and by 21.5 h (95% CI, −36.8 to −6.2; P < 0.01) in the DID analysis following the introduction of rapid PCR.
TABLE 3.
Median regression analysis showing changes in the median LOS following the introduction of rapid PCR
| Variable | No. of patients | Median LOS (h [95% CI]) | Change in LOS within the intervention groups (pre to post)a |
Adjusted DID analysisb |
||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedb |
|||||||
| Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |||
| Intervention group | ||||||||
| Regardless of test results | ||||||||
| Standard PCR | 953 | 100.9 (96.2 to 109.9) | Reference group | Reference group | Reference group | |||
| Rapid PCR | 1,209 | 101.8 (97.1 to 112.8) | 0.9 (−7.9 to 9.6) | 0.85 | −6.5 (−16.0 to 2.9) | 0.18 | −8.9 (−21.5 to 3.7) | 0.17 |
| Positive test results | ||||||||
| Standard PCR | 210 | 98.6 (94.5 to 116.9) | Reference group | Reference group | Reference group | |||
| Rapid PCR | 440 | 92.9 (81.5 to 98.6) | −5.5 (−19.9 to 8.8) | 0.45 | −21.2 (−38.0 to −4.5) | 0.01 | −21.5 (−36.8 to −6.2) | <0.01 |
| Negative test results | ||||||||
| Standard PCR | 743 | 101.4 (96.0 to 113.0) | Reference group | Reference group | Reference group | |||
| Rapid PCR | 769 | 116.2 (103.4 to 122.6) | 14.8 (2.4 to 27.1) | 0.02 | 1.4 (−10.8 to 13.5) | 0.83 | −1.2 (−14.9 to 12.4) | 0.86 |
| Subgroup analysis including only patients whose test results were returned during their stay in the hospital | ||||||||
| Regardless of test results | ||||||||
| Standard PCR | 773 | 126.0 (117.9 to 139.0) | Reference group | Reference group | Reference group | |||
| Rapid PCR | 1,182 | 104.0 (98.3 to 114.8) | −22.2 (−32.5 to −11.9) | <0.01 | −22.8 (−33.1 to −12.5) | <0.01 | −25.5 (−38.1 to −12.9) | <0.01 |
| Positive test results | ||||||||
| Standard PCR | 175 | 117.5 (100.5 to 142.8) | Reference group | Reference group | Reference group | |||
| Rapid PCR | 437 | 93.2 (82.2 to 98.6) | −24.3 (−42.0 to −6.6) | <0.01 | −27.9 (−45.2 to −10.5) | <0.01 | −33.4 (−49.5 to −17.3) | <0.01 |
| Negative test results | ||||||||
| Standard PCR | 598 | 129.7 (121.2 to 142.7) | Reference group | Reference group | Reference group | |||
| Rapid PCR | 745 | 117.9 (109.6 to 125.2) | −11.9 (−25.9 to 2.2) | 0.09 | −17.3 (−30.4 to −4.3) | <0.01 | −19.6 (−33.7 to −5.5) | <0.01 |
| Control group | ||||||||
| 2016 | 937 | 75.3 (71.0 to 80.5) | Reference group | Reference group | ||||
| 2017 | 1,102 | 78.9 (73.0 to 87.3) | 3.2 (−5.6 to 12.1) | 0.47 | 1.6 (−6.0 to 9.2) | 0.68 | Not applicable | |
A negative coefficient indicates a median decrease in the LOS compared to the reference group.
Adjusted for age, study hospital, the source of referral, intensive care admission status, mode of separation, Charlson comorbidity index and type of principal diagnosis. DID (difference-in-difference) = ΔLOS (h) in intervention group − ΔLOS (h) in control group.
When the analysis was limited to patients whose test results were returned during their stay in the hospital, rapid PCR was associated with a significant decrease in the median LOS, irrespective of test result. Adjusted analysis showed a significant reduction in the LOS both in the within-intervention-groups analysis (median, −22.8 h; 95% CI, −33.1 to −12.5; P < 0.01) and in the DID analysis (median, −25.5 h; 95% CI, −38.1 to −12.9; P < 0.01) following the introduction of rapid PCR. For patients with positive test results, the median LOS decreased by 27.9 h (95% CI, −45.2 to −10.5; P < 0.01) in the within-intervention-groups analysis and by 33.4 h (95% CI, −49.5 to −17.3; P < 0.01) in the DID analysis following the introduction of rapid PCR (Table 3). Of patients in the rapid PCR group, the median LOS of patients who received an additional standard multiplex PCR panel was almost 1 day longer than that of those not requiring the multiplex PCR.
Secondary outcome.
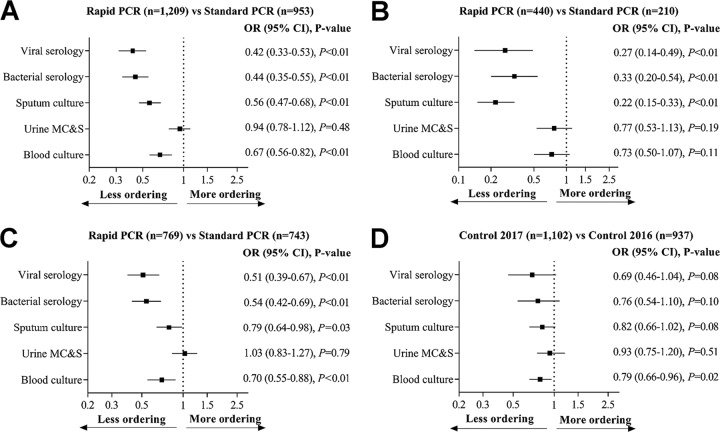
Figure 2 compares utilization of five common microbiology tests by intervention and control groups during the postimplementation versus baseline. Overall, after adjusting for confounding variables, patients in the rapid PCR group had significantly fewer blood culture, sputum culture, and respiratory bacterial and viral serology tests compared to standard PCR (Fig. 2A). For positive test results, patients in the rapid PCR group had significantly fewer sputum culture, and respiratory bacterial and viral serology tests but not fewer blood culture and urine MC&S tests compared to those in the standard PCR group (Fig. 2B). For negative test results, with the exception of urine MC&S, there were significantly fewer other tests ordered for the rapid PCR group compared to those in the standard PCR group (Fig. 2C). In the control group, with the exception of blood culture, there was no difference in ordering practices over time (Fig. 2C).
FIG 2.
Common microbiology test utilization. (A) Regardless of test results, (B) positive test results, (C) negative test results, and (D) control groups. The middle square shows the odds ratio (OR), and the horizontal line shows the 95% confidence interval (CI) of the OR. The inclusion of “1” in the 95% CI indicates a nonsignificant difference in the ordering practices between groups. All analyses were adjusted for baseline variables, including age, study hospital, the source of referral, intensive care admission status, mode of separation, Charlson comorbidity index, and type of principal diagnosis. MC&S, microscopy, culture, and sensitivity.
DISCUSSION
In this controlled, quasi-experimental study, we investigated the impact of rapid PCR testing of influenza A/B and RSV across three hospitals on hospital LOS and related microbiology test utilization. We found that the introduction of rapid PCR was associated with significant reductions in the hospital LOS among patients with positive test results and in the use of other common microbiology tests, including blood culture, sputum culture, and respiratory bacterial and viral serology tests, compared with those for patients in the preimplementation period who received standard multiplex PCR testing.
In our study, the impact of rapid PCR on hospital LOS was statistically significant among patients with positive test results. This finding is unsurprising, given that patients can leave the hospital earlier if a bacterial infection has been ruled out. For patients requiring antiviral therapy, treatment can be started more quickly in patients with positive results, which may expedite their discharge (21), whereas patients with negative results are more likely to stay in the hospital, as they may require further investigation and management. This was consistent with results of a previous U.S. study (11). Rappo et al. retrospectively assessed the impact of early diagnosis of respiratory infections using rapid PCR (FilmArray) on the outcomes of over 337 adult patients over two flu seasons at a tertiary care center in New York, NY, USA, and found a significant reduction in hospital LOS among influenza-positive patients with rapid PCR (11).
In the analysis that included all patients regardless of test results, there was no significant difference in LOS between rapid PCR and standard PCR groups. This was because more patients in the standard PCR group (18.9% versus 2.2%) were discharged from the hospital even before the test results were available. These patients had a shorter LOS compared with that of patients who were discharged after test results were available, confounding the effect of rapid PCR on hospital LOS. When the analysis was limited to patients whose test results were returned during their stay in the hospital, rapid PCR use was associated with a significant reduction in the median LOS, irrespective of test results.
In a recent large UK study, the use of a molecular point-of-care-test (POCT) resulted in a reduction in hospital LOS regardless of test results, although the effect was more noticeable among patients with positive test results (22). Brendish et al. conducted a pragmatic randomized controlled trial enrolling 720 adult patients (aged ≥18 years) at a large teaching hospital in the United Kingdom (the ResPOC trial). That study assessed the impact of molecular POCT testing for respiratory viruses compared with referral laboratory testing and found a significant reduction in hospital LOS by 1.1 days in the patients receiving POCT. In a subgroup analysis, patients with positive rapid PCR results had a hospital stay that was an average of 1.7 days shorter than that of patients with negative results (22).
In contrast to our findings and the results of the ResPOC trial (22) and Rappo et al. (11), the use of rapid PCR did not decrease hospital LOS in a study by Andrews et al. (21). The authors conducted a quasi-randomized trial to assess the impact of the FilmArray respiratory panel as a POCT compared to laboratory-based PCR, serology, or culture testing on a range of outcomes, including hospital LOS. The study enrolled 545 patients aged ≥16 years and presenting with influenza-like illness or other respiratory tract infections at an Acute NHS Hospital Trust in London, United Kingdom. Interestingly, the authors found an 11% increase in hospital LOS in the rapid PCR group, although it was not statistically significant. The authors attributed the lack of impact on LOS to a delay in initiating FilmArray testing. The median time to test result from admission was 19 h in their study. This was substantially longer than ours (the median time to test result from admission was 7.5 h), and this may explain the differences between our findings and those of Andrews et al. (21).
The limitation of the rapid PCR (Cepheid Xpert Flu/RSV XC) used in our study is its inability to diagnose other respiratory viruses. While it can accurately detect influenza A/B and RSV, other clinically relevant respiratory viruses, such as rhinovirus, coronavirus, metapneumovirus, and parainfluenza viruses, cannot be detected by our current rapid PCR method. This means that patients can still be ordered standard multiplex PCR following the results of rapid PCR if other respiratory viruses are suspected. Our analysis showed that 20% of the patients in the rapid PCR group also received multiplex PCR, and their median LOS was almost 1 day longer than that of those not requiring the multiplex PCR. Other studies implemented multiplex PCR respiratory panel (i.e., our version of the referral laboratory-based standard PCR) as a POCT test (11, 21, 22), avoiding the need for further respiratory viral investigations. The introduction of a broader multiplex PCR as a POCT in the future may offer better outcomes for patients.
Our results show that the utilization of blood culture, sputum culture, and respiratory bacterial and virus serology tests was significantly lower among patients tested with rapid PCR. To the best of our knowledge, no previous studies have studied the impact of PCR-based rapid tests on other laboratory test utilization in hospitalized adults. Previous studies that assessed other test ordering practices were conducted using antigen-based POCT in ED (23). Poehling et al. (13) and Blaschke et al. (14) reported significantly fewer blood culture tests when POCT was used, similar to our findings. Other studies have reported fewer biochemistry tests, including full blood count (14, 15), C-reactive protein (15), and urinalysis (14, 15), when POCT was used.
Our findings have some important implications. Reducing hospital LOS has large economic benefits. Cost savings for hospitals can be achieved through avoidance of unnecessary hospital resource utilization, including that of supplementary laboratory and imaging testing (24, 25). In a Spanish study by Soto et al., rapid PCR decreased the isolation time of hospitalized patients by 23.7 h, which resulted in a cost reduction of $70 per hospitalized patient (24). A half-day reduction in hospital LOS in one U.S. study was associated with an estimated annual savings of $500 to 900 million (26). Rapid diagnostic testing can have safety implications too. Reducing LOS in hospital leads to a reduction in hospital-acquired infections, including the disruption of Clostridium difficile transmission (27). According to a large U.S. study, reducing LOS by about 1 day lowers the risk of 30-day readmission through a reduction in infection risk (28).
The strengths of this study include its relatively large sample size, its utilization of real-world routinely collected clinical practice data, and multiple-center inclusion of three teaching hospitals, which enhances the generalizability of our findings. Unlike several prior studies (11, 12, 14, 29), we applied a quasi-experimental, controlled study design that allowed us to account for secular trends or sudden changes over time (30). Moreover, we used a number of strategies to reduce the potential impact of confounding variables. We used matching on key baseline variables to identify comparable control groups. To reduce seasonal effects, a similar time frame for both groups (i.e., July to December) was selected. To decrease the potential for selection bias, all hospitalized adults tested for influenza and RSV during the study period were included. Finally, we conducted within-intervention-groups and DID analyses, adjusting for differences in several baseline patient and clinical characteristics.
There are some limitations to consider when interpreting the findings of the current study. Given that this study was not a randomized controlled trial, our findings should not be interpreted as a cause and effect relationship. Due to a lack of randomization, there is always a risk of unidentified or hidden confounders in a quasi-experimental study (31). While our results indicated that the use of rapid PCR was associated with fewer other microbiology test utilizations, because of lack of data on regarding the time when the clinicians ordered the tests, our study did not examine whether the larger number of tests ordered with the standard PCR was associated with orders after results were returned to the patient. As we did not have access to treatment data, we were unable to assess the potential impact of rapid PCR testing on clinical decision making, including whether it influenced the decision to initiate antiviral therapy following positive results or to deescalate its use if patients were already using the medication and the test result returned negative. Although our current study result suggests economic benefits of rapid PCR testing, a formal health economic study is required.
Conclusion.
The introduction of rapid PCR testing of influenza and RSV viruses for hospitalized adults was associated with a significant reduction in hospital LOS for patients with positive results and a significant reduction in microbiology test use compared with those for patients who received standard multiplex PCR testing. These findings suggest there may be economic and patient outcome benefits from this intervention that should be tested in a future cost-effectiveness study.
Supplementary Material
ACKNOWLEDGMENTS
The study was part of a partnership project funded by the National Health and Medical Research Council (NHMRC) of Australia (project grant APP1111925).
Footnotes
For a commentary on this article, see https://doi.org/10.1128/JCM.01890-18.
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01727-18.
REFERENCES
- 1.Chen Y, Kirk MD. 2014. Incidence of acute respiratory infections in Australia. Epidemiol Infect 142:1355–1361. doi: 10.1017/S0950268813002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang D-A, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lázaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccalà G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida L-M, Yu H, Zar HJ, Campbell H, Nair H. 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varghese BM, Dent E, Chilver M, Cameron S, Stocks NP. 2018. Epidemiology of viral respiratory infections in Australian working-age adults (20-64 years): 2010-2013. Epidemiol Infect 146:619–626. doi: 10.1017/S0950268818000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J-P, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh P-H, Yip P, Zabetian A, Zheng Z-J, Lopez AD, Murray CJL. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2018. Up to 650 000 people die of respiratory diseases linked to seasonal flu each year. Saudi Med J 39:109–110. [Google Scholar]
- 6.Loeffelholz M, Chonmaitree T. 2010. Advances in diagnosis of respiratory virus infections. Int J Microbiol 2010:126049. doi: 10.1155/2010/126049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahony JB. 2008. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 21:716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahony J, Chong S, Merante F, Yaghoubian S, Sinha T, Lisle C, Janeczko R. 2007. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J Clin Microbiol 45:2965–2970. doi: 10.1128/JCM.02436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay IM. 2004. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect 10:190–212. [DOI] [PubMed] [Google Scholar]
- 10.Novak-Weekley SM, Marlowe EM, Poulter M, Dwyer D, Speers D, Rawlinson W, Baleriola C, Robinson CC. 2012. Evaluation of the Cepheid Xpert Flu Assay for rapid identification and differentiation of influenza A, influenza A 2009 H1N1, and influenza B viruses. J Clin Microbiol 50:1704–1710. doi: 10.1128/JCM.06520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rappo U, Schuetz AN, Jenkins SG, Calfee DP, Walsh TJ, Wells MT, Hollenberg JP, Glesby MJ. 2016. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol 54:2096–2103. doi: 10.1128/JCM.00549-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers BB, Shankar P, Jerris RC, Kotzbauer D, Anderson EJ, Watson JR, O'Brien LA, Uwindatwa F, McNamara K, Bost JE. 2015. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med 139:636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 13.Poehling KA, Zhu Y, Tang YW, Edwards K. 2006. Accuracy and impact of a point-of-care rapid influenza test in young children with respiratory illnesses. Arch Pediatr Adolesc Med 160:713–718. doi: 10.1001/archpedi.160.7.713. [DOI] [PubMed] [Google Scholar]
- 14.Blaschke AJ, Shapiro DJ, Pavia AT, Byington CL, Ampofo K, Stockmann C, Hersh AL. 2014. A national study of the impact of rapid influenza testing on clinical care in the emergency department. J Pediatric Infect Dis Soc 3:112–118. doi: 10.1093/jpids/pit071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitsch-Osuch A, Stefanska I, Kuchar E, Brydak LB, Pirogowicz I, Zycinska K, Wardyn K. 2013. Influence of rapid influenza test on clinical management of children younger than five with febrile respiratory tract infections. Adv Exp Med Biol 755:237–241. doi: 10.1007/978-94-007-4546-9_30. [DOI] [PubMed] [Google Scholar]
- 16.Huh HJ, Kim J-Y, Kwon HJ, Yun SA, Lee M-K, Lee NY, Kim J-W, Ki C-S. 2017. Performance evaluation of Allplex respiratory panels 1, 2, and 3 for detection of respiratory viruses and influenza A virus subtypes. J Clin Microbiol 55:479–484. doi: 10.1128/JCM.02045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbefeville S, Thonen-Kerr E, Ferrieri P. 2017. Prospective and retrospective evaluation of the performance of the FDA-approved Cepheid Xpert Flu/RSV XC assay. Lab Med 48:e53–e56. doi: 10.1093/labmed/lmx038. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Georgiou A, Vecellio E, Eigenstetter A, Toouli G, Wilson R, Westbrook JI. 2015. The effect of laboratory testing on emergency department length of stay: a multihospital longitudinal study applying a cross-classified random-effect modeling approach. Acad Emerg Med 22:38–46. doi: 10.1111/acem.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Cook B, Manning WG. 2013. Thinking beyond the mean: a practical guide for using quantile regression methods for health services research. Shanghai Arch Psychiatry 25:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee AH, Fung WK, Fu B. 2003. Analyzing hospital length of stay: mean or median regression? Med Care 41:681–686. doi: 10.1097/01.MLR.0000062550.23101.6F. [DOI] [PubMed] [Google Scholar]
- 21.Andrews D, Chetty Y, Cooper BS, Virk M, Glass SK, Letters A, Kelly PA, Sudhanva M, Jeyaratnam D. 2017. Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis 17:671. doi: 10.1186/s12879-017-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brendish NJ, Malachira AK, Armstrong L, Houghton R, Aitken S, Nyimbili E, Ewings S, Lillie PJ, Clark TW. 2017. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med 5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko F, Drews SJ. 2017. The impact of commercial rapid respiratory virus diagnostic tests on patient outcomes and health system utilization. Expert Rev Mol Diagn 17:917–931. doi: 10.1080/14737159.2017.1372195. [DOI] [PubMed] [Google Scholar]
- 24.Soto M, Sampietro-Colom L, Vilella A, Pantoja E, Asenjo M, Arjona R, Hurtado JC, Trilla A, Alvarez-Martínez MJ, Mira A, Vila J, Marcos MA. 2016. Economic impact of a new rapid PCR assay for detecting influenza virus in an emergency department and hospitalized patients. PLoS One 11:e0146620. doi: 10.1371/journal.pone.0146620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St John A, Price CP. 2013. Economic evidence and point-of-care testing. Clin Biochem Rev 34:61–74. [PMC free article] [PubMed] [Google Scholar]
- 26.Raut M, Schein J, Mody S, Grant R, Benson C, Olson W. 2009. Estimating the economic impact of a half-day reduction in length of hospital stay among patients with community-acquired pneumonia in the US. Curr Med Res Opin 25:2151–2157. doi: 10.1185/03007990903102743. [DOI] [PubMed] [Google Scholar]
- 27.Brain DC, Barnett AG, Yakob L, Clements A, Riley TV, Halton K, Graves N. 2018. Reducing length of stay to improve Clostridium difficile-related health outcomes. Infect Dis Health 23:87–92. doi: 10.1016/j.idh.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Kaboli PJ, Go JT, Hockenberry J, Glasgow JM, Johnson SR, Rosenthal GE, Jones MP, Vaughan-Sarrazin M. 2012. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med 157:837–845. doi: 10.7326/0003-4819-157-12-201212180-00003. [DOI] [PubMed] [Google Scholar]
- 29.Li-Kim-Moy J, Dastouri F, Rashid H, Khandaker G, Kesson A, McCaskill M, Wood N, Jones C, Zurynski Y, Macartney K, Elliott EJ, Booy R. 2016. Utility of early influenza diagnosis through point-of-care testing in children presenting to an emergency department. J Paediatr Child Health 52:422–429. doi: 10.1111/jpc.13092. [DOI] [PubMed] [Google Scholar]
- 30.Eccles M, Grimshaw J, Campbell M, Ramsay C. 2003. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 12:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahajan A. 2015. Limitations of CBA study: controlled before after study. Lung India 32:670–671. doi: 10.4103/0970-2113.168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.