The global expansion of dengue viruses (DENV-1 to DENV-4) has contributed to the divergence, transmission, and establishment of genetic lineages of epidemiological concern; however, tracking the phylogenetic relationships of these virus is not always possible due to the inability of standardized sequencing procedures in resource-limited public health laboratories. Consequently, public genomic data banks contain inadequate representation of geographical regions and historical periods.
KEYWORDS: E gene, Sanger, dengue, dengue virus, genotyping, sequencing
ABSTRACT
The global expansion of dengue viruses (DENV-1 to DENV-4) has contributed to the divergence, transmission, and establishment of genetic lineages of epidemiological concern; however, tracking the phylogenetic relationships of these virus is not always possible due to the inability of standardized sequencing procedures in resource-limited public health laboratories. Consequently, public genomic data banks contain inadequate representation of geographical regions and historical periods. In order to improve detection of the DENV-1 to DENV-4 lineages, we report the development of a serotype-specific Sanger-based method standardized to sequence DENV-1 to DENV-4 directly from clinical samples using universal primers that detect most DENV genotypes. The resulting envelope protein coding sequences are analyzed for genotyping with phylogenetic methods. We evaluated the performance of this method by detecting, amplifying, and sequencing 54 contemporary DENV isolates, including 29 clinical samples, representing a variety of genotypes of epidemiological importance and global presence. All specimens were sequenced successfully and phylogenetic reconstructions resulted in the expected genotype classification. To further improve genomic surveillance in regions where dengue is endemic, this method was transferred to 16 public health laboratories in 13 Latin American countries, to date. Our objective is to provide an accessible method that facilitates the integration of genomics with dengue surveillance.
INTRODUCTION
Dengue virus, a member of the Flaviviridae RNA virus family, consists of four distinct serotypes (DENV-1, DENV-2, DENV-3, and DENV-4), all of which have been transmitted in more than 100 countries. Approximately 390 million infections have estimated to occur annually (1, 2). Over the last 4 decades, the range of endemic transmission has increased significantly, as dengue is frequently introduced into regions previously unexposed to the virus, often causing epidemics (3–5), and consequently placing approximately 40% of the world population at risk (1, 6). This global spread, in addition to the characteristic high rate of mutation by flavivirus polymerases, has contributed to important evolutionary events that affect disease severity and epidemiology, such as the divergence of subserotypic genotypes and taxonomic lineages. Many genotypes of epidemiological relevance have been associated with transmission in a particular geographic region (7–9), disease severity (10–12), or even increased epidemic potential (13–16). Recent outbreak investigations have reported molecular evidence suggesting that the introduction of genetically distinct virus strains may lead to the emergence of new lineages directly related to epidemic transmission (5, 17). In addition, phylogenetic and molecular epidemiological studies have identified the formation, expansion, decline, and extinction of genotypes and subgenotypic lineages during periods of endemic and epidemic dengue transmission (18–20); nevertheless, the drivers of these dynamics and their impact on the epidemiology of disease are not well understood.
Even after decades of studying dengue molecular epidemiology and the modern advances in genomics, the capacity remains inaccessible for many public health laboratories running dengue surveillance in regions of endemicity. Although there is a wide array of published DENV sequencing methods, significant limitations have impaired implementation, such as high variability between methods, cost, lack of standardization with clinical specimens, inadequate method evaluation, and high protocol complexity, which often includes amplification of the virus by tissue culture. Only a limited number of laboratories run some sort of genomic surveillance and have the capacity to track the local traffic of circulating dengue strains using a diversity of partial sequencing methods (21–25). However, this function is not frequently performed or applied to complement surveillance. This is reflected in the public repository of dengue genome sequences, GenBank, which currently holds an unbalanced collection of DENV genomic data that limits phylogenetic studies. Although the GenBank collection includes abundant DENV envelope glycoprotein coding sequence (E) data, as historically targeted for DENV genotyping, this collection is handicapped by the abundance of redundant sequences and the misrepresentation of certain time periods and endemic geographical locations (Virus Pathogen Resource, https://www.viprbrc.org/brc/home.spg?decorator=flavi_dengue).
To increase surveillance and research laboratory capacity and support genomic surveillance of DENV, we developed the CDC DENV E gene sequencing assay, a standardized, serotype-specific, Sanger-based method for genotyping analyses by sequencing the envelope glycoprotein coding sequence (E) directly from clinical serum specimens. We developed this assay for broad implementation in surveillance laboratories, including an open-platform protocol that incorporates simple, cost-effective, and traditional molecular biology techniques to facilitate adaptability. Our assay includes DNA primers specifically tailored and validated to detect a wide variety of genotypes of epidemiological relevance and contemporary circulation globally. The E protein coding sequence was selected because it provides a convenient target considering the high abundance of E sequences published in GenBank; in addition to reports that validate E for genotyping analyses, this fragment contains sufficient phylogenetic signal to distinguish DENV from other flaviviruses and between serotypes, genotypes, and individual strains (26–28). Furthermore, DENV phylogenetic tree reconstructions based on E coding sequence render tree topologies similar to those based on complete genome sequences facilitating genotyping at a significantly reduced cost and effort (8, 26, 29, 30).
This method aims to facilitate DENV genomic surveillance, improve genotyping, and foster molecular epidemiology by providing laboratories with a convenient, cost-effective, and highly efficient tool for sequencing DENV circulating globally directly from clinical specimens.
MATERIALS AND METHODS
Sequencing target selection and primer design.
The DENV E protein coding sequence was selected as the target for amplification and sequencing considering that the length of this sequence, approximately 1,485 bp, contains sufficient conserved sequence regions to differentiate DENV from other flaviviruses and sufficient variability to further classify between serotypes, genotypes, and individual strains (8, 31); it is thus a historically preferred genome fragment for genotyping. The E coding sequence has been the target for genotyping DENV, so more E coding sequences are available than for any other gene in GenBank. These E coding sequences allow for wider sequence sampling for assay design and phylogenetic analysis. All amplification and sequencing primers were designed from multiple sequence alignments of each serotype considering approximately 300 sequences obtained from GenBank and comparing complementarity with 70% and 90% consensus sequences. Each alignment included representation of all clinically relevant genotypes, global geographical representation, and sequences isolated between 1990 to 2017. Each alignment was revised to exclude extensive geotemporal redundancy and sylvatic lineages. Up to three noncontiguous mismatches were considered acceptable if sequence changes did not affect melting point temperatures (Tm) significantly; however, we recognize that in silico matches may not predict efficient primer binding accurately. The final primer sets for RT-PCR are listed in Table 1 , and the sequencing primers are listed in Table 2.
TABLE 1.
RT-PCR oligonucleotides for DENV E gene amplification
| Serotype and primer | Sequence |
|---|---|
| DENV-1 PCR-F | AGC ACA TGC YAT AGG AAC ATC C |
| DENV-1 PCR-R | GCT GAT CGA ATT CCA CAC ACR CC |
| DENV-2 PCR-F | TCG CTC CTT CAA TGA CAA TGC |
| DENV-2 PCR-R | CAG CTC ACA ACG CAA CCA CTA |
| DENV-3 PCR-F | TAG CCC TAT TTC TTG CCC ATT AC |
| DENV-3 PCR-R | AGT TCA TTG GCT ATT TGC TTC CA |
| DENV-4 PCR-F | AAC AGG RAT CCA GCG AAC TGT |
| DENV-4 PCR-R | CTC GCT GGG GAC TCT GGT TG |
TABLE 2.
DENV E gene sequencing primers
| Serotype and primer | Sequence |
|---|---|
| DENV-1 | |
| D1SEQ1 | GCY ATA GGA ACA TCC ATC ACY CAG |
| D1SEQ2 | ACG TGT GCY AAG TTY AAG TGT GT |
| D1SEQ3 | AAG ACA GCT CAT GCA AAG AAR CAG |
| D1SEQ4 | TTG GTG AGA GYT ACA TCG TGR TAG |
| D1SEQ5 | GCT GAT CGA ATT CCA CAC ACR C |
| D1SEQ6 | CTG GTG TAC CAA TTT TCC CAC AG |
| D1SEQ7 | TGG GTC TCA GCC ACT TCY TTC T |
| D1SEQ8 | AGC CCT GTT CTA GGT GAG CAA TC |
| DENV-2 | |
| D2SEQ1 | TYG CTC CTT CAA TGA CAA TGC G |
| D2SEQ2 | ACA TGC AAA AAG AAC ATG GAA GGA |
| D2SEQ3 | AAT CCC CAY GCV AAG AAA CAG GAT |
| D2SEQ4 | CCA TTC GGR GAC AGC TAC ATC AT |
| D2SEQ5 | CAG CTC ACA ACG CAA CCA CTA |
| D2SEQ6 | TGA TGA TGT AGC TGT CTC CGA ATG |
| D2SEQ7 | CTG TGA GTG CCG TGT GCA TG |
| D2SEQ8 | CAA RTT TTC TGG TTG CAC GAC T |
| DENV-3 | |
| D3SEQ1 | AGG CAC TTC CTT GAC CCA GAA |
| D3SEQ2 | TGG CAA GGG AAG CTT GGT AA |
| D3SEQ3 | AAC AGG AAG GAG CTT CTT GTG ACA |
| D3SEQ4 | GAG CCT GTC AAT ATT GAG GCT GA |
| D3SEQ5 | TGT CAT GGA TGG GGT GAC CA |
| D3SEQ6 | TCC CTC TAT TGG TTC CAG GCA |
| D3SEQ7 | CTT GCG ATC CAA GGA CAA CTA CT |
| D3SEQ8 | GCG TTG TCT CCA ATT CCA ATY ACT |
| DENV-4 | |
| D4SEQ1 | TGT CCT AAT GAT GCT GGT CGC |
| D4SEQ2 | GAG GAG TTG TGA CAT GTG C |
| D4SEQ3 | ATG GTG ACA TTT AAG GTT CCT CAT |
| D4SEQ4 | GCT GAG AAT ACC AAC AGT GTA ACC A |
| D4SEQ5 | CTC GCT GGG GAC TCT GGT TG |
| D4SEQ6 | GCC ATD CGY TTT GCA CCT CT |
| D4SEQ7 | TTG AGA TGT CCT GCA AAC ATG T |
| D4SEQ8 | ATG GTG ACC TGG GRG TTA TCG TC |
Viral strains and clinical samples.
A panel of 25 presequenced DENV international isolates was obtained from the Centers for Disease Control and Prevention (CDC) Dengue Branch archived collection (Table 3). These 25 strains represent the four serotypes and genotypes of contemporary circulation globally. Sylvatic strains were not included due to the sporadic clinical relevance and the high sequence divergence from the urban-endemic strains, which results in an average of four mismatches per reverse transcription-PCR (RT-PCR) or sequencing primer and reduces the probability of amplification or sequencing. Each isolate was amplified by tissue culture on Aedes albopictus-derived C6/36 cells, as previously described (32). In addition, a panel of 29 clinical serum specimens RT-PCR positive for all DENV serotypes was obtained from the reference specimen archive of the CDC Dengue Branch collected as part of the island-wide passive surveillance system in collaboration with the Puerto Rico Department of Health and from a dengue outbreak in American Samoa investigated by the CDC Dengue Branch in 2017 (Table 4). The CDC Institutional Review Board (IRB) authorized the use of deidentified samples for this study, protocol 6874. Serotypes and the relative RNA concentration (threshold cycle [CT] ≤ 30) were confirmed by real-time RT-PCR (33).
TABLE 3.
Panel of sequenced virus isolates
| Serotype | Strain | Genotype | GenBank no. | Contig size (bp)a |
|---|---|---|---|---|
| DENV-1 | BR/DB001/2003 | American-African | JF804014 | 1,667 |
| MX/DB006/2007 | American-African | JF804019 | 1,726 | |
| PH/DB007/2004 | South Pacific | JF804020 | 1,729 | |
| PR/DB008/1998 | American-African | JF804021 | 1,622 | |
| TH/DB047/2006 | Asian | JF812097 | 1,738 | |
| TI/DB011/2001 | South Pacific | JF804024 | 1,732 | |
| DENV-2 | BR/DB015/2006 | American-Asian | JF804028 | 1,529 |
| CI/DB016/2007 | American-Asian | JF804029 | 1,504 | |
| VN/DB014/1988 | Asian I | JF804027 | 1,499 | |
| DR/DB018/2003 | American-Asian | JF804031 | 1,521 | |
| CR/DB017/2003 | American-Asian | JF804030 | 1,503 | |
| IQT2133 | American | AY577439 | 1,525 | |
| 1349 | Cosmopolitan | EU056810 | 1,522 | |
| GU/DB019/2001 | Asian II | JF804032 | 1,529 | |
| IN/DB020/2006 | Cosmopolitan | JF804033 | 1,531 | |
| DENV-3 | BR/DB028/2003 | Indian | JF804041 | 1,780 |
| SA/DB028/1995 | South Pacific | JF804049 | 1,777 | |
| TH/DB055/2006 | Thailand | JF812105 | 1,777 | |
| EC/DB032/2000 | Indian | JF804045 | 1,775 | |
| CK/DB030/1991 | South Pacific | JF804043 | 1,772 | |
| DENV-4 | CI/DB039/2006 | Indonesian | JF804052 | 1,648 |
| MX/DB042/2006 | Indonesian | JF804055 | 1,647 | |
| SC/DB045/1994 | Indonesian | JF804058 | 1,666 | |
| EC/DB041/1999 | Indonesian | JF804054 | 1,616 | |
| TH/DB060/2006 | Southeast Asian | JF812110 | 1,625 |
Contig size was determined using SeqMan Pro (DNASTAR Lasergene 14).
TABLE 4.
Panel of clinical specimens sequenced
| Serotype | Sequence label | Origin | Collection date (yr) | GenBank no. |
|---|---|---|---|---|
| DENV-1 | US/DB224/2013 | Puerto Rico | 2013 | MK039554 |
| US/DB225/2013 | Puerto Rico | 2013 | MK039555 | |
| DENV-2 | US/DB226/2009 | Puerto Rico | 2009 | MK040384 |
| US/DB227/2009 | Puerto Rico | 2009 | MK040385 | |
| US/DB228/2009 | Puerto Rico | 2009 | MK040386 | |
| AS/DB243/2017 | American Samoa | 2017 | MK244386 | |
| AS/DB244/2017 | American Samoa | 2017 | MK244387 | |
| AS/DB245/2017 | American Samoa | 2017 | MK244388 | |
| AS/DB246/2017 | American Samoa | 2017 | MK244389 | |
| AS/DB247/2017 | American Samoa | 2017 | MK244390 | |
| AS/DB248/2017 | American Samoa | 2017 | MK244391 | |
| AS/DB249/2017 | American Samoa | 2017 | MK244392 | |
| AS/DB250/2017 | American Samoa | 2017 | MK244393 | |
| AS/DB251/2017 | American Samoa | 2017 | MK244394 | |
| AS/DB252/2017 | American Samoa | 2017 | MK244395 | |
| DENV-3 | US/DB229/2003 | Puerto Rico | 2003 | MK040387 |
| US/DB230/2003 | Puerto Rico | 2003 | MK040388 | |
| US/DB231/2003 | Puerto Rico | 2003 | MK040389 | |
| US/DB232/2003 | Puerto Rico | 2003 | MK040390 | |
| US/DB233/2003 | Puerto Rico | 2003 | MK040391 | |
| US/DB234/2003 | Puerto Rico | 2003 | MK040392 | |
| US/DB235/2003 | Puerto Rico | 2003 | MK040393 | |
| US/DB236/2003 | Puerto Rico | 2003 | MK040394 | |
| US/DB237/2003 | Puerto Rico | 2003 | MK040395 | |
| US/DB238/2003 | Puerto Rico | 2003 | MK040396 | |
| DENV-4 | US/DB239/2014 | Puerto Rico | 2014 | MK040397 |
| US/DB240/2014 | Puerto Rico | 2014 | MK040398 | |
| US/DB241/2014 | Puerto Rico | 2014 | MK040399 | |
| US/DB242/2014 | Puerto Rico | 2014 | MK040400 |
Viral RNA extraction.
For our evaluation of the CDC DENV E gene sequencing assay, we extracted viral RNA from clinical specimens (serum) or tissue culture supernatant using a QIAamp viral RNA minikit (Qiagen, catalog no. 52904) or other automated method, including the MagNA Pure LC 2.0 instrument (Roche, catalog no. 05197686001) or the MagNA Pure 96 instrument (Roche, catalog no. 05195322001), according to the manufacturer’s recommended protocols.
E coding sequence amplification.
E coding sequence was amplified by endpoint RT-PCR using a SuperScript III Platinum one-step RT-PCR system (Invitrogen, catalog no. 12574026) and one set of serotype-specific amplification primers (Table 1). These primers were designed to detect DENV genotypes of contemporary circulation globally and amplify the complete E coding sequence flanked by a small portion of the premembrane (preM) and nonstructural protein 1 (NS1) coding sequences. RT-PCRs were assembled by mixing 13.0 µl of nuclease-free PCR-grade water, 25.0 µl of 2× SuperScript reaction mix, 20.0 µM concentrations of each DENV forward and reverse primer, 2.0 µl of SuperScript III RT/platinum Taq mix, and 8.0 µl of template RNA elution to a final reaction volume of 50.0 µl. RT-PCR analysis was performed in a conventional thermal cycler (SimpliAmp; Thermo Fisher) programmed as follows: reverse transcription (RT) at 55°C for 30 min and 94°C for 2 min; followed by 40 cycles of 94°C for 15 s, 55°C for 30 s, and 68°C for 2 min; and then a final extension at 68°C for 5 min. RT-PCR products were detected and visualized by agarose gel electrophoresis. A 0.8% UltraPure agarose gel (Invitrogen, catalog no. 16500500) was prepared in 1× Bionic buffer (Sigma-Aldrich, catalog no. B6186-4L) stained with SYBR Safe DNA stain (Invitrogen, catalog no. S33102) with wells sufficiently thick to contain the complete RT-PCR and loading buffer (approximately 55.0 µl). A 1-kb ladder DNA marker was used to determine the amplicon size. The RT-PCR amplicon length will vary by serotype: approximately 1,700 bp for DENV-1, 1,500 bp for DENV-2, 1,800 bp for DENV-3, and 1,600 bp for DENV-4. This RT-PCR procedure was tested with serial dilutions of reference strains of the four DENV serotypes and dilutions with a real-time RT-PCR CT value of <30 produced a band with sufficient DNA for successful sequencing (33). Reagents for RT-PCR and electrophoresis can be substituted for other compatible reagents (with the exception of the primers) of similar performance, if available.
RT-PCR amplicon purification.
RT-PCR amplicon bands should be of the expected size with a brightness equal to or greater than the bands in the 1-kb DNA ladder marker. The amplicon bands were excised with a clean scalpel under low-intensity UV light, eliminating as much agarose as possible. The excised slice of gel was placed in a 1.7-ml nuclease-free microcentrifuge tube. DNA from the gel was extracted and purified using a QIAquick gel extraction kit (Qiagen, catalog no. 28706) with the following modifications to the manufacturer’s recommended protocol: the microcentrifuge tube containing the gel slice was filled to capacity with QG buffer and incubated at 50°C for 10 min with frequent vortexing until the gel was completely melted and dissolved in the buffer. Isopropanol was not added to the tube. Half of the contents of the tube was transferred to the Qiagen spin columns by pipetting, and the columns were centrifuged for 1 min at 14,000 rpm. The flowthrough was discarded, and the previous step was repeated until the entire volume of the samples passed through the column. The columns were then washed with 800 µl of QG buffer and centrifuged for 1 min at 14,000 rpm. The manufacturer’s recommended protocol continues at this point. Alternatively, the amplicon can be purified using enzymatic methods such as ExoSAP-IT PCR product cleanup reagent (Thermo-Fisher, catalog no. 78201.1.ML). The purified products were quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific).
Sanger sequencing.
Sequencing reactions were assembled using a BigDye Terminator v3.1 cycle sequencing kit (Thermo-Fisher, catalog no. 4337455). Optimal purified product input was determined experimentally to range between 40 and 60 ng/reaction. Eight sequencing reactions were performed per sample using serotype-specific primer sets: four primers that generate tiled, overlapping sequences from 5′ to 3′ and four primers that generate overlapping sequence from 3′ to 5′ for a minimum 2× coverage across the E coding sequence (Table 2). Sequencing reactions were assembled according to the manufacturer’s recommended protocol: 8.0 µl of BigDye Terminator 3.1 Ready Reaction mix, a 3.3 µM concentration of the corresponding single sequencing primer, 60.0 ng of purified DNA product and nuclease-free PCR-grade water to complete a final reaction volume of 20.0 µl. Sequencing reactions were placed on a conventional thermocycler programmed with the following protocol: denaturation at 96°C for 1 min, followed by 25 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. The ramp rate was adjusted to 1°C/s at each step. Variations of the protocol for the BigDye sequencing reactions should be evaluated and adjusted for optimal results considering the sequencing instrument available and laboratory experience. Variations of the protocol may include dilution of the BigDye Terminator 3.1 Ready Reaction mix with 5× sequencing buffer and DNA input. BigDye reactions were purified using a BigDye XTerminator purification kit (Thermo-Fisher, catalog no. 4376484) to remove unincorporated dyes following the manufacturer’s recommended protocol. Alternatively, sequencing reactions can be purified by spin column methods like DyeEx 2.0 spin kit (Qiagen, catalog no. 63204). Sequencing was performed on an ABI 3130 XL genetic analyzer (Thermo-Fisher, catalog no. 4359571).
Trace sequence analysis and assembly.
Sequence trace files were analyzed individually using BioEdit software (www.mbio.ncsu.edu/BioEdit/bioedit.html) or Chromas 2.6.5 software (Technelysium). A minimum of 400 bp of high-quality sequence (determined by size of fluorescence signal and clarity of the peak) are expected per trace file. Contig assembly was performed with the eight trace files per sample using the SeqMan Pro program found in the DNASTAR Lasergene 14 software package. The software reports the number of contigs assembled and the number of trace files used to assemble the contigs and displays the trace file alignment to assemble the contig consensus sequence. Initially, we confirm that we obtained a minimum of 2× coverage across the length of the E coding sequence, followed by a base-by-base curation of the sequence data to guarantee sequence quality. If an ambiguous base is called in the contig consensus sequence, the trace files are analyzed at the specific sequence position to determine the correct base. Once the contig is assembled and the sequence is curated, the consensus sequence is analyzed by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm sequence identity and retrieve similar sequences. The top 5 to 10 sequences from the BLAST results were selected and aligned with our sample consensus sequence using Clustal W or MUSCLE in MEGA 7 software (www.megasoftware.net) to determine consensus sequence orientation and trim sequence data flanking the E coding sequence. This step also serves to identify additional sequences of high similarity that can be used in downstream phylogenetic analyses and provides a general idea of the genotype, as well as the geographic-temporal origin of the query sequence. The resulting DNA sequence was translated to amino acid sequence and compared to similar annotated amino acid sequences retrieved from GenBank. We also confirmed the absence of internal stop codons that might have been introduced by sequencing errors. There are numerous software packages that perform these functions, and we defer software selection to the user considering availability and computational capacity.
Phylogenetic analysis.
The sequences obtained with this method were grouped by serotype and aligned with other genotype reference sequences obtained from GenBank (see Table S1 in the supplemental material) using the MUSCLE module in MEGA 7. The multiple sequence alignments were trimmed to the length of the shortest sequence (1,480 to 1,485 bp). Phylogenetic relationships were inferred using the maximum likelihood method based on the Tamura-Nei model and topologies tested with 1,000 bootstrap replicates using IQ-TREE software (http://www.iqtree.org/). Genotypes are marked by brackets and labeled accordingly (7).
Data availability.
Sequence data were deposited in GenBank under the accession numbers listed in Table 4.
RESULTS
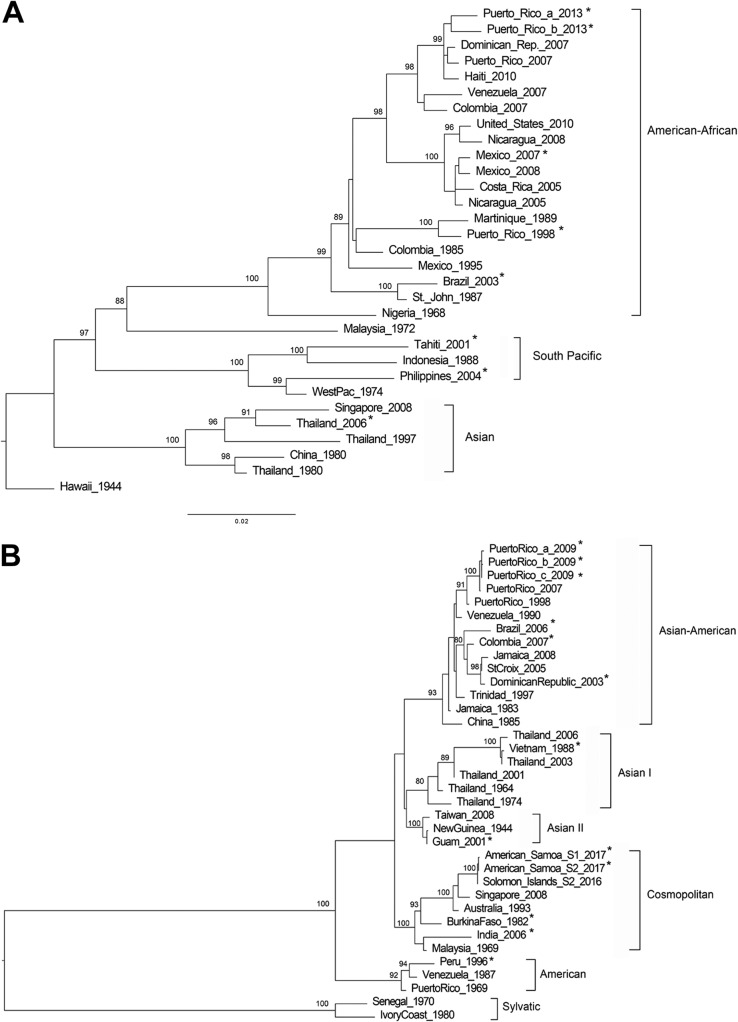
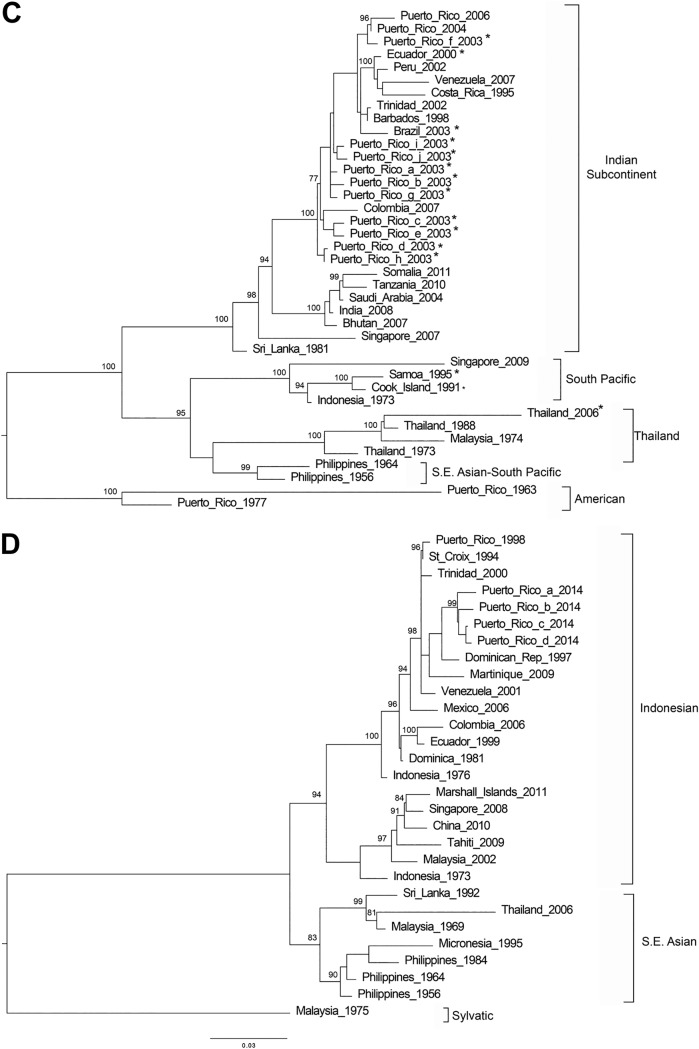
DENV E coding sequence amplification was successful in the 54 clinical serum and tissue culture samples evaluated (Tables 3 and 4). Amplicons containing E coding sequence flanked by preM and NS1 coding sequence fragments were sequenced with the CDC DENV E gene sequencing assay. The assay produced the eight expected sequence trace files with a minimum of 400 bp of high-quality data for 29 of 29 clinical samples and 24 of the 25 virus isolates, which assembled single contigs of more than 1,500 bp with a minimum of 2× coverage across the E coding sequence segment (Table 3). The overlapping sequencing reactions and the bidirectional sequencing strategy increased the probability of covering the coding sequence with high-quality sequence data and facilitated base-to-base calling. For DENV-4 isolate Ecuador 1999, GenBank accession number JF804054, the assay produced seven of the eight sequence trace files with the expected minimal size of 400 bp in length; however, a single contig of 1,438 bp was assembled with 2× coverage across 80% of the E coding sequence fragment. A multiple sequence alignment with 10 similar sequences showed that the missing sequence data were at the 3′ end of the E coding sequence. To address this issue, we resequenced this isolate and succeeded obtaining all eight sequence trace files assembling a contig of 1,616 bp. A highly conserved region of the E coding sequence across similar sequences was evaluated. Maximum-likelihood phylogenetic trees were inferred for each DENV serotype using the E coding sequences obtained from this method in addition to other E coding sequences considered as references for each genotype (Fig. 1). Each of the 54 E coding sequences obtained grouped with the expected genotypes and relatedness to genotype reference sequences were supported by bootstrap scores of higher than 75%. In addition, our genotyping results concurred with the results obtained from a recently developed web-based online Genome Detective Dengue Virus Typing Tool for dengue virus genotyping (https://www.genomedetective.com/app/typingtool/dengue/introduction) which aligns any DENV-1 to DENV-4 sequence with preselected genotype reference sequences and outputs a phylogenetic tree.
FIG 1.
Genotyping DENV using E gene sequence. Phylogenetic reconstruction was used to determine DENV genotypes by the maximum-likelihood method based on the Tamura-Nei model of nucleotide substitution. (A) DENV-1; (B) DENV-2; (C) DENV-3; (D) DENV-4. Asterisk-labeled taxa indicate sequences obtained by this method. Due to the sequence similarity and space limitations, only two of the ten DENV-2 American Samoa 2017 sequences were included in the maximum-likelihood tree.
DISCUSSION
The transmission of DENV continues to expand globally, increasing the number of regions exposed and people at risk of infection. Real-time monitoring of transmission and epidemics is necessary to forecast further virus spread, which will impact the design of preventive interventions and treatment measures. Several studies have shown how, occasionally, foreign virus introductions or even endemic transmission can generate dengue outbreaks regardless of previous exposure to the virus (5, 17, 34). This supports the importance of genomic surveillance to understand the epidemiology of dengue in a region. The CDC DENV E gene sequencing assay was developed to provide a simple and convenient standardized tool for DENV partial sequencing, for genotyping, and to harmonize genomic surveillance of dengue viruses. Such a tool will be readily adaptable to public health laboratories and provide the opportunity to monitor genetic changes associated with circulating genotypes or other epidemiological factors. The selection of the E coding sequence as sequencing target provides sufficient data to perform phylogenetic inferences for genotyping and lineage classification with tree topologies that closely resemble those obtained from complete genome sequences (7, 26, 35).
This assay was developed to sequence directly from diagnostic serum specimens using a simple approach to amplify the target region and obtain reliable sequence data taking advantage of the Sanger sequencing method. Despite the advantages that next-generation sequencing technologies offer, the Sanger method is more accessible to laboratories in regions where dengue is endemic and provides a nimble strategy to process smaller numbers of samples with small genomes with high accuracy. Our evaluation of this assay demonstrated the capacity to sequence a wide variety of international DENV genotypes within each serotype and with wide sequence variation. Only on one occasion was a DENV-4 isolate not sequenced entirely, with a contig formed by seven sequence trace files, and we speculate that performance of the eighth sequencing reaction could have been affected by insufficient loading of amplicon product into the reaction. However, this was corrected by resequencing the isolate with the proper concentration of DNA template. A simple genomic analysis demonstrated that the sequences obtained by this method readily apply to phylogenetic studies relevant to molecular and genomic surveillance such as genotype detection. Although whole-genome sequences are more suitable for evolutionary studies and provide higher phylogenetic resolution for lineage classification, the E coding region has been a more popular target historically; hence, there is a larger availability and representation in public databases. Consequently, the accuracy of all phylogenetic analyses based on this or any other sequencing method is restricted by the sequence sampling and availability in public domain. Because all national laboratories in the Americas are currently running the same RT-PCR procedures for DENV-1 to DENV-4 detection (33), the RNA extracts already obtained can be readily sequenced using this procedure. Therefore, we have deployed this procedure in collaboration with the Pan American Health Organization, the National Health Institute of Surveillance and Reference in Mexico, and the Institute Fiocruz in Brazil. These efforts have made it possible to incorporate genomic surveillance components in 16 public health laboratories in 13 countries in the region.
The success rate of this assay when used directly on diagnostic serum specimens is limited by the sample quality, i.e., maintenance of the cold chain, the time of specimen collection (i.e., acute stage 0 to 5 days after onset of symptoms), and the viral RNA concentration (i.e., CT < 30 determined by real-time RT-PCR, although a low CT value may not reflect the integrity of the RNA genome molecule). Further in silico analyses of the primer sequences showed several mismatches with modern sequences of Asian origin. To address this, we offer a list of the primers with IUPAC nucleic acid codes in positions where mismatches were detected (Table S2); however, due to the unavailability of Asian dengue virus isolates in our laboratory, we were unable to validate these primer sequences with PCR testing. For further evolutionary and molecular epidemiological studies, we recommend methods that provide complete genome sequence.
In conclusion, surveillance and research laboratories can use this method to collect dengue genomic information to strengthen the understanding of DENV genotypes, diversity, and transmission dynamics and to improve disease prevention strategies.
Supplementary Material
ACKNOWLEDGMENT
G.A.S. is a research scientist at the Centers for Disease Control and Prevention, Dengue Branch, San Juan, Puerto Rico. His research is focused on the development of molecular diagnostic tests and the study of the molecular epidemiology of arboviruses.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01957-18.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. 2006. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 277:3–16. discussion 16-22, 71-3, 251-3. [DOI] [PubMed] [Google Scholar]
- 3.Leduc-Galindo D, Rincón-Herrera U, Ramos-Jiménez J, Garcia-Luna S, Arellanos-Soto D, Mendoza-Tavera N, Tavitas-Aguilar I, Garcia-Garcia E, Galindo-Galindo E, Villarreal-Perez J, Fernandez-Salas I, Santiago GA, Muñoz-Jordan J, Rivas-Estilla AM. 2015. Characterization of the dengue outbreak in Nuevo Leon state, Mexico, 2010. Infection 43:201–206. doi: 10.1007/s15010-014-0700-7. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DL, Santiago GA, Abeyta R, Hinojosa S, Torres-Velasquez B, Adam JK, Evert N, Caraballo E, Hunsperger E, Munoz-Jordan JL, Smith B, Banicki A, Tomashek KM, Gaul L, Sharp TM. 2016. Reemergence of dengue in southern Texas, 2013. Emerg Infect Dis 22:1002–1007. doi: 10.3201/eid2206.152000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz-Jordan JL, Santiago GA, Margolis H, Stark L. 2013. Genetic relatedness of dengue viruses in Key West, Florida, USA, 2009-2010. Emerg Infect Dis 19:652–654. doi: 10.3201/eid1904.121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rico-Hesse R. 2003. Microevolution and virulence of dengue viruses. Adv Virus Res 59:315–341. doi: 10.1016/S0065-3527(03)59009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes EC, Twiddy SS. 2003. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 3:19–28. doi: 10.1016/S1567-1348(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 9.Rico-Hesse R. 1990. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology 174:479–493. doi: 10.1016/0042-6822(90)90102-W. [DOI] [PubMed] [Google Scholar]
- 10.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de C, Ramos C, Rico-Hesse R. 1999. Dengue virus structural differences that correlate with pathogenesis. J Virol 73:4738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cologna R, Armstrong PM, Rico-Hesse R. 2005. Selection for virulent dengue viruses occurs in humans and mosquitoes. J Virol 79:853–859. doi: 10.1128/JVI.79.2.853-859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OhAinle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborio S, Nunez A, Lennon NJ, Birren BW, Gordon A, Henn MR, Harris E. 2011. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 3:114ra128. doi: 10.1126/scitranslmed.3003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong PM, Rico-Hesse R. 2001. Differential susceptibility of Aedes aegypti to infection by the American and Southeast Asian genotypes of dengue type 2 virus. Vector Borne Zoonotic Dis 1:159–168. doi: 10.1089/153036601316977769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JR, Rico-Hesse R. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am J Trop Med Hyg 75:886–892. doi: 10.4269/ajtmh.2006.75.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villabona-Arenas CJ, Zanotto PM. 2013. Worldwide spread of dengue virus type 1. PLoS One 8:e62649. doi: 10.1371/journal.pone.0062649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manokaran G, Finol E, Wang C, Gunaratne J, Bahl J, Ong EZ, Tan HC, Sessions OM, Ward AM, Gubler DJ, Harris E, Garcia-Blanco MA, Ooi EE. 2015. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 350:217–221. doi: 10.1126/science.aab3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Melo FL, Romano CM, de Andrade Zanotto PM. 2009. Introduction of dengue virus 4 (DENV-4) genotype I into Brazil from Asia? PLoS Negl Trop Dis 3:e390. doi: 10.1371/journal.pntd.0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElroy KL, Santiago GA, Lennon NJ, Birren BW, Henn MR, Munoz JJL. 2011. Endurance, refuge, and reemergence of dengue virus type 2, Puerto Rico, 1986–2007. Emerg Infect Dis 17:64–71. doi: 10.3201/eid1701.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiago GA, McElroy-Horne K, Lennon NJ, Santiago LM, Birren BW, Henn MR, Muñoz-Jordán JL. 2012. Reemergence and decline of dengue virus serotype 3 in Puerto Rico. J Infect Dis 206:893–901. doi: 10.1093/infdis/jis426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carneiro AR, Cruz ACR, Vallinoto M, Melo D. d V, Ramos RTJ, Medeiros DBA, Silva E. V P d, Vasconcelos P. F d C. 2012. Molecular characterization of dengue virus type 1 reveals lineage replacement during circulation in Brazilian territory. Mem Inst Oswaldo Cruz 107:805–812. doi: 10.1590/S0074-02762012000600016. [DOI] [PubMed] [Google Scholar]
- 21.Dash PK, Sharma S, Soni M, Agarwal A, Parida M, Rao PV. 2013. Complete genome sequencing and evolutionary analysis of Indian isolates of dengue virus type 2. Biochem Biophys Res Commun 436:478–485. doi: 10.1016/j.bbrc.2013.05.130. [DOI] [PubMed] [Google Scholar]
- 22.Koo C, Nasir A, Hapuarachchi HC, Lee KS, Hasan Z, Ng LC, Khan E. 2013. Evolution and heterogeneity of multiple serotypes of dengue virus in Pakistan, 2006–2011. Virol J 10:275. doi: 10.1186/1743-422X-10-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar SR, Patil JA, Cecilia D, Cherian SS, Barde PV, Walimbe AM, Yadav PD, Yergolkar PN, Shah PS, Padbidri VS, Mishra AC, Mourya DT. 2010. Evolution, dispersal, and replacement of American genotype dengue type 2 viruses in India (1956–2005): selection pressure and molecular clock analyses. J Gen Virol 91:707–720. doi: 10.1099/vir.0.017954-0. [DOI] [PubMed] [Google Scholar]
- 24.Patil JA, Cherian S, Walimbe AM, Patil BR, Sathe PS, Shah PS, Cecilia D. 2011. Evolutionary dynamics of the American African genotype of dengue type 1 virus in India (1962–2005). Infect Genet Evol 11:1443–1448. doi: 10.1016/j.meegid.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Patil JA, Cherian S, Walimbe AM, Bhagat A, Vallentyne J, Kakade M, Shah PS, Cecilia D. 2012. Influence of evolutionary events on the Indian subcontinent on the phylogeography of dengue type 3 and 4 viruses. Infect Genet Evol 12:1759–1769. doi: 10.1016/j.meegid.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Koo C, Tien WP, Xu H, Ong J, Rajarethinam J, Lai YL, Ng LC, Hapuarachchi HC. 2018. Highly selective transmission success of dengue virus type 1 lineages in a dynamic virus population: an evolutionary and fitness perspective. iScience 6:38–51. doi: 10.1016/j.isci.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allicock OM, Lemey P, Tatem AJ, Pybus OG, Bennett SN, Mueller BA, Suchard MA, Foster JE, Rambaut A, Carrington CV. 2012. Phylogeography and population dynamics of dengue viruses in the Americas. Mol Biol Evol 29:1533–1543. doi: 10.1093/molbev/msr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett SN, Holmes EC, Chirivella M, Rodriguez DM, Beltran M, Vorndam V, Gubler DJ, McMillan WO. 2006. Molecular evolution of dengue 2 virus in Puerto Rico: positive selection in the viral envelope accompanies clade reintroduction. J Gen Virol 87:885–893. doi: 10.1099/vir.0.81309-0. [DOI] [PubMed] [Google Scholar]
- 29.Klungthong C, Putnak R, Mammen MP, Li T, Zhang C. 2008. Molecular genotyping of dengue viruses by phylogenetic analysis of the sequences of individual genes. J Virol Methods 154:175–181. doi: 10.1016/j.jviromet.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Zhang Y, Hamoudi R, Yan G, Chen X, Zhou Y. 2014. Spatiotemporal characterizations of dengue virus in mainland China: insights into the whole genome from 1978 to 2011. PLoS One 9:e87630. doi: 10.1371/journal.pone.0087630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twiddy SS, Holmes EC, Rambaut A. 2003. Inferring the rate and time-scale of dengue virus evolution. Mol Biol Evol 20:122–129. doi: 10.1093/molbev/msg010. [DOI] [PubMed] [Google Scholar]
- 32.Medina F, Medina JF, Colon C, Vergne E, Santiago GA, Munoz-Jordan JL. 2012. Dengue virus: isolation, propagation, quantification, and storage. Curr Protoc Microbiol Chapter 15:Unit 15D.2. [DOI] [PubMed] [Google Scholar]
- 33.Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, Medina F, Colon C, Margolis H, Munoz JJL. 2013. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis 7:e2311. doi: 10.1371/journal.pntd.0002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villabona-Arenas CJ, Miranda-Esquivel DR, Jimenez RE. 2009. Phylogeny of dengue virus type 3 circulating in Colombia between 2001 and 2007. Trop Med Int Health 14:1241–1250. doi: 10.1111/j.1365-3156.2009.02339.x. [DOI] [PubMed] [Google Scholar]
- 35.Weaver SC, Vasilakis N. 2009. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol 9:523–540. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data were deposited in GenBank under the accession numbers listed in Table 4.