The World Health Organization End Tuberculosis (TB) strategy has called for the development of—and increased access to—effective tools for diagnosis and treatment of TB disease. Mycobacterium tuberculosis , the causative agent of TB, is categorized as a highly infectious agent.
KEYWORDS: heat inactivation, molecular tests, Mycobacterium tuberculosis, RNA preservation
ABSTRACT
The World Health Organization End Tuberculosis (TB) strategy has called for the development of—and increased access to—effective tools for diagnosis and treatment of TB disease. Mycobacterium tuberculosis , the causative agent of TB, is categorized as a highly infectious agent. Consequently, diagnostic tests that involve comprehensive manipulation of specimens from presumed tuberculosis cases must be performed in a category 3 laboratory. We have evaluated the use of heat inactivation to render TB samples safe to work with while preserving RNA for downstream molecular tests. Using Mycobacterium bovis bacillus Calmette-Guérin (BCG) cultures and TB-positive sputum samples, we show that boiling for 20 min at 80, 85, and 95°C inactivates all M. tuberculosis bacilli. The efficiency of inactivation was verified by culturing heat-treated and untreated (live) fractions of BCG and TB sputum samples for 42 days. No growth was observed in the cultures of heat-treated samples. In contrast, the optical density of untreated BCG in Middlebrook 7H9 broth rose from 0.04 to 0.85, and the untreated sputum samples flagged positive at 3 days of incubation in mycobacterial growth indicator tubes. Quantification of reference genes 16S rRNA, transfer-messenger RNA (tmRNA), pre-16S rRNA, and rpoB by reverse transcriptase quantitative PCR (RT-qPCR) showed minimal loss in estimated bacterial load. The loss was RNA species dependent, <1 log10, 1.1 log10, 1.3 log10, and 2.4 log10 estimated CFU/ml for 16S rRNA, tmRNA, pre-16S rRNA, and rpoB, respectively. The RNA loss was independent of inactivation temperature. These findings show that heat inactivation could obviate the need for category 3 laboratories to perform RNA-based testing of TB samples.
INTRODUCTION
Tuberculosis (TB) caused by Mycobacterium tuberculosis is a leading infectious disease killer claiming over 1 million lives every year worldwide. Close to 10 million new cases were reported per year in 2016 and 2017 (1, 2). The development of effective diagnostic and treatment tools is the main aim of pillar 3 of the End TB strategy (3). M. tuberculosis is classified under category 3 infectious organisms, requiring most research and diagnostic procedures to be conducted in high-containment laboratories, especially when the organism is to be cultured. The construction and maintenance of category 3 laboratories are costly, and, consequently, most high-burden low- and middle-income countries consolidate such services at the regional or national level. Consequently, culture laboratories are hundreds of kilometers away from most people that need the service. This severely limits access to these facilities, slowing or preventing the effective diagnosis and treatment of tuberculosis. In addition, health care facilities are forced to rely on less-sensitive or -specific methods, such as microscopic examination of sputum smears, that are limited by low sensitivity and specificity failing to distinguish viable from dead bacilli.
Molecular tests like Xpert MTB/RIF have approval from the WHO for implementation at the district hospital level to provide rapid diagnosis of TB (4, 5). The main challenge of Xpert MTB/RIF is the detection of DNA, a stable molecule that hangs around long after cell death and cannot, therefore, be used for monitoring treatment response (5, 6). RNA-based assays have been developed to overcome this challenge (7–13). There are different species of RNA, ribosomal, transfer, and messenger, which vary in stability and copies per cell. mRNA is the least stable, degrading rapidly after cell death (14). rRNA and tRNA are structural RNAs and are relatively more stable than mRNA (14). By this definition, mRNA is the most ideal marker for cell viability; however, its fast degradation and existence as a low copy molecule compromises its utility as a marker in a diagnostic test.
We have published a method, the molecular bacterial load assay (MBLA), that used rRNA to identify M. tuberculosis and quantify the total viable count in a single molecular reaction (15). This showed that the amount of 16S rRNA proportionally increased with bacterial growth measured by CFU counts (11, 13, 16). In response to treatment, the fall in CFU counts was matched by a corresponding decline of 16S rRNA measured by a semiquantitative reverse transcriptase PCR, suggesting that the latter is a good marker of cell viability (13, 16). The current MBLA protocol requires that the first steps of TB sample processing be performed in a high-containment laboratory until all M. tuberculosis cells have been lysed. It also includes sample preservation with guanidine thiocyanate (GTC), a hazardous class 4 chemical that requires special precautions to work with, and samples should be maintained at −80°C if they are to be tested later. The need for simple and user friendly but safe TB sample handling cannot be more emphasized.
The MBLA is currently a research use only (RUO) test. It is being used by a range of research groups in Eastern and Southern Africa, United Kingdom, Netherlands, Germany, Thailand, and Vietnam that are using the test to monitor response to anti-TB therapy in clinical trials of standard and test regimens and/or diagnostic evaluation studies. Although developed and optimized to detect and quantify TB in sputum samples, groups in Public Health England and Vietnam have successfully applied the MBLA to quantify bacterial load in Guinea pig lung tissue and cerebral spinal fluid samples from TB meningitis patients, respectively (unpublished data). Results from multisite evaluation in Africa (unpublished data) and from previous publications (9, 11) show that the sensitivity of MBLA is consistent with that of MGIT liquid culture and higher than that of solid culture. An important difference between MBLA and liquid culture is that MBLA is not affected by non-TB contaminants in the specimen and gives quantitative bacterial burden results in real time. This means that the results can inform clinical decisions for patient management. Based on these findings, the MBLA was recently recognized by the World Health Organization as a biomarker for TB treatment monitoring with potential to replace smear and culture (2).
Heat treatment is an established technique that has been used to decontaminate medical devices, ensure aseptic inoculation, and aid in therapeutic preparations (17, 18). However, for M. tuberculosis, reports have shown that short slide flaming or drying on a hot block is insufficient to completely inactivate all bacilli (19, 20). Heating M. tuberculosis cultures at 80°C for 20 min was shown to be effective at inactivating M. tuberculosis without compromising the integrity of DNA for downstream manipulation (21). Currently, a number of DNA isolation techniques use heating at 95°C as part of their procedure, suggesting such heat is not detrimental to nucleic acid integrity. However, studies have shown DNA as a stable molecule that survives long after cell death, which makes it a poor marker of cell viability and monitoring of bacteriologic response to therapy (6).
This study aimed to evaluate whether samples containing Mycobacterium tuberculosis complex organisms can be heat inactivated without compromising the detection of different RNA species that could be used to estimate bacterial load. Our data report a simple method to render TB samples noninfectious, potentially obviating the need for a high-containment laboratory while performing molecular assays like MBLA.
MATERIALS AND METHODS
Study site and samples.
This study was conducted at the University of St Andrews in the United Kingdom and the Mozambique National Tuberculosis Reference Laboratory (NTRL) in Maputo.
Two types of samples were used, clinical sputum samples from TB patients and in vitro cultures of Mycobacterium bovis bacillus Calmette-Guérin (BCG). Smear-positive TB sputum samples were obtained from the routine and emergency TB laboratories at Mavalane Health Centre and Maputo Central Hospital in Maputo, Mozambique. At the NTRL, the presence of M. tuberculosis in the specimens was further confirmed by Xpert MTB/RIF. None of specimens was rifampin resistant. The sputum samples (from different patients) were then pooled and homogenized, and 1-ml aliquots were prepared for the different downstream test conditions, heat inactivation, and decontamination with N-acetyl-l-cystein (NALC)–NaOH for culture. Eight 1-ml aliquots of pooled sputum were prepared for inactivation at each temperature. Two repeats were performed, each involving a batch of pooled sputum from the patients.
BCG cultures were cultivated and processed at the University of St Andrews to explore the effect of heat inactivation on RNA prior to processing clinical sputum.
Culture.
BCG was propagated in Middlebrook 7H9 broth at 37°C for 19 days prior to use. The 7H9 medium was made of 7H9 broth with 2% (vol/vol) glycerol, 1% (vol/vol) Tween 80, and 10% (vol/vol) ADC supplement. The cultures were harvested into 15-ml centrifuge tubes (Thermo Fisher Scientific, UK), and the tubes were tightly closed. Eight 2-ml aliquots were processed per culture batch for each condition. Three independent batches of culture were processed.
Sample inactivation.
Clinical sputum samples and BCG cultures were inactivated for 20 min in a nonshaking water bath at 80°C, 85°C, and 95°C to kill all mycobacteria and assess the impact on the bacterial load (CFU/ml) estimated from the total RNA harvested from the cells. The inactivation temperatures were selected based on previous study (21) and temperatures that are now commonly used in laboratory molecular preparations. Sample boiling was done in tightly closed 15-ml centrifuge tubes, which were left to stand for 10 min to allow any aerosols to settle prior to opening. Controls were clinical sputum or culture aliquots that were not exposed to heat (untreated). Inactivation was confirmed by cultivating the heat-killed fractions and controls in Middlebrook 7H9 broth. Controls and inactivated BCG cultures were inoculated at 1.0 ml into the growth medium while clinical sputum samples were inoculated at 0.5 ml into mycobacterial growth indicator tube (MGIT) growth medium (BD Ltd). The optical density (OD) of the liquid culture was measured at a 600-nm wavelength before and weekly during incubation at 37°C for 42 days to confirm no growth in heat-inactivated fractions. Growth or no growth of clinical sputum samples was automatically determined by MGIT over 42 days of incubation.
RNA extraction and quantitative PCR.
RNA extraction and reverse transcriptase quantitative PCR (RT-qPCR) were performed according to the proposed procedures of Honeyborne et al. (9). Briefly, BCG cultures and Xpert MTB/RIF-positive sputum samples were spiked with a standard internal control as described in Honeyborne et al. (9) and Gillespie et al. (15) and centrifuged at 3,000 × g for 30 min. The sediment was suspended in lysis buffer, RNApro blue solution (MP Biomedicals, UK), and bead homogenized for 40 s at 6,000 rpm using the Precellys 24 (Peqlab, UK) homogenizer. RNA was isolated using a FastPrep RNA kit (MP Biomedicals, UK) according to the manufacturer’s instructions. Genomic DNA was removed from the extracts by a 1-h DNase treatment at 37°C using the Ambion Turbo DNase kit (Life Technologies, UK).
RT-qPCR was performed on a Rotor-Gene 5plex platform (Qiagen, UK) using primers and dually labeled hydrolysis probes (TaqMan) targeting M. tuberculosis complex 16S rRNA, transfer-messenger RNA (tmRNA), precursor 16S (pre-16S) rRNA, and RNA polymerase B (rpoB) genes and the internal control. All primers and probes were procured from MWG Eurofins, Germany. The optimal PCR conditions and translation of quantification cycle (Cq) into bacterial load (estimated CFU [eCFU]/ml) were as described in Honeyborne et al. (9) and Gillespie et al. (15). Briefly, the MBLA RT-qPCR limit of detection is 10 CFU/ml, equivalent to a 30 Cq cut off. RNase-free molecular grade and no reverse transcriptase samples were included in each assay run as negative and DNA contamination controls, respectively.
Note that the internal control, primers, and probes have now been incorporated into the MBLA kit under the trademark Vitalbacteria. Consequently, the internal control, the primer, and probe sequences cannot be published.
Statistical analysis.
All analyses were performed using GraphPad Prism v.6. A one-way analysis of variance (ANOVA) test was used to calculate the difference in the mean bacterial load (log10 eCFU/ml) of the control (from live BCG or M. tuberculosis) and that of heat-inactivated fractions. Sidak’s multiple-comparison test was used to test whether the mean bacterial loads at 80°C, 85°C, and 95°C were different. The Sidak’s test was selected because of its power to compare a set of means and provide a P value of the difference; a P value less than 0.05 was considered significant.
Ethics.
The study was nested in the Pan-Africa Biomarkers expansion program (PANBIOME) for performance evaluation of the molecular bacterial load assay (MBLA). The study was approved by the University of St Andrews teaching and research ethics committee and by the Institutional Review Board of Instituto Nacional de Saúde and the National Bioethical Committee (CNBS) in Mozambique.
RESULTS
Heat inactivates all mycobacteria.
The OD of heat-inactivated BCG samples remained unchanged over the 42 days of incubation, whereas the OD of untreated samples increased from 0.04 to 0.85 (Fig. 1). Similarly, untreated clinical sputum samples flagged positive in MGIT at day 3 of incubation while the heat-inactivated sputum samples remained negative throughout the culture period. The presence of Mycobacterium tuberculosis in the positive MGIT culture was confirmed with Ziehl-Neelsen microscopy and antigen MPT64 (22). None of the heat killed sputum samples grew positive for 42 days of incubation.
FIG 1.

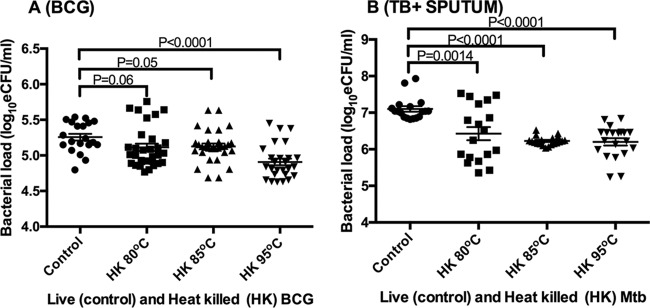
Verification of BCG inactivation at 80°C, 85°C, and 95°C. The control was live (unheated) BCG culture inoculated into the same growth medium. Growth in the control was confirmed by the increase in the OD of the culture over the incubation period.
Effect of heat-based sample inactivation on bacterial load measured by 16S rRNA as the marker.
Compared to the control (live) BCG mean bacterial load of 5.26 ± 0.21 log10 eCFU/ml, the mean bacterial load significantly reduced to 5.11 ± 0.29, 5.13 ± 0.23, and 4.91 ± 0.24 log10 eCFU/ml when cells were killed at 80°C, 85°C, and 95°C, respectively (ANOVA P < 0.0001 between controls and heat-inactivated samples). Pairwise comparison revealed no significant difference between the control and 80°C and 85°C, implying that the ANOVA P value was driven by the substantially lower bacterial load at 95°C than in the control. The reduction in measured bacterial load at the same temperatures was 0.14, 0.13, and 0.35 log10 eCFU/ml, resulting in an average reduction of 0.21 ± 0.12 log10 eCFU/ml for all temperatures combined (Fig. 2A). A similar trend was observed when the conditions were applied to clinical sputum samples from TB patients. The control M. tuberculosis bacterial load was 7.10, reducing to 6.43 ± 0.76, 6.23 ± 0.12, and 6.20 ± 0.45 log10 eCFU/ml at 80°C, 85°C, and 95°C (ANOVA P < 0.0001), respectively. This resulted in measured bacterial load reductions of 0.67, 0.88, and 0.89 log10 eCFU/ml and a combined average reduction of 0.82 ± 0.12 log10 eCFU/ml (Fig. 2B).
FIG 2.
The effect of heat killing on bacterial load estimated by 16S rRNA as a marker. Bacterial load estimated from in vitro BCG cultures (A) and bacterial load estimated from tuberculosis-positive sputum samples (B). Error bars are standard error of the mean (n = 18 and 20 replicates for A and B, respectively).
Using Sidak’s multiple-comparison test, we asked whether the mean bacterial loads were different between the three heat inactivation temperatures. We found that the mean bacterial load of 4.91 log10 eCFU/ml for BCG cultures inactivated at 95°C was significantly smaller than those of 5.26 and 5.11 log10 eCFU/ml at 80°C and 85°C (P = 0.001), respectively. In contrast, there was no difference in the bacterial loads of M. tuberculosis samples at all of the three temperatures, P = 0.77.
Effect of heat-based sample inactivation on bacterial load measured by other RNA species: tmRNA, pre-16S rRNA, and rpoB.
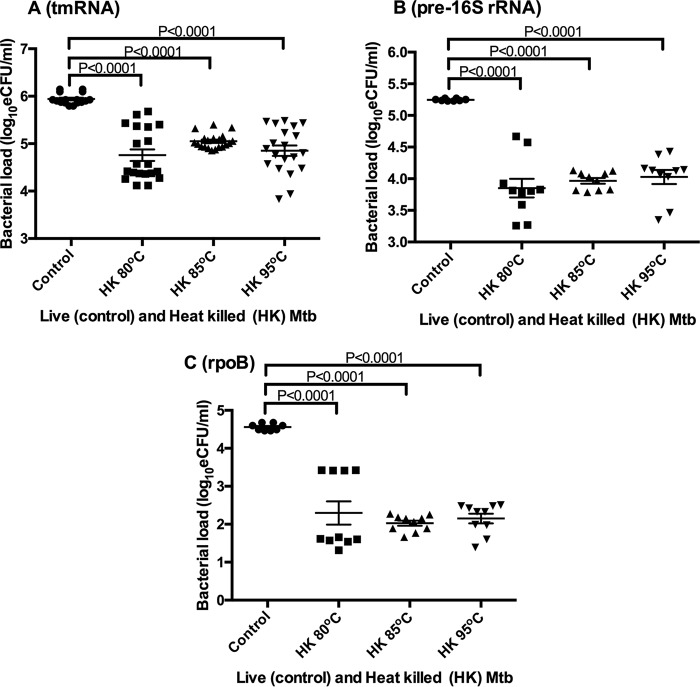
We explored what effect similar heat killing conditions had on other RNA species that are potential markers for quantifying tuberculosis bacterial load. The mean bacterial loads of the control were 5.94 ± 0.12, 5.25 ± 0.02, and 4.56 ± 0.09 log10 eCFU/ml for tmRNA, pre-16S rRNA, and rpoB, respectively. The bacterial loads at 80°C, 85°C, and 95°C were 4.76 ± 0.54, 5.05 ± 0.16, and 4.85 ± 0.59 log10 eCFU/ml, respectively, for tmRNA, 3.85 ± 0.47, 3.97 ± 0.14, and 4.03 ± 0.35 log10 eCFU/ml, respectively, for pre-16 rRNA, and 2.29 ± 0.09, 2.03 ± 0.22, and 2.15 ± 0.39 log10 eCFU/ml, respectively, for rpoB. Like 16S rRNA, the bacterial loads of the control and the heat-killed samples were significantly different (ANOVA P < 0.0001) for all three RNA species (Fig. 3A, B, and C). However, according to Sidak’s multiple-comparison test, the bacterial loads did not vary significantly between the different heat killing temperatures within each RNA species.
FIG 3.
The effect of heat killing on bacterial load estimated by non-16S rRNA RNA species as markers. Bacterial load measured by transfer-messenger RNA (tmRNA) (A), pre-16S rRNA (B), and RNA polymerase B (rpoB) (C). Error bars are standard error of the mean (n = replicates per RNA species per temperature). Mtb, M. tuberculosis.
The rate of bacterial load loss varies with RNA species.
While there was no difference in inter-RNA species’ bacterial load losses at different heat killing temperatures, these varied between the RNA species tested. 16S rRNA had the lowest loss in both pure BCG culture (0.21 ± 0.12 log10 eCFU/ml) and in TB sputum samples (0.82 ± 0.12 log10 eCFU/ml). The highest loss was observed with rpoB at 2.40 ± 0.13 log10 eCFU/ml in TB sputum samples. Transfer-messenger RNA and pre-16S rRNA were in between with losses of 1.05 ± 0.15 and 1.30 ± 0.09 log10 eCFU/ml, respectively (Fig. 4).
FIG 4.
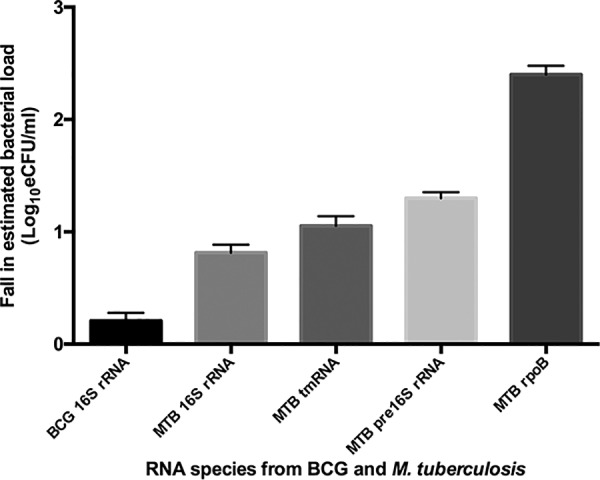
A plot of the RNA species specific average bacterial load loss following heat killing of cells at different temperatures. The lowest loss was in the 16S rRNA in BCG pure culture and the highest was in rpoB in sputum. BCG, BCG pure culture; MTB, M. tuberculosis in patient sputum samples. Errors are standard error of the mean.
DISCUSSION
We have shown that heat treatment for 20 min at 80°C, 85°C, and 95°C inactivates tuberculosis specimens effectively, making it possible for downstream molecular tests to occur without risk of infection. These findings concur with the conclusions of Doig et al. (21) and contrast with those of Zwadyk et al. (23) that temperatures less than 100°C cannot consistently kill M. tuberculosis. In line with the observations of Zwadyk et al., some studies have indicated that 80°C may not completely inactivate samples with a large bacterial load or high-density cultures of M. tuberculosis (21, 24–26). To remove the effect of high-density inoculum in our study, all sputum samples and pure cultures were heated at a 1-ml volume per 15-ml centrifuge tube, providing adequate space to expose every part of the sample to boiling.
Unlike the two studies that evaluated DNA as a molecular marker, our study has evaluated the preservation of RNA following heat inactivation. Our study adds evidence to studies which showed that RNA could be preserved following the heat inactivation of bacteria (12, 27). We show that the amounts of RNA preserved are sufficient for downstream qualitative and potentially for quantitative molecular tests like MBLA and other reverse transcriptase PCR diagnostics of bacterial pathogens based on the same principle. Modifications of the M. tuberculosis MBLA principle could be used to quantify the viable bacterial loads of different pathogens. Heat inactivation may obviate the need to use high-containment laboratories for RNA-based tests of category 3 bacterial pathogens.
By analyzing four RNA markers, 16S rRNA, tmRNA, pre-16S rRNA, and rpoB, we demonstrate that the amount of RNA preserved depends on the RNA species and is independent of temperature. Of the four RNA species, 16S rRNA was most resilient with <1 log bacterial load loss compared to that of rpoB, the most vulnerable with >2 logs of bacterial load loss.
The bacterial loads measured in the control (live) samples were consistently larger than those of the heat-inactivated samples. However, the loss did not increase with higher temperatures. This suggests that the loss is most likely not due to heat-induced RNA degradation, as this would in principle increase with increasing temperature. We hypothesize that heating at these temperatures lyses some of the cells, exposing RNA to RNases present in the sample, and the extent of degradation is dependent on how susceptible the RNA species are to these enzymes. The RNA species-dependent loss could be explained by the different susceptibilities that the species have to RNase and the amount of contaminant RNase present in the sample. For instance, it is notable that the rate of RNA loss in BCG cultures was lower than that in TB sputum samples for 16S rRNA. The lower degradation of 0.23 log10 eCFU/ml of 16S rRNA in BCG pure cultures could be explained by low concentrations of RNase in pure cultures compared to those in clinical sputum samples in which both host- and M. tuberculosis-generated RNases are most likely present (28, 29).
16S rRNA is a structural RNA constituting the smaller unit of prokaryotic ribosome, which potentially makes it less susceptible to RNase (30, 31). Furthermore, a single M. tuberculosis cell contains hundreds of ribosomes, ≈700/0.1 μm3 of cytoplasm (30), implying larger quantities of rRNA for which small quantities of RNase may have less impact (31). Transfer-messenger RNA is a combination of two RNA species, transfer and messenger, and has also been shown to have higher structural stability than mRNA (32). It is not clear what structural stability pre-16S rRNA has since it is a precursor (transition) molecule. Drawing on the results of the rate of RNA loss in this study, the structural stability of pre-16S rRNA could be between mRNA and tRNA. RNA polymerase B (rpoB), which is mRNA, is more susceptible. It was shown that M. tuberculosis mRNA has a half-life of 9 min at 37°C; however, when temperatures were reduced to 20°C, the half-life was significantly increased to more than 5 h (33). The 9-min rate of degradation at 37°C is expected since this is the temperature at which most physiological reactions take place. Likely, the reason that we have not seen fast degradation at temperature of 80°C and above is that they are not optimal temperatures for RNase function.
Heat inactivation has been used for centuries for many functions, including disinfection of medical devises and therapeutic preparations, aseptic inoculation and preparation smears for microscopy in microbiology laboratories, and in the pasteurization of milk. This means that heat inactivation as a technique already has a place in the clinical laboratory and can easily be deployed to processing samples for RNA-based tests. The current standard-of-care TB culture uses a decontamination step with NaOH to remove nonmycobacterial flora in sputum samples, but this unfortunately reduces the viable M. tuberculosis load by 1 to 2 logs (34, 35). The degree of loss that we show here with heat inactivation is less than that of NaOH-induced loss of viability, which places the impact of this procedure in context especially as the MBLA obviates the need for NaOH treatment of sputum samples.
This study has shown that the heat treatment of sputum samples renders them safe while preserving RNA for downstream laboratory tests. This potentially obviates the need for category 3 laboratories to manipulate TB specimens for molecular tests. Since the amount of RNA preserved is RNA species dependent, it is crucial for species-specific optimization to be conducted prior to adoption for routine application. RNA is more susceptible to degradation than DNA but could survive longer if not for the universally present and highly stable RNases (36). Future studies will explore the impact of heat inactivation on samples with a range of bacterial loads to understand the number that might change from positive to negative, i.e., those with fewer bacteria.
ACKNOWLEDGMENTS
This study was made possible by funding from the European and Developing Countries Clinical Trials Partnership (EDCTP) Pan African Biomarker Expansion program (PanBIOME) under grant SP.2011.41304.008. Support was also obtained from a University of St Andrews School of Medicine research grant.
We thank the Maputo Maternal hospital and Mavalane Health Centre, which provided the clinical sputum specimens.
REFERENCES
- 1.World Health Organization. 2017. END TB global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.World Health Organization. 2014. Towards TB elimination in low-incidence countries. An action framework for low incidence countries. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2010. Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.Friedrich SO, Rachow A, Saathoff E, Singh K, Mangu CD, Dawson R, Phillips PPJ, Venter A, Bateson A, Boehme CC, Heinrich N, Hunt RD, Boeree MJ, Zumla A, McHugh TD, Gillespie SH, Diacon AH, Hoelscher M. 2013. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 1:462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 7.Desjardin LE, Perkins MD, Wolski K, Haun S, Teixeira L, Chen Y, Johnson JL, Ellner JJ, Dietze R, Bates J, Cave MD, Eisenach KD. 1999. Measurement of sputum Mycobacterium tuberculosis messenger RNA as a surrogate for response to chemotherapy. Am J Respir Crit Care Med 160:203–210. doi: 10.1164/ajrccm.160.1.9811006. [DOI] [PubMed] [Google Scholar]
- 8.Hellyer TJ, DesJardin LE, Teixeira L, Perkins MD, Cave MD, Eisenach KD. 1999. Detection of viable Mycobacterium tuberculosis by reverse transcriptase-strand displacement amplification of mRNA. J Clin Microbiol 37:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honeyborne I, Mchugh TD, Phillips PPJ, Bannoo S, Bateson A, Carroll N, Perrin FM, Ronacher K, Wright L, Helden PD, Van Walzl G, Gillespie SH. 2011. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol 49:3905–3911. doi: 10.1128/JCM.00547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Mahan CS, Palaci M, Horter L, Loeffelholz L, Johnson JL, Dietze R, Debanne SM, Joloba ML, Okwera A, Boom WH, Eisenach KD. 2010. Sputum Mycobacterium tuberculosis mRNA as a marker of bacteriologic clearance in response to antituberculosis therapy. J Clin Microbiol 48:46–51. doi: 10.1128/JCM.01526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honeyborne I, Mtafya B, Phillips PPJ, Hoelscher M, Ntinginya EN, Kohlenberg A, Rachow A, Rojas-Ponce G, McHugh TD, Heinrich N. 2014. The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol 52:3064–3067. doi: 10.1128/JCM.01128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellyer TJ, DesJardin LE, Hehman GL, Cave MD. 1999. Quantitative analysis of mRNA as a marker for viability of Mycobacterium tuberculosis. J Clin Microbiol 37:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aellen S, Que Y, Guignard B, Haenni M, Moreillon P. 2006. Detection of live and antibiotic-killed bacteria by quantitative real-time PCR of specific fragments of rRNA. Antimicrob Agents Chemother 50:1913–1920. doi: 10.1128/AAC.00869-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deutscher MP. 2006. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res 34:659–666. doi: 10.1093/nar/gkj472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie SH, Sabiiti W, Oravcova K. 2017. Mybacterial load assay, p. 107–120. In Bishop-Lilly KA. (ed), Diagnostic bacteriology: methods and protocols. Humana Press, New York, NY. [Google Scholar]
- 16.Sheridan GEC, Masters CI, Shallcross JA, Mackey BM. 1998. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol 64:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juffs H, Deeth H. 2007. Scientific evaluation of pasteurisation for pathogen reduction in milk and milk products. Food Standards Australia Newsland, Wellington, New Zealand. [Google Scholar]
- 18.Holmes CJ, Degremont A, Kubey W, Straka P, Man NK. 2004. Effectiveness of various chemical disinfectants versus cleaning combined with heat disinfection on Pseudomonas biofilm in hemodialysis machines. Blood Purif 22:461–468. doi: 10.1159/000080791. [DOI] [PubMed] [Google Scholar]
- 19.Chedore P, Th’ng C, Nolan DH, Churchwell GM, Sieffert DE, Hale YM, Jamieson F. 2002. Method for inactivating and fixing unstained smear preparations of Mycobacterium tuberculosis for improved laboratory safety. J Clin Microbiol 40:4077–4080. doi: 10.1128/JCM.40.11.4077-4080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardoso CL, Giacomelli LR, Helbel C, Sant’Ana JJ, Martins FM, Barreto AM. 2001. Survival of tubercle bacilli in heat-fixed and stained sputum smears. Mem Inst Oswaldo Cruz 96:277–280. doi: 10.1590/S0074-02762001000200024. [DOI] [PubMed] [Google Scholar]
- 21.Doig C, Seagar AL, Watt B, Forbes KJ. 2002. The efficacy of the heat killing of Mycobacterium tuberculosis. J Clin Pathol 55:778–779. doi: 10.1136/jcp.55.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar VG, Urs TA, Ranganath RR. 2011. MPT 64 Antigen detection for Rapid confirmation of M. tuberculosis isolates. BMC Res Notes 4:79. doi: 10.1186/1756-0500-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwadyk P, Down JA, Myers N, Dey MS. 1994. Rendering of mycobacteria safe for molecular diagnostic studies and development of a lysis method for strand displacement amplification and PCR. J Clin Microbiol 32:2140–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackwood KS, Burdz TV, Turenne CY, Sharma MK, Kabani AM, Wolfe JN. 2005. Viability testing of material derived from Mycobacterium tuberculosis prior to removal from a containment level-III laboratory as part of a laboratory risk assessment program. BMC Infect Dis 5:4. doi: 10.1186/1471-2334-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somerville W, Thibert L, Schwartzman K, Behr MA. 2005. Extraction of Mycobacterium tuberculosis DNA: a question of containment. J Clin Microbiol 43:2996–2997. doi: 10.1128/JCM.43.6.2996-2997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bemer-Melchior P, Drugeon HB. 1999. Inactivation of Mycobacterium tuberculosis for DNA typing analysis. J Clin Microbiol 37:2350–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKillip JL, Jaykus LA, Drake M. 1998. rRNA stability in heat-killed and UV-irradiated enterotoxigenic Staphylococcus aureus and Escherichia coli O157:H7. Appl Environ Microbiol 64:4264–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blank A, Dekker CA. 1981. Ribonucleases of human serum, urine, cerebrospinal fluid, and leukocytes. Activity staining following electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. Biochemistry 20:2261–2267. doi: 10.1021/bi00511a030. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary TJ. 1999. Reducing the impact of endogenous ribonucleases on reverse transcription-PCR assay systems. Clin Chem 45:449–450. [PubMed] [Google Scholar]
- 30.Yamada H, Yamaguchi M, Chikamatsu K, Aono A, Mitarai S. 2015. Structome analysis of virulent Mycobacterium tuberculosis, which survives with only 700 ribosomes per 0.1 fl of cytoplasm. PLoS One 10:e0117109. doi: 10.1371/journal.pone.0117109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Chang JY, Cui Z, Li X, Meng R, Duan L, Thongchol J, Jakana J, Huwe CM, Sacchettini JC, Zhang J. 2017. Structural insights into species-specific features of the ribosome from the human pathogen Mycobacterium tuberculosis. Nucleic Acids Res 45:10884–10894. doi: 10.1093/nar/gkx785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huter P, Müller C, Arenz S, Beckert B, Wilson DN. 2017. Structural basis for ribosome rescue in bacteria. Trends Biochem Sci 42:669–680. doi: 10.1016/j.tibs.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Rustad TR, Minch KJ, Brabant W, Winkler JK, Reiss DJ, Baliga NS, Sherman DR. 2013. Global analysis of mRNA stability in Mycobacterium tuberculosis. Nucleic Acids Res 41:509–517. doi: 10.1093/nar/gks1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdz TVN, Wolfe J, Kabani A. 2003. Evaluation of sputum decontamination methods for Mycobacterium tuberculosis using viable colony counts and flow cytometry. Diagn Microbiol Infect Dis 47:503–509. doi: 10.1016/S0732-8893(03)00138-X. [DOI] [PubMed] [Google Scholar]
- 35.Yajko DM, Wagner C, Tevere VJ, Kocago T, Hadley WK, Chambers HF. 1995. Quantitative culture of Mycobacterium tuberculosis from clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J Clin Microbiol 33:1944–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto T, Okano S, Kasai N. 2009. Irreversible thermoinactivation of ribonuclease-A by soft-hydrothermal processing. Biotechnol Prog 25:1678–1685. doi: 10.1002/btpr.267. [DOI] [PubMed] [Google Scholar]