Abstract
Obesity is a risk factor for breast cancer and also predicts poor clinical outcomes regardless of menopausal status. Contributing to the poor clinical outcomes is the suboptimal efficacy of standard therapies due to dose limiting toxicities and obesity related complications, highlighting the need to develop novel therapeutic approaches for treating obese patients. We recently found that obesity leads to an increase in tumor-infiltrating macrophages with activated NLRC4 inflammasome and increased interleukin (IL)-1β production. IL-1β, in turn, leads to increased angiogenesis and cancer progression. Using Next Generation RNA sequencing, we identified an NLRC4/IL-1β-dependent upregulation of angiopoietin-like 4 (ANGPTL4), a known angiogenic factor in cancer, in tumors from obese mice. ANGPTL4-deficiency by genetic knockout or treatment with a neutralizing antibody led to a significant reduction in obesity-induced angiogenesis and tumor growth. At a mechanistic level, ANGPTL4 expression is induced by IL-1β from primary adipocytes in a manner dependent on NF-κB- and MAP kinase-activation, which is further enhanced by hypoxia. This report shows that adipocyte-derived ANGPTL4 drives disease progression under obese conditions and is a potential therapeutic target for treating obese breast cancer patients.
Keywords: Breast cancer, obesity, inflammation, ANGPTL4, Angiogenesis
Introduction
Obesity is associated with an increased risk of estrogen receptor-positive breast cancer in postmenopausal women and a worse clinical outcome regardless of menopausal status1. While outcomes for obese patients are worse, treatment options are the same despite often being less efficacious due to dose limiting toxicities and obesity-related complications, thus underlying the need to develop specific therapies for better treating obese patients. We recently found that obesity promotes breast cancer progression by inducing the activation of NLRC4 inflammasome and the consequent IL-1β production from macrophages. IL-1β then promotes angiogenesis and disease progression2. An association between obesity and increased tumor angiogenesis has been reported in several other studies3–5, underscoring the potential importance of increased angiogenesis to obesity-driven breast cancer progression.
Antiangiogenic therapies have been approved for use in several types of cancer; however, survival benefits have been minimal6. In breast cancer, the anti-vascular endothelial growth factor (VEGF) antibody, bevacizumab, failed approval following phase 3 clinical trials due to a lack of overall survival benefits7. Thus, identifying patients which may benefit from anti-angiogenic therapies and understanding mechanisms of resistance is important. Studies have shown that anti-VEGF therapies may be beneficial in breast cancer patients with high levels of vascular density. Obese breast cancer patients may represent one such cohort that may respond to anti-angiogenic therapies. However, obesity may promote resistance to ani-VEGF therapy8.
Angiopoietin-like 4 (ANGPTL4) is a secreted protein that is cleaved into two active peptides9. The N-terminus domain is a potent inhibitor of lipoprotein lipase (LPL) activity and modulates lipid composition and energy homeostasis9. The C-terminus (cANGPTL4) domain is involved in wound healing, vessel permeability, and angiogenesis10. ANGPTL4 plays a role in promoting the progression of several types of cancer11, 12 and is correlated with poor response to anti-VEGF therapy13. Here we show that obesity-associated NLRC4 inflammasome activation/IL-1β leads to the upregulation of ANGPTL4, primarily in adipocytes. This increase in ANGPTL4 is required for obesity-driven breast cancer progression and angiogenesis and targeting cANGPTL4 with a neutralizing antibody inhibits obesity-driven tumor progression.
Results
Obesity-associated NLRC4-inflammasome activation/IL-1β upregulates Angptl4
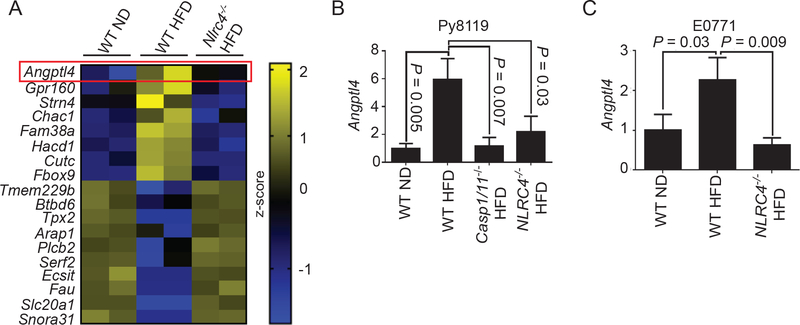
We recently found that obesity promotes breast cancer progression via NLRC4-inflammasome activation and subsequent IL-1β secretion in tumor-infiltrating macrophages2. To further understand how NLRC4-inflammasome promotes cancer progression, we performed Next Generation RNA sequencing on Py8119 orthotopic tumors from wild-type (wt) mice given a normal diet (ND), WT mice with high-fat diet (HFD), or the HFD-fed Nlrc4−/− mice used in our previous study2. Using the criterion described in the materials and methods, we identified 8 genes that were upregulated in an NLRC4-dependent manner in tumors from obese mice and 10 that were downregulated (Figure 1A). Among the most highly upregulated genes was Angptl4, whose expression was further verified by real-time PCR showing an increase in Py8119 (Figure 1B) and E0771 (Figure 1C) tumors from obese mice but not in those from obese NLRC4 inflammasome-deficient mice (Nlrc4−/− HFD and Casp1/11−/−HFD, the common effector enzyme for inflammasomes).
Figure 1.
NLRC4-inflammasome upregulates ANGPTL4 in tumors from diet-induced obese mice
A) Heatmap showing differentially regulated genes in tumors from diet-induced obese mice that are NLRC4-dependent. Next generation RNA sequencing was performed using tumor RNAs from WT mice fed a normal diet (WT ND), WT mice fed a high-fat diet (WT HFD), or Nlrc4−/− mice fed a HFD (Nlrc4−/− HFD). Each lane represents a combination of equal amount of RNAs from 3 individual tumors.
B, C) Average Angptl4 mRNA relative to Ppia in tumors from the indicated mice presented as a fold change compared to WT ND. (n=3 for all groups)
One-way ANOVA with multiple comparisons correction using Dunnett’s test was performed to determine significance in B-C.
ANGPTL4 promotes obesity-driven breast cancer progression and angiogenesis
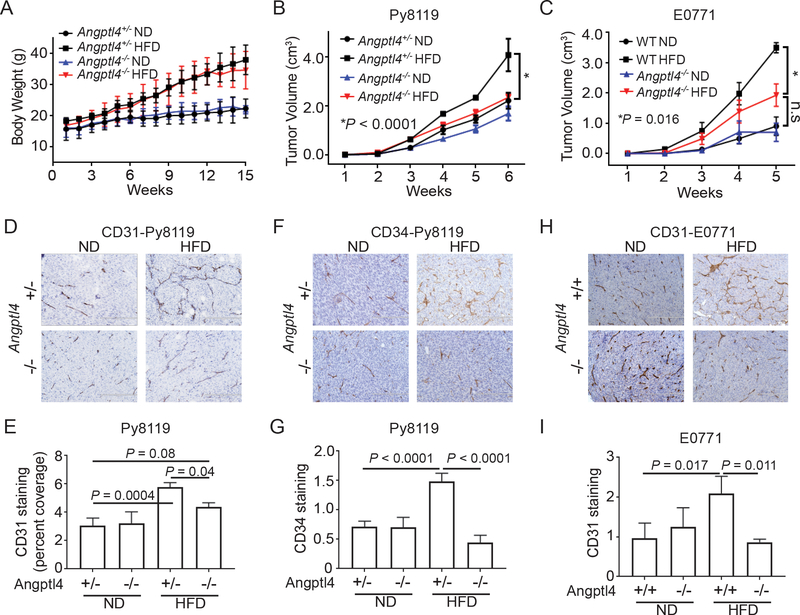
To determine if ANGPTL4 plays a role in obesity-driven breast cancer progression, we fed Angptl4−/− and Angptl4+/ - littermates an ND or HFD for 10 weeks prior to implantation of Py8119 cells. We observed no significant difference in weight gain between the Angptl4−/− and Angptl4+/ - mice (Figure 2A). Tumor growth in Angptl4+/− mice fed with the HFD was significantly higher than that in Angptl4+/− mice fed with ND (Figure 2B). This obesity-driven tumor progression was significantly reduced in Angptl4−/− mice (Figure 2B). Tumor growth in Angptl4−/− mice fed HFD was similar to that of Angptl4+/− and Angptl4−/− mice fed ND, suggesting that only in the obese setting is tumor growth dependent on ANGPTL4. We next verified the role of ANGPTL4 in obesity-driven breast cancer progression using E0771 breast cancer cells and found similar results (Figure 2C). Obesity led to an increase in tumor growth in WT mice and was partially, but significantly, reduced (P=0.016) in obese Angptl4-deficient mice (Figure 2C). These data support that the upregulation of ANGPTL4 is critical in promoting cancer progression under obesity.
Figure 2.
ANGPTL4 promotes tumor growth and angiogenesis in obese mice
A, B) Angptl4+/− and Angptl4−/− littermates were fed either a normal diet (ND) or high fat diet (HFD) for 10 weeks, followed by implantation of 1×105 Py8119 cells into the #4 mammary fatpad. Graphs depict the average body weight ±s.e.m. (A) and tumor volumes (B) ± s.e.m. from the indicated mice (n=5 Angptl4−/− ND; n=6 Angptl4−/− HFD; n=6 Angpt4l+/− ND; n=8 Angptl4+/− HFD).
C) The indicated mice were given an ND or HFD for 10 weeks and then implanted with 1×105 E0771 cells. Graph depicts the average tumor volumes ± s.e.m. for the indicated mice (n=5 for all groups).
D-E) IHC staining of CD31 from tumors used in Figure 2B. (D) Representative images from the indicated mice. Scale bars: 200 μm. (E) Average CD31-positive ± s.d. staining as a percent of total pixels in tumors from the indicated mice (At least 4 fields per sample and 3 samples per group were quantified).
F-G) IHC staining of CD34 from tumors used in Figure 2B. (F) Representative images from the indicated mice. Scale bars: 200 μm. (G) Average CD34-positive ± s.d. staining in tumors from the indicated mice (At least 4 fields per sample and 3 samples per group were quantified).
H-I) IHC staining of CD31 from tumors used in Figure 2C. (H) Representative images from the indicated mice. Scale bars: 200 μm. (I) Average CD31-positive ± s.d. staining in tumors from the indicated mice (At least 4 fields per sample and 3 samples per group were quantified).
Two-way ANOVA was used to determine significance in A-C. One-way ANOVA with multiple comparisons correction using Dunnett’s test was performed to determine significance in E, G, and I.
It has been previously reported that Angptl4−/− mice given a diet high in saturated fatty acids, such as the one used in our studies, have a systemic inflammatory response eventually leading to intestinal fibrosis and cachexia14. The authors noted that Angptl4−/− mice given a diet high in unsaturated fatty acids did not present any of these clinical abnormalities. To determine if this inflammatory response may effect tumor growth in our model, we fed WT and Angptl4−/− mice a safflower oil-based HFD consisting of primarily 18:2 unsaturated fatty acids (HFD-Saff); however, the Angptl4−/− mice failed to gain weight making this an inappropriate model to study obesity-driven breast cancer progression (Supplementary Figure S1A). This lack of weight gain from 18:2 unsaturated fatty acids is an intriguing future avenue of investigation for ANGPTL4’s role in regulation of LPL. Furthermore, tumors from Angptl4−/− mice given a HFD had similar numbers of tumor-infiltrating macrophages and IL-1β levels as those in Angptl4+/− mice (Supplementary Figure S1B-D).
In our previous study, we found that NLRC4-inflammasome promotes tumor angiogenesis in obese mice2. As previous studies have shown that ANGPTL4 can promote angiogenesis15, we next determined if upregulation of ANGPTL4 in obese mice led to increased angiogenesis. Immunohistochemical staining for CD31, a marker for endothelial cells, showed a significant increase in CD31-positive staining in tumors from Angptl4+/− HFD mice compared to Angptl4+/− ND mice (Figure 2D-E). This increase in CD31-positive staining was partially reduced in Angptl4−/− HFD mice, suggesting that obesity-driven tumor angiogenesis is at least partially dependent on ANGPTL4. While obese Angptl4−/− mice had increased CD31 staining compared to Angptl4+/− or Angptl4−/− ND mice, the difference was not significant (Figure 2D-E). Similar results were found using a second endothelial cell marker, CD34, wherein tumors from obese wild-type mice had increased CD34-positive staining compared to ND mice but not in those from obese ANGPTL4-deficient mice (Figure 2F-G). In Figure 2D-G, the tumors were collected at the same time and hence the tumors from the HFD group were larger (Figure 2B). To determine if this difference in size played a role in the increased angiogenesis, we collected tumors from ND and HFD mice when they reached 2 cm in diameter and stained for CD31. Tumors from obese mice still had increased CD31 staining compared to tumors from ND mice, indicating that the difference in angiogenesis was not due to the larger tumors (Supplementary Figure S2A). This ANGPTL4-dependent increase in angiogenesis was also seen in E0771 tumors (Figure 2H-I), further supporting the role of obesity-associated ANGPTL4 in promoting tumor angiogenesis. We also stained tumor sections for Ki-67, a proliferation marker, and found no difference between tumors from obese and ND mice (Supplementary Figure S2B).
IL-1β promotes the upregulation of Angptl4 in adipocytes
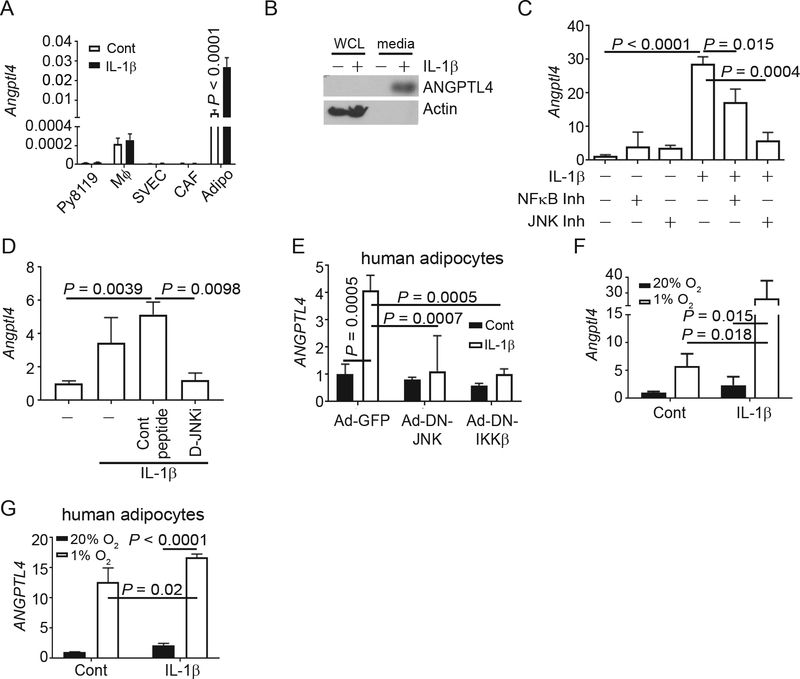
To determine the source of ANGPTL4 in response to IL-1β in obese tumor, we treated various cell types found in the tumor microenvironment with IL-1β and measured Angptl4 expression. IL-1β did not induce Angptl4 expression in Py8119 cancer cells, bone-marrow-derived macrophages (BMDM, Mφ), cancer-associated fibroblasts (CAFs), or endothelial cells (SVEC; Figure 3A). In contrast, IL-1β induced a significant, 28-fold increase in Angptl4 mRNA in primary mouse adipocytes (Figure 3A). It should also be noted that the relative expression of Angptl4 is 200x or higher in primary adipocytes than in the other cell types tested (Figure 3A). The high expression of Angptl4 in mouse adipocytes corresponds with RNA-sequencing data from human tissues showing that adipose tissue has the highest expression of ANGPTL4 (Supplementary Figure S3A). Western Blotting analysis of whole cell lysates and media from human adipocytes, obtained by differentiation of an immortalized preadipocyte cell line18, showed that IL-1β induced ANGPTL4 protein in human adipocytes mainly as the secreted form in media (Figure 3B). Adipocytes are an important cell type in the breast tumor microenvironment16 and are frequently found in mammary tumors. The number of adipocytes was increased in tumors from obese mice regardless of whether ANGPTL4 was present (Supplementary Figure S3B-C). The high expression of ANGPTL4 in primary adipocytes compared to other stromal cells indicates that ANGPTL4 is primarily produced from adipocytes in the obese microenvironment. To determine which signaling pathway is involved in the upregulation of Angptl4, we treated adipocytes with IL-1β in the presence or absence of either an NF-κB- or a JNK-inhibitor. Inhibition of NF-κB partially reduced IL-1β-induced upregulation of Angptl4 expression in primary adipocytes, while inhibition of JNK attenuated IL-1β-induced Angptl4 (Figure 3C), suggesting that ANGPTL4 expression is induced through NF-κB- and JNK-mediated signaling pathways. We also treated primary mouse adipocytes with IL-1β in the presence or absence of D-JNKi, a cell-permeable peptide inhibitor of JNK17, or control peptide and confirmed that inhibition of JNK signaling abolished IL-1β-induced Angptl4 (Figure 3D). The inhibition of IL-1β-induced JNK activation was confirmed by Western Blotting for phospho-c-JUN downstream of JNK activation (Supplementary Figure 3D). We transduced differentiated human adipocytes with adenovirus expressing GFP (Ad-GFP), dominant-negative (DN) JNK (Ad-DN-JNK) or DN IKKβ (Ad-DN-IKKβ), following with IL-1β treatment. Infectivity was verified by expression of GFP (Supplementary Figure S3E). IL-1β-induced expression ANGPTL4 in human adipocytes was inhibited by the expression of DN JNK and DN IKKβ (Figure 3E), further confirming the role of NF-κB- and JNK-mediated signaling in the regulation of ANGPTL4 downstream of IL-1β.
Figure 3.
IL-1β directly upregulates ANGPTL4 in adipocytes
A) The indicated cells were treated with 100 ng/ml IL-1β for 6 hours. Graph depicts the average Angptl4 mRNA relative to Ppia ± s.d. (n=3 for all groups).
B) Human preadipocytes were differentiated into adipocytes and treated with 100 ng/ml of IL-1β for 4 hours. Then the media was collected and cells were lysed. Immunoblots for ANGPTL4 is shown. β-ACTIN was included as a loading control.
C) Primary mouse adipocytes were treated as indicated. Graph depicts the average Angptl4 mRNA relative to Ppia ± s.d. as a fold change compared to control (n=3 for all groups).
D) Primary mouse adipocytes were treated with 30 μM D-JNKi or control peptide 1 hour prior to treatment with 100 ng/ml IL-1β for 6 hours. Graph depicts the average Angptl4 mRNA relative to Ppia ± s.d. (n=3 for all groups).
E) Differentiated human adipocytes were transduced with adenovirus expressing GFP (Ad-GFP), dominant-negative JNK (Ad-DN-JNK) or dominant-negative IKKβ (Ad-DN-IKKβ) for 16 hours then treated with 100 ng/ml IL-1β for 6 hrs. Graph depicts the average ANGPTL4 mRNA relative to ACTB ± s.d. (n=3 for all groups).
F) Primary adipocytes were treated with or without IL-1β and incubated at 20% or 1% O2 for 6 hours. Graph depicts the average Angptl4 mRNA relative to Ppia ± s.d. as a fold change compared to control (n=3 for all groups).
G) Immortalized human preadipocytes were differentiated into mature adipocytes then treated as in C. Graph depicts the average ANGPTL4 mRNA relative to PPIA ± s.d. as a fold change compared to control (n=3 for all groups).
In (C, D and F) data depicts the results of a single experiment using adipocytes from one litter of mice treated in triplicate. Experiments were repeated using different batches of adipocytes with similar results. One-way ANOVA with multiple comparisons correction using Dunnett’s test was performed to determine significance.
ANGPTL4 is a known to be regulated by hypoxia-inducible factor 1 (HIF1)19. We stained tumor sections with HIF1α and counted the number of cells with nuclear HIF1α staining as a marker for hypoxia. While we didn’t see an increase in nuclear HIF1α staining in tumors from obese mice, both the tumor microenvironment and obese adipose tissues tend to have regional hypoxia (Supplementary Figure S3F-G). We hence determined the effect of hypoxia on IL-1β-induced Angptl4. While both hypoxia (incubation of cells at 1% O2) and IL-1β induced the expression of Angptl4, the combination of IL-1β and hypoxia induced a further upregulation of Angptl4 relative to either alone: 4.7-fold increase over hypoxia alone and 11.1-fold increase over IL-1β alone (Figure 3F). Human adipocytes were treated as in Figure 3F and similar to mouse adipocytes, hypoxia induced a significant increase in ANGPTL4 expression, which is further increased by IL-1β (Figure 3G). We previously showed that vascular endothelial growth factor (VEGF) A is upregulated by IL-1β in obese tumors in our model2. As such, we examined the IL-1β induced Vegfa in adipocytes in conjunction with hypoxia. In primary adipocytes, while both IL-1β and hypoxia induced Vegfa expression individually, the combination did not significantly increase its expression compared to hypoxia alone (Supplementary Figure S3H). These data indicate that the upregulation of IL-1β in obese tumors acts collectively with hypoxia to induce Angptl4 expression in adipocytes.
ANGPLT4 in human obesity and breast cancer
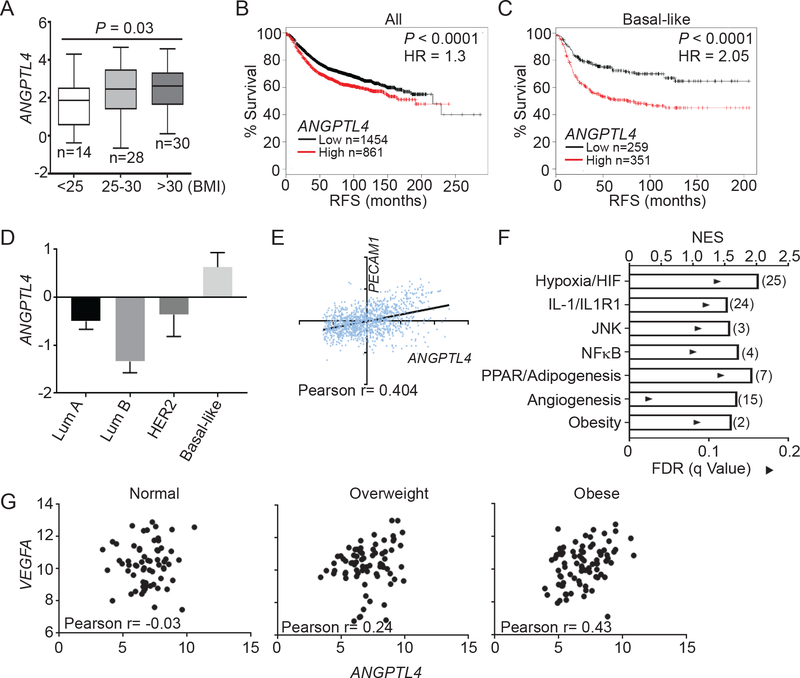
We next examined the relevance of ANGPTL4 in human obesity and breast cancer. Analysis of the publicly available dataset (GSE33256) showed a significant increase in the expression of ANGPTL4 in normal breast tissue from obese women compared to normal weight women (Figure 4A). Using a meta-dataset with 2315 samples, we found that ANGPTL4 expression was inversely correlated with recurrence-free survival (RFS; hazard ratio HR=1.3) across all breast cancer subtypes (Figure 4B), which became much more pronounced within basal-like breast cancer (HR=2.05; Figure 4C). Examining TCGA breast cancer expression data, we also found that ANGPTL4 expression was highest in aggressive basal-like breast cancer compared to Luminal A, Luminal B, and HER2 subtypes (Figure 4D).
Figure 4.
ANGPTL4 in human obesity and breast cancer.
A) Box plots depicting ANGPTL4 mRNA with 95% confidence interval (CI) in normal mammary tissue in normal weight (BMI<25), overweight (BMI 25–30) and obese (BMI>30) patients. Data were from GSE33256. Number of samples is indicated. Welch’s t-test was used to determine significance.
B, C) Correlation between ANGPTL4 mRNA and recurrence-free survival (RFS) in all breast cancer samples (B) and basal-like breast cancer (C). Data generated using KMplot meta-dataset of invasive ductal breast carcinoma. Number of cases and Log-rank P values are shown.
D) Mean centralized ANGPTL4 mRNA with 95% CI in PAM50 subtypes of breast cancer. Data from TCGA invasive breast cancer cohort.
E) Correlation between ANGPTL4 and PECAM1 mRNA in basal like breast cancers. Data is from the basal-like breast cancer subtype of the TCGA invasive breast cancer cohort. Pearson r is indicated.
F) Gene Set Enrichment Analysis of basal-like breast cancer samples comparing ANGPTL4 high expression tertile (n=66) to ANGPTL4 low expression tertile (n=66) from GEO datasets GSE76275. Graph depicts the nominal enrichment score (NES; bar) and FDRs (solid triangles) of a representative gene set related to the indicated pathway enriched in the ANGPTL4 high tertile group. The number of pathways enriched related to the indicated pathways is labeled in parenthesis. Only gene sets with FDRs < 0.25 were considered significantly enriched.
G) Correlation between VEGFA and ANGPTL4 mRNA in breast cancer samples from normal (BMI<25), overweight (BMI 25–30), and obese (BMI>30) patients from GSE20194. Pearson r is indicated.
We analyzed basal-like breast cancers as they had the highest expression of ANGPTL4, and found that there is a positive correlation between ANGPTL4 and PECAM1 (CD31) (Figure 4E), suggesting that ANGPTL4 is positively correlated with angiogenesis in human breast cancer. Using a publically available dataset of 198 basal-like breast cancer samples (GSE76275), we performed Gene Set Enrichment Analysis (GSEA) comparing transcriptomes with high expression of ANGPTL4 versus those with low expression of ANGPTL4, to identify pathways and gene sets associated with ANGPTL4. We found an enrichment for gene sets related to all of the ANGPTL4 regulatory pathways, including those of hypoxia and HIF signaling, IL-1 signaling, JNK signaling, NFκB signaling, and PPAR/Adipogenesis (Figure 4F, Supplementary Table 1). We also found the enrichment for gene sets related to angiogenesis and obesity, further corroborating an association between ANGPTL4, obesity and angiogenesis in breast cancer (Figure 4F, Supplementary Table 1). We previously reported an upregulation of VEGFA in response to NLRC4 inflammasome/IL-1β2. We also determined if VEGFA was associated with enrichment for gene sets related to the same pathways associated with ANGPTL4 expression. While high VEGFA expression was associated with fewer pathways overall compared to pathways altered in high ANGPTL4 specimens, VEGFA expression was associated with enrichment for pathways related to Hypoxia/HIF signaling as well as NFκB and PPAR/adipogenesis, but not pathways related to JNK, IL-1 or obesity (Supplementary Figure S4A, Supplementary Table 2). As expected, cancer specimens with high expression of VEGFA exhibited enrichment for gene sets related to angiogenesis and VEGFA signaling (Supplementary Figure S4A, Supplementary Table 2). We also found that cancer specimens with high expression of ANGPTL4 had enrichment for gene sets related to the same pathways, regardless of whether the samples were first separated into groups with high or low expression of VEGFA (Figure 4F, Supplementary Figure S4B, Supplementary Tables 3–4), suggesting that expression of ANGPTL4 is associated with these pathways independently of VEGFA expression. Previous studies have found that both VEGFA and ANGPTL4 are required for angiogenesis19 in certain situations and that high expression of ANGPTL4 is correlated with poor response to anti-VEGF therapies13. Interestingly, we found a body-mass index (BMI)-dependent correlation pattern between VEGFA and ANGPTL4, with significant correlation between VEGFA and ANGPTL4 expression (Pearson r=0.43) in obese breast cancer patients, a trend to positive correlation in overweight patients (Pearson r=0.24), and no correlation in normal weight patients (Figure 4G). These analyses suggest that ANGPTL4 and VEGF cooperatively promote angiogenesis in obese patients.
Targeting cANGPTL4 inhibits obesity-driven breast cancer progression
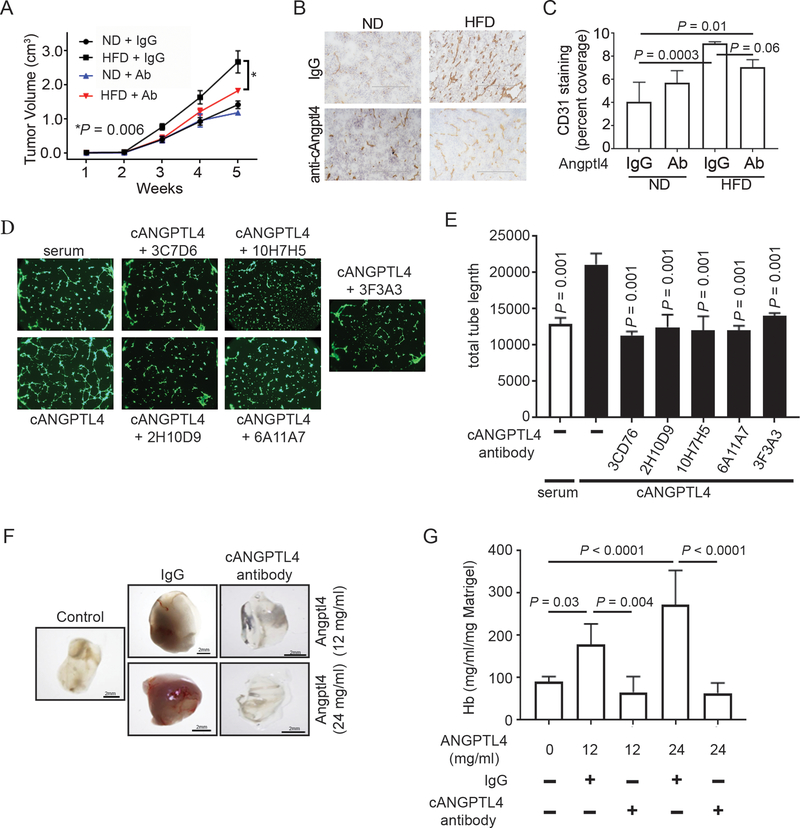
To see if targeting ANGPTL4 would inhibit obesity-driven breast cancer progression, we used a neutralizing antibody against the C-terminus of ANGPTL420, 21. Mice were given either a ND or HFD for 10 weeks then implanted with Py8119 cells as in Figure 2. Once tumors were palpable, the mice were treated with the anti-cANGPTL4 blocking antibody or IgG as a control (Figure 5A). Blocking cANGPTL4 reduced obesity-driven tumor growth compared to obese mice treated with IgG control (Figure 5A). Anti-cANGPTL4 antibody treatment in mice fed ND had no effect on tumor growth (Figure 5A). Treating obese mice with anti-cANGPTL4 antibody also reduced tumor angiogenesis indicated by CD31 IHC staining as compared to obese mice treated with IgG, though the difference was barely insignificant (Figure 5B-C). This data indicates that targeting cANGPTL4 with a neutralizing antibody can inhibit obesity-driven breast cancer progression and angiogenesis.
Figure 5.
Targeting cANGPTL4 inhibits obesity-driven cancer progression and angiogenesis.
A) WT mice were fed and implanted with Py8119 cells as described in Figure 2. Mice were treated with anti-cANGPTL4 antibody (Ab) or rabbit IgG twice weekly once tumors were palpable. Graph depicts the average tumor volumes ± s.e.m. from the indicated mice (n=5 for all groups except ND IgG, n=4). Two-way ANOVA was used to determine significance.
B-C) IHC staining for CD31 from tumors used in Figure 5A. (B) Representative images from the indicated mice. Scale bars: 200 μm. (C) Average CD31-positive staining ± s.d. as a percent of total pixels in tumors from the indicated mice (At least 4 fields per sample and 3 samples per group were quantified). One-way ANOVA with multiple comparisons correction using Dunnett’s test was performed to determine significance
D-E) Endothelial cell tube formation assay. Endothelial cells were plated on matrigel and treated as indicated. 6 hour later cells were stained with Calcein AM and imaged (D). Images were analyzed and total tube length was determined. Graph (E) depicts the average total tube length ± s.d. (n=3 for all groups). One-way ANOVA with multiple comparisons correction using Dunnett’s test was performed to determine significance.
F-G) Matrigel plug assay. Athymic nude mice were implanted with matrigel containing the indicated doses of recombinant human cANGPTL4 and 48 ng/ml anti-cANGPTL4 antibody or rat IgG. After 6 days, the matrigel plugs were removed and hemoglobin was measured as a redout for angiogenesis. Representative images (F) are shown. Graph (G) depicts the average hemoglobin concentration ± s.d. (n=6 for all groups).
We had 5 monoclonal antibodies against human cANGPTL4 developed which bound to both denatured and folded human cANGPTL4. To determine if these antibodies could block cANGPTL4 function, we performed an endothelial cell tube formation assay22. Treatment of endothelial cells with recombinant human cANGPTL4 induced tube formation (Figure 5D-E). cANTPTL4-induced tube formation was inhibited by co-treating cells with antibodies targeting human cANGPTL4 (Figure 5D-E). Importantly, these antibodies did not inhibit serum-induced tube formation (Figure S5). We next performed an in vivo matrigel plug assay using hemoglobin as a readout for angiognesis23. As shown in Figure 5F-G, recombinant cANGPTL4 induced angiogenesis in a dose dependent manner in matrigel plugs from athymic nude mice (Figure 5F, middle two panels Vs control panel) and quantitated by hemoglobin protein expression (Figure 5G, bar 1, 2, and 4). The cANGPTL4-induced angiogenesis in vivo was completely blocked by treatment with our new anti-cANGPTL4 antibody (Figure 5F, right two panels Vs middle two panels) and further quantitated by hemoglobin protein expression (Figure 5G, bar 3 Vs 2 and bar 5 Vs 4). These data indicate that cANGPTL4 can directly induce angiogenesis and can be blocked with anti-ANGPTL4 antibody against cANGPTL4.
Discussion
Previously we showed that obesity-associated NLRC4-inflammasome activation in infiltrating macrophages drove increases in angiogenesis and breast cancer progression2, suggesting that obese breast cancer patients may represent a patient cohort that might benefit from VEGFA targeted therapy. Bevacizumab (Avastin) is an FDA-approved neutralizing antibody for human VEGFA to treat several solid cancers, but it failed in breast cancer due to lack of overall survival benefit. There is no doubt that angiogenesis is required for tumor growth and progression. Here we identified ANGPTL4, a different angiogenic factor, as critical for angiogenesis under obesity. Obesity is known to be associated with increased VEGFA expression and angiogenesis. As depicted (Supplementary Figure S6), we found much more pronounced increase in ANGTPL4 expression from adipocytes that can be regulated by several pathways: 1) (purple) IL-1β produced by macrophages directly acts on adipocytes and induces Angptl4 transcription via JNK and NF-κB pathways; and 2) (blue) Hypoxia within tumor microenvironment and obesity synergizes with IL-1β to induce Angptl4 transcription likely via HIF1α. These multifaceted Angptl4 regulations further implicate its important role in tumor growth and progression under obesity. Our animal data further demonstrate the critical role of ANGPTL4 in obesity-driven tumor angiogenesis and growth in two breast cancer models, suggesting that ANGPTL4 is one of the major obesity-induced angiogenic factors downstream of NLRC4-inflammasome activation and IL-1β release from macrophages.
Based on the expression pattern of ANGPTL4 in human tissues (Supplementary Figure S3A) and the high expression of Angptl4 in primary adipocytes compared to other stromal cells found in the tumor microenvironment (Figure 3A), we hypothesize that adipocytes are the primary source of ANGPTL4 in the obese breast tumor microenvironment. Moreover, we observed a reduction in obesity-driven breast cancer progression when ANGPTL4 was lost in the microenvironment but intact in the cancer cells (Figure 2), indicating that the source of ANGPTL4 was from the tumor associated stroma. Previous studies have shown that cancer cells express ANGPTL4. In breast cancer, expression of TGFβ can induce expression of ANGPTL4, which can promote lung metastasis12. Others have shown that ANGPTL4 is expressed in various tumors and the loss of ANGPTL4 in squamous cell carcinoma and melanoma cells can reduce tumor growth in mouse models24. However, here we only focused on the relevance of stromal ANGPTL4 and our data indicate that the major source of ANGPTL4 in primary breast cancer under obesity could be adipocytes.
Our current focus is how obesity-associated microenvironment promotes tumor growth and progression. Based on our and others’ results, it is likely that hypertrophic adipocytes from obese individuals recruit macrophages via CCL2 production5. The activated macrophages produce IL-1β via NLRC4-inflammaome activation, which in turn works on the above pathways to induce ANGPTL4 from adipocytes and consequent angiogenesis. Based on phenotypic resemblance between ANGPTL4-deficiency and NLRC4-inflammasome inactivation, we conclude that ANGPTL4 is the major downstream effector involved in obesity-driven tumor growth. As several mechanisms have been proposed, we reason that angiogenesis is one of the major pathways in the obesity-driven tumor growth as ANGPTL4 is critical in obesity-associated angiogenesis. We cannot exclude other pathways, but relevant to obesity in these models we did not find significant difference in apoptosis (data not shown) and proliferation (Supplementary Figure S2) between tumors from normal weight mice and those from obese mice. Several recent studies have found that ANGPTL4 can promote survival and proliferation of cancer cells through various mechanisms24–27, providing possible mechanisms how ANGPTL4 promotes cancer progression that may be irrelevant of obesity. ANGPTL4-deficient mice have a systemic inflammatory response leading to intestinal fibrosis and cachexia when given a HFD that is high in saturated fatty acids, such as the one used in these studies, but not when given a HFD composed primarily of unsaturated fatty acids14. This inflammatory response may have an effect on tumor growth in our model. Since ANGPTL4-deficient mice failed to gain weight when given a safflower oil-based HFD consisting of primarily 18:2 unsaturated fatty acid (Supplementary Figure S1A), we cannot conclusively determine what effect, if any, this inflammatory response to a diet high in saturated fatty acids has on obesity-driven tumor growth in our model. However, we saw no difference in the number of tumor infiltrating macrophages and IL-1β between tumors from control HFD mice compared to Angptl4−/− HFD mice (Supplementary Figure S1B-D). Furthermore, obesity-associated tumor growth was abrogated by treating mice with a neutralizing antibody targeting cANGPTL4 (Figure 5A), suggesting that this inflammatory response in ANGPTL4-deficient mice was not responsible for the reduced tumor growth observed in obese Angptl4−/− mice (Figure 2B-C).
While anti-VEGF therapies have shown little therapeutic promise in breast cancer, they may be beneficial to patients with high levels of neoangiogenesis28. Due to the increase in angiogenesis in obesity-associated breast cancer, we have postulated that anti-VEGF therapies may be a viable therapy in obese breast-cancer patients2. However, a recent publication found that obesity promotes resistance to anti-VEGF therapies possibly through the upregulation of IL-6 and FGF-28. Here we found that ANGPTL4 is required for obesity-driven breast cancer progression and angiogenesis, and that its expression is inversely correlated with relapse-free survival (Figure 4). Moreover, we found that VEGFA and ANGPTL4 expression are correlated only in obese patients (Figure 4G). Previous studies have shown that expression of ANGPTL4 is correlated with poor response to anti-VEGFA therapies13. Thus, the upregulation of ANGPTL4 represents another mechanism for the resistance to anti-VEGF therapies in obese mice reported previously8. Therefore, targeting ANGPTL4 alone or in combination with anti-VEGF therapies may be a better potential therapy for obese breast cancer patients. In particular, the high expression in basal-like breast cancer and its secretive nature make ANGPTL4 an ideal target for antibody-based therapy. Indeed, treating mice with a neutralizing antibody against cANGPTL4 inhibits obesity-driven breast cancer progression in obese mice (Figure 5A), further highlighting the potential of ANGPTL4 as a therapeutic target for obese breast cancer patients. One caveat is the critical role of its N-terminus in inhibiting LPL regulation that could be detrimental for lipid homeostasis if inhibited. It is unknown if antibodies targeting cANGPTL4 deplete the pool of whole length ANGPTL4 leading to a potential reduction of its N-terminal peptide. Further investigations are warranted to address if cANGPTL4 represents a viable target for therapy in particular to obese patients with basal-like breast cancer.
Materials and Methods
Cell lines, primary adipocytes and cell culture
Py8119 and E0771 cells have been described and validated previously2, 29, 30. Cancer associated fibroblast cells were described previously31. SVEC endothelial cells were purchased from American Type Culture Collection (ATCC)32. Cell lines were routinely tested for mycoplasma contamination. Human preadipocytes (Lonza, Basel Switzerland) were immortalized, cultured and differentiated into mature adipocytes as described previously18. Primary mouse preadipocytes were isolated from the mammary fat pad of 4 to 6 day old pups and differentiated into mature adipocytes as described previously33. For experiments involving treatment with IL-1β, cells were cultured with 100 ng/ml recombinant mouse human IL-1β (R&D Systems, Minneapolis MN) for 6 hours. For co-treatment with IL-1β, adipocytes were treated with 5 μM BMS345541 (Sigma-Aldrich, St. Louis, MO), an NF-κB inhibitor, 40 μM SP600125 (Sigma Aldrich) a JNK inhibitor, 30 μM D-JNKi (GenScript, Pascataway NJ) or 30 μM control peptide (GenScript), 1 hour prior to treatment with IL-1β. For transduction of differentiated human adipocytes, cells were incubated with purified adenovirus expressing GFP, DN JNK (University of Iowa Viral Vector Core) or DN IKKβ for 16 hrs prior to treatment with IL-1β. Each individual experiment involving primary adipocytes were performed using a batch of adipocytes from a single litter of mice plated in triplicate for each experimental group. These experiments were then repeated at least twice using different batches of primary adipocytes.
Mouse colony and orthotopic transplant model
Animal experiments were approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC) and performed in accordance with IACUC guidelines. All mice used were female mice of C57BL/6N background. Angptl4−/− (B6;129S5-Angptl4Gt (OST352973)Lex/Mmucd) mice were obtained from the Mutant Mouse Resource and Research Center (mmrrc.org) and backcrossed 10 generations with C57BL/6N background. For Py8119 transplant models, Angptl4+/− and Angptl4−/− littermates were used. Tumors from Casp1/11−/−34 and Nlrc4−/−35 mice were from our previous publication2. Athymic nude mice of both genders were purchased from Charles River Laboratories for matrigel plug assays (San Diego, CA).
The orthotopic transplant model was done as described previously2. Briefly, 6-week old mice were randomly grouped and given a normal diet provided by our vivarium, or a high fat diet (HFD, 60% kCal from fat, S3282, Bio-Serv, Flemington, NJ) for 10 weeks, then Py8119 or E0771 cells in Matrigel/PBS were implanted into the #4 mammary gland. Experiments were terminated when the largest tumors reached 2 cm in diameter. A custom safflower oil-based HFD (60 kCal from fat) where in the fat content was 76.6% 18:2 fatty acid was obtained from Research Diets Inc. (New Brunswick, NJ). For experiments involving treatment of mice with antibody, mice were treated with 10mg/kg anti-cANGPTL4 or rabbit IgG (BioXcell, West Lebanon, NH) twice weekly by intraperitoneal (i.p.) injection once tumors were palpable. The anti-cANGPTL4 antibody was described previously 20, 21. For inbred mice, we expect to see variability representing ≤ 40% of the mean of each group, thus, a minimum of 5 mice/group was used to exceed a confidence level of 95% for transplant models and repeated. Animal studies were not performed blinded due to the use of different diets and genotypes.
Western blotting and ELISA
For Western blotting analysis, adipocyte cells were lysed with RIPA buffer. Proteins in cell lysates or media were separated by SDS PAGE and detected by immunoblot analysis. The following antibodies were used: phospho-c-JUN (K-M-1, Santa Cruz Biotechnologies, Dallas TX), rat anti-cANGPTL4 clone 6A11A7 (GenScript), and mouse anti-β-Actin (8H10D10, Cell Signaling Technology, Danvers MA). For ELISA, tissue was lysed in buffer consisting of 100 mM Tris pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol-bis(β-aminoethy ether) N, N, N’, N’-tetraacetic acid, 1% Triton X-100, and 0.5% sodium deoxycholate plus protease inhibitors. The ELISA was done using the following protein pair: rat anti-mouse IL-1β (30311, R&D Systems, Minneapolis MN) and goat anti-mouse IL-1β biotinylated (polyclonal, R&D). The ELISA was developed using TMB substrate (Thermo Fisher Scientific, Waltham MA).
Immunohistochemistry (IHC)
Tumor sections were preserved in OCT compound, frozen and sectioned. 5 μm thick sections were fixed in 35/65 methanol/acetone. CD31 was detected with anti-mouse CD31 antibody (MEC13.3, Biolegend, San Diego CA). For the remaining IHC staining, Formalin-fixed tissue samples were paraffinized and sectioned by University of Iowa Comparative Pathology Core. Sections were then de-paraffinized in a series of xylene washes and rehydrated with ethanol and water. Antigen retrieval was accomplished by immersing slides in Citrate Buffer pH 6.0 in a decloaker (Biocare Medical, Concord CA). Tissue samples were incubated with 3% hydrogen peroxide followed by blocking with horse serum (Biocare Medical). Slides were then incubated with rabbit anti-mouse Ki-67 antibody (D2H10, Cell Signaling Technology), rat anti-CD34 antibody (MEC14.7, Novus Biologicals, Littleton CO) or rabbit anti-HIF1α (polyclonal, Abcam, Cambridge UK). Slides were then stained with Rat-on-mouse HRP polymer and probe (Biocare,) or secondary rabbit envision (DAKO, Santa Clara CA). The slides were developed with 3,3’-diaminobenzadine (DAB and 0.3% H2O2 in PBS) developing buffer, followed by counterstaining with hematoxylin. Positive staining was quantified using Image J software. All analysis of IHC was done blinded in regards to group allocation.
Endothelial cell tube formation assay
SVEC cells were serum-starved (0.3% serum) for 12 hrs, prior to plating 2×104 cells in DMEM in a growth factor reduced matrigel (Corning Inc., Corning NY) coated 48-well plate. 3% serum, recombinant human cANGPTl4 antibodies in hybridoma supernatant diluted 1:50 (GenScript, Piscataway NJ), and 20 μg/ml of cANGPTL4 was added to both the matrigel and the cell suspension as indicated. Rat anti-cANGPTL4 monoclonal antibodies were generated by GenScript against human cANGPTL4 protein. The ability of these proteins to bind to both denatured and folded cANGPTL4 was verified by GenScript by Western blotting after SDS-PAGE and ELISA, respectively. Cells were then incubated for 6 hours at 37°C. Following incubation cells were stained with 2μg/ml Calcein AM (Thermo Fisher Scientific) for 30 minutes followed by visualization using a fluorescent microscope. Tube formation was quantified using AngioTool software 36.
Matrigel plug assay
Athymic nude mice (Charles River Laboratories, San Diego, CA) were implanted with 0.4 ml growth factor reduced matrigel/PBS (Corning Inc.) containing the indicated concentration of recombinant human cANGPTL4 (Genescript) and 48 μg/ml rat IgG or purified rat anti-cANGPTL4 antibody clone 6A11A7 (Genescript) or PBS. After 6 days, mice were euthanized and plugs were used for the measurement of hemoglobin content using the Drabkin’s reagent kit according to the manufacturer’s instructions (Sigma-Aldrich). The concentration of hemoglobin was based on a set of standards.
Real-time PCR and RNA-seq
RNA was isolated using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). For real-time PCR, cDNA was generated using Superscript III First Strand cDNA Synthesis Kit (ThermoFisher Scientific). mRNA expression was quantified by real-time PCR using a ViiA7 Real-Time PCR System (ThermoFisher Scientific). The following primers for ANGPTL4 were used: for mouse, 5’-GGAAAAGTCCACTGTGCCTC and 5’-TAGATGACCCAGCTGATTG-3’; for human 5’-TAGTCCCACTCTGCCTCTCCC-3’ and 5’-GAGTTGGCCCAGCCAGTT-3’. The following primers for mouse Il1b were used” 5’- GCAACTGTTCCTGAACTCAACT-3’ and 5’- ATCTTTTGGGGTCCGTCAACT-3’. For RNA-seq, samples were submitted to the Iowa Institute of Human Genomics for quality assessment and samples with a RNA Integrity Number score > 8.0 were submitted to the University of Chicago Genomics for singe-end 50bp on the Illumina HiSeq 2000. Data was aligned using the Usegalaxy web platform and TopHat 37–39. The aligned data was further processed using cufflinks workflow 40. The raw data were shown as fragments per kilobase of transcript per million mapped reads (FPKM). Z-scores and fold change were calculated using the resulting expression data converted to Log2 (FPKM+1) values. Due to the small sample size, 2 per group, determining statistical significance for differentially regulated genes was not possible. Instead, differentially regulated genes in tumors from obese that were NLRC4-dependent were identified as genes with a fold change of greater than or equal to |1.8|. The list was further narrowed to only genes that were upregulated in the HFD group and had a Z-score of greater than 0.5 while the Z-score for the other 4 samples (ND and Nlrc4−/− HFD) were less than 0.25. For down regulated genes we included samples where the Z-score was less than 0.25 for the HFD samples and greater than 0.5 for the other 4. A heatmap of differentially regulated genes was made using Graphpad Prism Software (Graphpad Software Inc., San Diego, CA).
Survival and transcriptomics analysis
Recurrence-free survival based on the expression of ANGPTL4 were generated using data from a published meta-dataset of 3554 breast cancer patient specimens and the KMplot online tools 41. Expression analyses of the TCGA BRCA cohort were performed using the mean-centralized level 3 Illumina HiSeq2000 RNA-seq data separated into PAM50 subtypes. RNA-seq data from 122 human individual representing 32 tissues was obtained from Human Protein Atlas 42 and ANGPTL4 expression in different tissues was graphed using Graphpad Prism Software (Graphpad Software Inc). Correlation between ANGPTL4 and PECAM1 mRNA was generated using Graphpad Prism Software (Graphpad Software Inc) using TCGA BRCA expression data from basal-like PAM50 subtype. GSE33256, GSE20914 and GSE76275 microarray data were obtained from NCBI Geo Datasets. BMI data for GSE20914 was provided by Dr. Sai-Ching Yeung at MD Anderson Cancer Center. For pathway analysis, basal-like breast cancer samples from GSE76275 were separated into high and low expressing tertiles of ANGPTL4 (probe 221009_s_at) or VEGFA (probe 210512_s_at). Further analysis was done by first separating the samples by VEGFA expression into high tertile or low tertile, then further separating each group into high and low ANGPTL4 expressing tertiles. Geneset Enrichment Analysis (Broad Institute of MIT and Harvard, Boston) was used comparing low expressing tertiles to high expressing tertile for each group. Genesets with a false discovery rate (FDR) <0.25 was considered significant enrichment.
Statistical Analysis
All statistical tests are described in the Figure legends. For animal models, statistical significance for body weight and tumor growth was determined by two-way ANOVA. Statistical significance in expression from human microarray or TCGA data was determined by a Welch’s t-test. Pearson r is reported for correlation between the expression of two genes. Long-rank test was ued to determine significance for survival curves. All other experiments used a One-way ANOVA with multiple comparisons correction using Dunnett’s test to determine significance. P-values of less than 0.05 were considered significant.
Data Availability
The processed RNA-sequencing data shown as fragments per kilobase of transcript per million mapped reads (FPKM) and their original FASTQ files are currently being submitted to GEO datasets.
Supplementary Material
Acknowledgements
We would like to thank the Comparative Histopathology Core in the Department of Pathology, University of Iowa for the processing of fixed tissue and CD31 immunohistochemistry. We thank Dr. Mikhail Kolonin (UT health science center at Houston) and Dr. Leslie Ellies (UCSD) for sharing E0771 and Py8119 cells, respectively. Financial Support: RK: NIH T32 AI007260; WZ: NIH grant CA200673, the V Scholar award, Oberley Award (National Cancer Institute Award P30CA086862) from Holden Comprehensive Cancer Center at the University of Iowa; NB: NIH F30CA206255; AK: Mark Stinski Developmental Grant from the Department of Microbiology, University of Iowa; BD: NIH R01HL130146; NST: grant from Ministry of Education, Singapore (MOE2014-T2–1-012); FSS: NIH RO1 AI118719.
Financial Support: RK: NIH T32 AI007260; WZ: NIH R01 grants CA200673 and CA203834, Oberley Award (National Cancer Institute Award P30CA086862) from Holden Comprehensive Cancer Center at the University of Iowa; NB: NIH F30 CA206255; AK: Mark Stinski Developmental Grant from the Department of Microbiology, University of Iowa; BD: NIH R01HL130146; NST: grant from Ministry of Education, Singapore (MOE2014-T2–1-012); FSS: NIH R01 AI118719.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol 2016; 29: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski AM et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun 2016; 7: 13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu JW, Young E, Patterson SG, Makey KL, Wells J, Huang M et al. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther 2011; 11: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukumura D, Incio J, Shankaraiah RC, Jain RK. Obesity and Cancer: An Angiogenic and Inflammatory Link. Microcirculation 2016; 23: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res 2013; 73: 6080–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 2014; 26: 605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmann AE, Chia S. Patients with metastatic breast cancer using bevacizumab as a treatment: is there still a role for it? Curr Treat Options Oncol 2012; 13: 249–262. [DOI] [PubMed] [Google Scholar]
- 8.Incio J, Ligibel JA, McManus DT, Suboj P, Jung K, Kawaguchi K et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Science Translational Medicine 2018; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei X, Shi F, Basu D, Huq A, Routhier S, Day R et al. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem 2011; 286: 15747–15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: a decade of research. Biosci Rep 2012; 32: 211–219. [DOI] [PubMed] [Google Scholar]
- 11.Tan MJ, Teo Z, Sng MK, Zhu P, Tan NS. Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer Res 2012; 10: 677–688. [DOI] [PubMed] [Google Scholar]
- 12.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 2008; 133: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai L, Wang F, Zhang DS, Li C, Jin Y, Wang DS et al. A plasma cytokine and angiogenic factor (CAF) analysis for selection of bevacizumab therapy in patients with metastatic colorectal cancer. Sci Rep 2015; 5: 17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab 2010; 12: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab 2008; 295: E1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res 2018; 69: 11–20. [DOI] [PubMed] [Google Scholar]
- 17.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes 2001; 50: 77–82. [DOI] [PubMed] [Google Scholar]
- 18.Vu BG, Gourronc FA, Bernlohr DA, Schlievert PM, Klingelhutz AJ. Staphylococcal superantigens stimulate immortalized human adipocytes to produce chemokines. PLoS One 2013; 8: e77988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu K, Babapoor-Farrokhran S, Rodrigues M, Deshpande M, Puchner B, Kashiwabuchi F et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget 2016; 7: 7816–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L et al. Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol 2010; 177: 2791–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Chong HC, Ng SY, Kwok KW, Teo Z, Tan EH et al. Angiopoietin-like 4 Increases Pulmonary Tissue Leakiness and Damage during Influenza Pneumonia. Cell Rep 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCicco-Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J Vis Exp 2014: e51312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinda KM. In vivo matrigel migration and angiogenesis assay. Methods Mol Biol 2009; 467: 287–294. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Ge C, Zhao F, Yan M, Hu C, Jia D et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin beta1 signaling in human hepatocellular carcinoma. Hepatology 2011; 54: 910–919. [DOI] [PubMed] [Google Scholar]
- 25.Hata S, Nomura T, Iwasaki K, Sato R, Yamasaki M, Sato F et al. Hypoxia-induced angiopoietin-like protein 4 as a clinical biomarker and treatment target for human prostate cancer. Oncol Rep 2017. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Park YY, Kim SW, Lee JS, Wang D, DuBois RN. ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res 2011; 71: 7010–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, Xie J, Lin S, Li S, Huang Z, Wang Y et al. The downregulation of ANGPTL4 inhibits the migration and proliferation of tongue squamous cell carcinoma. Arch Oral Biol 2016; 71: 144–149. [DOI] [PubMed] [Google Scholar]
- 28.Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci U S A 2015; 112: 14325–14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas T, Gu X, Yang J, Ellies LG, Sun LZ. Attenuation of TGF-beta signaling supports tumor progression of a mesenchymal-like mammary tumor cell line in a syngeneic murine model. Cancer Lett 2014; 346: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiura K, Stock CC. Studies in a tumor spectrum. I. Comparison of the action of methylbis (2-chloroethyl)amine and 3-bis(2-chloroethyl)aminomethyl-4-methoxymethyl −5-hydroxy-6-methylpyridine on the growth of a variety of mouse and rat tumors. Cancer 1952; 5: 382–402. [DOI] [PubMed] [Google Scholar]
- 31.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011; 470: 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol 1990; 144: 521–525. [PubMed] [Google Scholar]
- 33.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014; 63: 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 1995; 267: 2000–2003. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med 2006; 203: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One 2011; 6: e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goecks J, Nekrutenko A, Taylor J, Galaxy T. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol 2010; 11: R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blankenberg D, Von Kuster G, Coraor N, Ananda G, Lazarus R, Mangan M et al. Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol 2010; Chapter 19: Unit 19 10 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res 2005; 15: 1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 2012; 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010; 123: 725–731. [DOI] [PubMed] [Google Scholar]
- 42.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed RNA-sequencing data shown as fragments per kilobase of transcript per million mapped reads (FPKM) and their original FASTQ files are currently being submitted to GEO datasets.