Abstract
Objectives:
To determine the prevalence of nonalcoholic fatty liver disease (NAFLD) in children with obesity because current estimates range from 1.7% to 85%. A second objective was to evaluate the diagnostic accuracy of alanine aminotransferase (ALT) for NAFLD in children with obesity.
Study Design:
We evaluated children ages 9–17 years with obesity for the presence of NAFLD. Diseases other than NAFLD were excluded by history and laboratories. Hepatic steatosis was measured by liver magnetic resonance imaging (MRI) proton density fat fraction (PDFF). The diagnostic accuracy of ALT for detecting NAFLD was evaluated.
Results:
The study included 408 children with obesity that had a mean age of 13.2 years and mean BMI percentile of 98.0. The study population had a mean ALT of 32 U/L and median hepatic MRI-PDFF of 3.7%. The estimated prevalence of NAFLD was 26.0% (95% CI 24.2 – 27.7), 29.4% in males (CI 26.1 – 32.7%) and 22.6% in females (CI 16.0 – 29.1%). Optimal ALT cut-point was 42 U/L (47.8% sensitivity, 93.2% specificity) for males and 30 U/L (52.1% sensitivity, 88.8% specificity) for females. CART model with sex, ALT, and insulin had 80% diagnostic accuracy for NAFLD.
Conclusions:
NAFLD is common in children with obesity, but NAFLD and obesity are not concomitant. In children with obesity, NAFLD is present in nearly one-third of boys and one-fourth of girls.
Keywords: Pediatric, NAFLD, BMI
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in children,1 and the most common indication for liver transplantation in young adults in the United States.2 Because obesity is the largest risk factor for NAFLD,3 current recommendations for screening focus on children with obesity.4 However, there is not a uniform agreement regarding the prevalence of NAFLD in children with obesity as reported estimates range from 1.7% to 85%.5,6,7 This wide range is due in part to variations in study design including sample size, geography, race, ethnicity, and setting. In addition, prevalence estimates differ based upon the reference method used to determine whether a child had NAFLD.
Obesity is recognized as a growing epidemic among youth in the United States; however, the ability to assess the impact of NAFLD on children is incomplete due to the large range in the estimated prevalence of NAFLD in children with obesity. The Centers for Disease Control and Prevention estimate that the prevalence of pediatric obesity in the US is 17%, which represents 12.7 million children.8 When combining this estimate with the range of estimates for the prevalence of NAFLD in children with obesity, there could be as few as 216,000 or as many as 10.8 million children with obesity in the US with NAFLD. This lack of understanding has ramifications for public health initiatives which include developing optimal screening guidelines for NAFLD in children, determining the utility and cost effectiveness of such guidelines, and designing effective health care policy. Improvement estimates of the prevalence of NAFLD may also advance research by guiding the design of studies to further our understanding of the genetics, pathophysiology, and pathogenesis of the overlapping but discrete conditions of obesity and NAFLD.
Therefore, the primary aim of this study was to estimate the prevalence of NAFLD in children ages 9–17 years with obesity. Because serum alanine aminotransferase (ALT) is widely used to screen for NAFLD in children with obesity, a secondary aim was to evaluate the diagnostic accuracy of ALT for detecting NAFLD in such children.
METHODS
Subject selection
We evaluated children ages 9 to 17 years with obesity, defined as body mass index (BMI) ≥ 95th percentile for age and sex. Children were recruited in the County of San Diego from community health centers, community health fairs, and primary care practices. Exclusion criteria were as follows: (1) inability to complete an MRI evaluation (claustrophobia, metal implants, or body circumference greater than the imaging chamber); (2) established diagnosis of chronic liver disease, (3) the use of medications that can cause steatosis or raise liver chemistry, (4) diagnosis of other chronic diseases that may have secondary effects on the liver, (5) substance abuse, and (6) pregnancy. The protocol was approved by the Institutional Review Board of the University of California, San Diego. The parents of all subjects provided written informed consent for their children. Written assent was obtained from all children.
Clinical evaluation
Clinical data were obtained for each participant from a single fasting intake visit at the Altman Clinical and Translational Research Institute at the University of California, San Diego. Age, sex, and self-identified race and ethnicity were recorded. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the subjects standing, wearing light clothing without shoes. BMI was calculated as weight (kg) divided by height squared (m2). Fasting laboratory assays included: ALT, aspartate aminotransferase (AST), glucose, insulin, high-density lipoprotein cholesterol (HDL), and triglyceride (TG) levels.
MRI evaluation
Participants were scanned at 3T using an advanced magnitude-based liver fat quantification MRI technique to measure proton density fat fraction (PDFF), a biomarker for hepatic steatosis.9 T1 bias was avoided by using a gradient-recalled-echo sequence with a low flip angle (10°) and a repetition time of ≥ 150 milliseconds.10,11 Six gradient-recalled echoes were acquired at sequential out-of-phase and in-phase echo times to measure the fractional fat signal while correcting for T2* signal decay.10,12,13 A multipeak fat spectral model was applied to correct for multifrequency interference effects of fat proton signals. A 1-cm radius circular region of interest (ROI) was placed in each of the nine Couinaud liver segments, and a composite PDFF value was calculated for each MRI exam by averaging the individual PDFF values from the nine ROIs.
Data Analysis
Descriptive statistics and exploratory graphing were used to assess the normality of the data in terms of the presence of skew and/or outliers. All data were within normal range and none were transformed. All missing data were examined to assess randomness. Data were analyzed using analyses of variance (ANOVAs) for continuous variables and Chi-square for dichotomous variables. Fatty liver was defined as MRI-PDFF ≥ 5.0% for the primary analysis.14,15 Overall prevalence estimates for NAFLD were directly standardized for the regional distribution of obesity by sex and ethnicity. Sex-specific estimates of prevalence with directly standardized for ethnicity. Optimal ALT cut points were determined using receiver operating characteristics (ROC) analysis. The classification and regression tree (CART) method was utilized to construct a decision tree for classifying children with obesity as having or not having NAFLD. The following variables were considered: age, sex, BMI z-score, ALT, AST, triglycerides, HDL, glucose, and insulin. The decision tree with the highest predictive power was selected. A secondary analysis of the prevalence was performed using other PDFF cut points that have been proposed in the literature (3.5%, 6.4% and 9.0%).16,17,18 All statistical tests were two-tailed and conducted using SPSS version 23 (IBM, Armonk, NY). Differences were considered statistically significant at a p-value < 0.05.
RESULTS
Demographics
There were 408 children with obesity ages 9 to 17 years included in this study. The study population characteristics are presented in Table 1. The mean age was 13.2 (± 4.0) years and was not significantly different in those with and without NAFLD. The mean BMI percentile was 98.0 and was significantly higher in children with NAFLD than those without NAFLD (98.5 vs 97.8, p<0.001). The mean ALT was 32 (± 32) U/L and was also significantly higher in children with NAFLD than those without NAFLD (53 vs 24, p<0.0001). As shown in Table 1, the mean values of AST, Triglycerides, HDL, and insulin all differed significantly in children with and without NAFLD.
Table 1.
Subject Characteristics by Fatty Liver Status
| All Participants N=408 | Normal Liver N = 291 | Fatty Liver N = 117 | p-value | |
|---|---|---|---|---|
| Sex, N (%) | 0.137 | |||
| Male | 217 (53.2) | 148(50.9) | 69 (59.0) | |
| Female | 191 (46.8) | 143 (41.1) | 48 (41.0) | |
| Age, mean (SD), yrs | 13.2 (4) | 13.9 (2.3) | 13.7 (2.4) | 0.343 |
| Weight, mean (SD), kg | 82.2 (19.3) | 80.1 (18.2) | 87.2 (20.8) | 0.001 |
| Height, mean (SD), cm | 161.2 (11.8) | 160.7 (12.1) | 162.4 (11.1) | 0.172 |
| BMI, mean (SD), kg/m2 | 31.3 (4.9) | 30.7(4.8) | 32.6 (4.9) | 0.001 |
| BMI percentile, mean (SD) | 98.0 (1.3) | 97.8 (0.1) | 98.5 (1.2) | 0.000 |
| BMI z-score, mean (SD) | 2.1 (0.3) | 2.0 (0.3) | 2.2 (0.3) | 0.000 |
| Race, N (%) | 0.476 | |||
| American Indian | 7 (1.7) | 5 (1.7) | 2 (1.2) | |
| Asian | 14 (3.4) | 7 (2.4) | 7 (6.0) | |
| Black | 9 (2.2) | 7 (2.4 | 2 (1.2) | |
| White | 241 (59) | 175 (60.1) | 66 (56.4) | |
| Other | 137 (33.6) | 97 (33.3) | 40 (34.1) | |
| Ethnicity, N (%) | 0.038 | |||
| Hispanic | 314 (77.0) | 216 (74.2) | 98 (83.8) | |
| Non-Hispanic | 94 (23.0) | 75 (25.8) | 19 (16.2) | |
| ALT, mean (SD), U/L | 32.0 (32.3) | 23.6 (19.2) | 53.1 (46.0) | 0.000 |
| AST, mean (SD), U/L | 30.0 (18.9) | 26.5 (12.1) | 38.7 (28.0) | 0.000 |
| TG, mean (SD), mg/dL | 117.4 (75.6) | 111.1 (77.4) | 132.9 (68.9) | 0.008 |
| HDL, mean (SD), mg/dL | 43.2 (10.0) | 44.5 (10.0) | 40.2 (9.3) | 0.000 |
| Glucose, mean (SD), mg/dL | 87.3 (10.1) | 86.9 (9.7) | 87.9 (11.0) | 0.389 |
| Insulin, mean (SD), uU/ml | 25.5 (24.0) | 21.3 (21.5) | 35.6 (26.7) | 0.000 |
Prevalence of NAFLD
The overall estimated prevalence of NAFLD in children with obesity was 26.0% (95% CI 24.2 – 27.7). As shown in Table 2, the prevalence was 29.4% in males (CI 26.1 – 32.7%) and 22.6% in females (CI 16.0 – 29.1%).
TABLE 2:
Prevalence of NAFLD by MRI PDFF Threshold Value in children with obesity
| PDFF Threshold | Prevalence of Fatty Liver | 95 Percent CI |
|---|---|---|
| ≥ 5.0% | ||
| All | 26.0% | 24.2 – 27.7% |
| Boys | 29.4% | 26.1 – 32.7% |
| Girls | 22.6% | 16.0 – 29.1% |
| ≥ 3.5% | ||
| All | 49.3% | 47.9–50.7% |
| Boys | 50.0% | 49.0 – 51.9% |
| Girls | 48.5% | 48.5 – 52.8% |
| ≥ 6.4% | ||
| All | 19.1% | 16.7–21.4% |
| Boys | 23.5% | 19.1 – 27.9% |
| Girls | 14.7% | 5.5 – 23.8% |
| ≥ 9.0% | ||
| All | 11.5% | 8.5–14.4% |
| Boys | 15.2% | 9.2 – 21.3% |
| Girls | 7.7% | 0.2 – 19.6% |
Diagnostic accuracy of ALT value for NAFLD
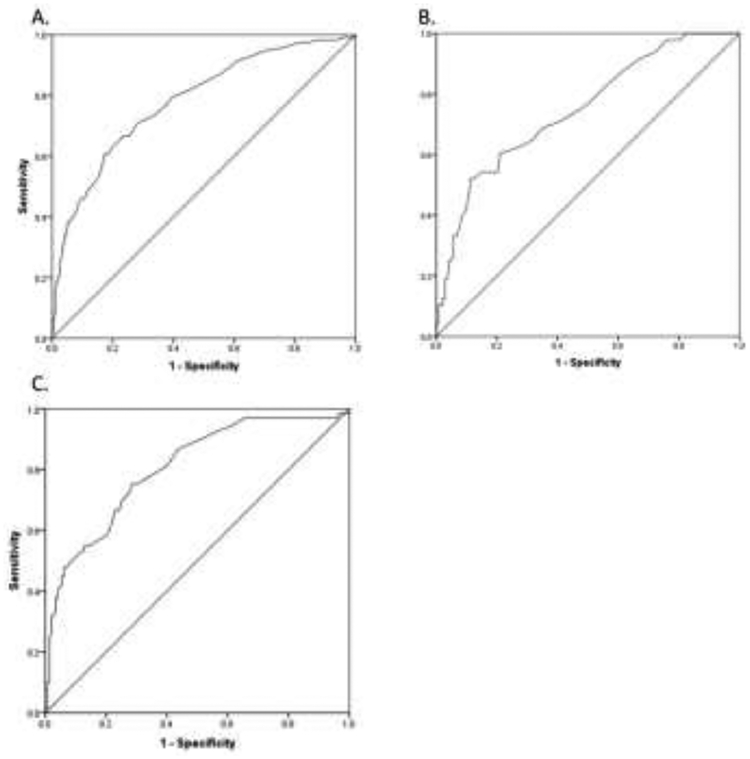
For males with obesity, the optimal ALT cut point was 42 U/L (47.8% sensitivity, 93.2% specificity), which provided a diagnostic accuracy of 80%. A diagnostic accuracy of 80% was also achieved for females with obesity using an optimal ALT cut point of 30 U/L (52.1% sensitivity, 88.8% specificity). See ROC curves in figure 1.
Figure 1.
Shown are ROC curves for ALT as a diagnostic tool for hepatic steatosis in children with obesity. Panel A shows the ROC for all children with an AUROC of 0.78. Panel B shows the ROC for girls with an AUROC of 0.75. Panel C shows the ROC for boys with an AUROC of 0.81.
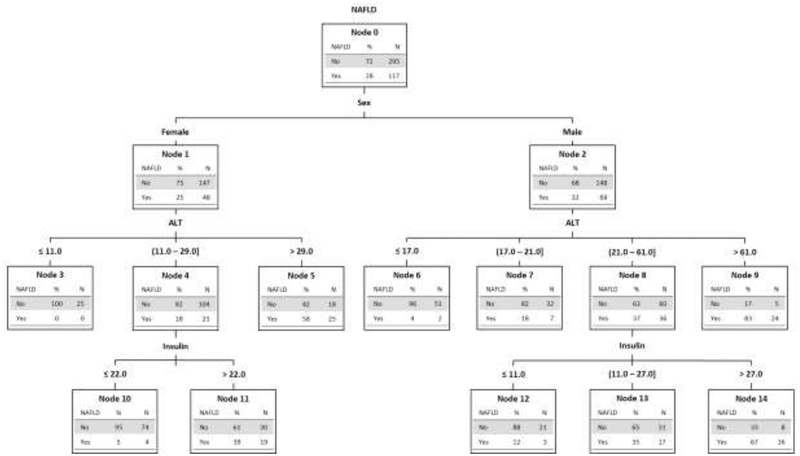
Decision tree for classification of NAFLD status
The CART model was based upon sex, ALT, and insulin. As shown in Figure 2, for optimal classification, ALT was separated into 3 categories for females and 4 categories for males. Insulin was used to better sort females with ALT > 11 through 29 U/L and to better sort males with ALT > 21 through 61 U/L. The overall diagnostic accuracy of the model was 80%. Each node shows the probability of correct classification based upon sex and a given ALT and insulin.
Figure 2.
Decision tree algorithm for NAFLD in children with obesity developed by machine learning via classification and regression tree modeling. The tree separates by sex (nodes 1 and 2), then by ALT U/L (nodes 3–9) and then by fasting insulin uU/ml (nodes 10–14). Each node shows the separation of NAFLD and not NAFLD based upon the specific parameters by total number and percentage.
Impact of using alternate MRI-PDFF cut points on prevalence
As shown in Table 2, using an MRI-PDFF cut point of 3.5%, the prevalence of NAFLD in children with obesity was 49.3% (95% CI: 47.9, 50.7%) with a similar estimate for males and females. Using a PDFF cut point of 6.4%, the prevalence of NAFLD in children with obesity was 19.1%, with higher rates in males than females (23.5% in males; 14.7% in females). Finally, using a PDFF cut point of 9.0%, the prevalence of NAFLD in children with obesity was 11.5% (95% CI: 8.5, 14.4%) with a higher prevalence in males (15.2%; 95%CI: 9.2, 21.3%) than in females (7.7%; 95% CI: 0.2, 19.6%).
DISCUSSION
We performed a large, community-based study to determine the prevalence of NAFLD in children ages 9 to 17 years with obesity. Using the most commonly reported threshold of liver MRI PDFF of 5.0%, the estimate for the prevalence of NAFLD in children with obesity was 26.0%. We identified sex-specific values for ALT for the classification of NAFLD in children with obesity. In addition, we developed a diagnostic tree that added insulin to sex and ALT to classify children with obesity as having or not having NAFLD. In addition, we evaluated the impact of alternate proposed MRI-PDFF thresholds and demonstrated that the choice of threshold has a substantial effect on the resulting prevalence estimate for NAFLD in children with obesity.
The estimate of the prevalence of NAFLD is influenced by the choice of study population, the sample size, and the accuracy of the diagnostic modality used. Prior studies have reported a wide range for the prevalence of NAFLD in children, from < 2% to greater than > 80% depending on the study.5,6,7 For example, in a study of 181 children with obesity drawn from a general pediatrics clinic that predominantly served children of black race, the prevalence of NAFLD was 8%.19 Factors that may have accounted for a relatively low prevalence estimate included a lower severity of obesity in general pediatrics, lower rates of NAFLD in children of black race, and the use of ALT as the diagnostic tool.1 Studies that have used ALT to detect NAFLD, in general, have lower prevalence estimates. In contrast, a study of 84 children with obesity in a tertiary referral clinic in China reported a prevalence of NAFLD of 77%.20 The high prevalence was likely influenced by the smaller sample size, the greater severity of obesity, higher rates of NAFLD in Asian children, and the use of ultrasound as the diagnostic tool. In general, studies utilizing ultrasound as the diagnostic modality have higher prevalence estimates. In addition to the diagnostic modality utilized, sample size influences estimate of prevalence such that studies with small sample sizes tend to have extreme values (< 20% and > 50%) and those with sample sizes > 300 have prevalence estimates that are in between (20–40%).21, 22, 23 Therefore, our observed prevalence of NAFLD of 28.7% in children with obesity was consistent with expected prevalence given that our study included over 400 children selected from the general community and used MRI-PDFF as the diagnostic modality.9, 24, 25
NASPGHAN guidelines recommend ALT as the best screening test for NAFLD in children.4 These guidelines propose an ALT ≥ 80 U/L on initial screening or ALT greater than or equal to twice the upper limit of normal (ALT ≥ 44 U/L for females and ALT ≥ 52 U/L for males) on repeated screening as an indication for further evaluation.4, 26 The optimal cut-points from our study are lower than the current recommended evaluation points. In this study, we determined that ALT ≥ 30 U/L in females with obesity and ≥ 42 U/L in males with obesity provided a good diagnostic accuracy for determining the presence of NAFLD. Of note, these ALT levels are only slightly higher than the 95th percentile for all children. As demonstrated in the SAFETY study, for the general population including children with obesity, the 95th percentile for ALT was 26.0 U/L in females and 37.2 U/L in males.24 On one hand, strong diagnostic accuracy reinforces that ALT is an effective screening tool. It is worth noting that there is not another commonly available marker with equivalent diagnostic accuracy. Moreover, of the other laboratory values commonly obtained clinically, only insulin improved the classification of an individual child with obesity as having or not having NAFLD. However, ALT alone is not a sufficient diagnostic tool. No matter what threshold is used, some children with obesity and ALT levels above a given threshold will have reasons for liver chemistry elevation other than NAFLD. In addition, ALT remains unable to differentiate disease severity.27,28 Thus, for patient care purposes, ALT should be used as a screening tool and not as a diagnostic tool.
In this study, NAFLD was defined as an MRI-PDFF ≥ 5.0%, which is a threshold for hepatic steatosis that has been utilized in other studies.28,29 Because a non-invasive diagnostic tool for NAFLD is of broad interest, having a set point for dichotomous determination of NAFLD is appealing for clinicians and patients alike. The data from the current study demonstrate that the choice of cut-point used can have a large impact on whether an individual child is considered to have NAFLD. As multiple cut points have been used previously to delineate grade 0 from grade 1 steatosis, and no single MRI-PDFF has been proven to be diagnostic for pediatric NAFLD,9 we also examined multiple MRI-PDFF cut points to demonstrate the impact on estimated point prevalence. We observed large differences in estimated NAFLD prevalence in children with obesity resulted from changing the MRI-PDFF cut point by small increments; a decrease of 1.5 percentage points in PDFF cutoff (e.g., from 5.0% to 3.5% PDFF) resulted in an increase of estimated NAFLD prevalence from 28.7% to 53.2%. Moreover, response to treatment and natural history may be framed by the choice of MRI-PDFF cut point. Thus, as the field continues to increasingly utilize MRI-PDFF as a non-invasive diagnostic tool for NAFLD, it is imperative that there be more uniform criteria established for diagnostic thresholds.
A major strength of this study was the large sample size and detailed phenotyping of the participants. Participants were obtained from a broad, community sample which decreased the selection bias present in a sample from a tertiary referral center. Additionally, the use of MRIPDFF provided an accurate, reproducible measure of hepatic steatosis. One study limitation was the lack of liver histology; however, this is not feasible for population-based epidemiology. Additionally, this study had a large prevalence of Hispanic children and thus may not represent all communities. We adjusted our prevalence estimates for ethnicity, but similar data from other regions would be useful.
NAFLD is common in children with obesity, but NAFLD and obesity are not concomitant. We estimate that in children with obesity, NAFLD is present in nearly one-third of boys and one-fourth of girls. These data should be useful for future initiatives in the treatment and prevention of NAFLD. Moreover, the study data highlight the need to develop standardized diagnostic cutoffs for MRI measured hepatic steatosis. Finally, we propose new, evidence based ALT thresholds for the detection of NAFLD derived from a community sample without prior knowledge of presence of liver disease.
Acknowledgments
Financial Support: The project was partially supported by the National Institutes of Health, Grants UL1TR000100 and UL1TR001442. The funders did not participate in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used in this paper:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
Body mass index
- HDL
high-density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- PDFF
proton density fat fraction
- ROI
region of interest
- TG
triglyceride
Footnotes
Conflict of Interest: Michael Middleton, MD consults for Bracco, Kowa, Median, Merge Healthcare, Novo Nordisk, Quantitative Insights; is a stockholder with General Electric and Pfizer, and has grant funding from Gilead and Guerbet.
Claude B. Sirlin, MD has industry research support from Bayer, GE, Philips and Siemens; consults for AMRA, Boehringer and Guerbet; is on the speaker’s bureau for Resoundant and has lab service agreements with Gilead, ICON, Intercept, Shire and Synageva.
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006; 118:1388–1393. [DOI] [PubMed] [Google Scholar]
- 2.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J Clin Gastroenterol. 2018; 52:339–346. [DOI] [PubMed] [Google Scholar]
- 3.Giorgio V, Prono F, Graziano F, Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013:13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohil R, et al. NASPGHAN Clinical Practice Guildeline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease In Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017; 64:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha R, Cotrim HP, Bitencourt AG, Barbosa DB, Santos AS, Almeida Ade M, et al. Nonalcoholic fatty liver disease in asymptomatic Brazilian adolescents. World J Gastroenterol. 2009; 15:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Rivera C, Hadjiyannakis S, Davila J, Hurteau J, Aglipay M, Barrowman N, et al. Prevalence and risk factors for non-alcoholic fatty liver in children and youth with obesity. BMC Pediatr. 2017; 17:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xanthakos SA, Jenkins TM, Kleiner DE, Boyce TW, Mourya R, Karns R, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. Gastroenterology. 2015; 149:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015; 61:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008; 26:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007; 58:354–364. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007; 26:1153–1161. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008; 60:1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016; 64:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014; 5:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Martino M, Pacifico L, Bezzi M, Di Miscio R, Sacconi B, Chiesa C, et al. Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver setaosis in children and adolescents. World J Gastroenterol. 2016; 22:8812–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013; 267:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease – MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012; 36:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louthan MV, Theriot JA, Zimmerman E, Stutts JT, McClain CJ. Decreased prevalence of nonalcoholic fatty liver disease in black obese children. J Pediatr Gastroenterol Nutr. 2005; 41:426–429. [DOI] [PubMed] [Google Scholar]
- 20.Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, et al. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004; 28:1257–1263. [DOI] [PubMed] [Google Scholar]
- 21.Guzzaloni G, Grugni G, Minocci A, Moro D, Morabito F. Liver steatosis in juvenile obesity: correlations with lipid profile, hepatic biochemical parameters and glycemic and insulinemic response to an oral glucose tolerance test. Int J Obes Relat Metab Disord. 2000; 24:772–776. [DOI] [PubMed] [Google Scholar]
- 22.Tazawa Y, Noguchi H, Nishinomiya F, Takada G. Serum alanine aminotransferase activity in obese children. Acta Paediatr. 1997; 28:385–388. [DOI] [PubMed] [Google Scholar]
- 23.Quiros-Tejeira RE, Rivera CA, Aiba TT, Mehta N, Smith CW, Butte NF. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI > or = 95th percentile. J Pediatr Gastroenterol Nutr. 2007; 44:227–236. [DOI] [PubMed] [Google Scholar]
- 24.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010; 138:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, et al. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One 2015;10: e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, Ellis LL, Vanderwall K and Fontanesi J Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 1013; 38: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwimmer JB. Clinical advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2016; 63:1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies From Adults With Nonalcoholic Steatohepatitis. Gastroenterology. 2017; 153:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Middleton MS, Van Natta ML, Heba ER, Alazraki A, Trout AT, Masand P, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology. 2018; 67:858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]