Abstract
An area within the ventral occipitotemporal cortex (vOTC), the “visual word form area” (VWFA), typically exhibits a strongly left-lateralized response to orthographic stimuli in skilled readers. While individual variation in VWFA lateralization has been observed, the behavioral significance of laterality differences remains unclear. Here, we test the hypothesis that differences in VWFA lateralization reflect differing preferences for holistic orthographic analysis. To examine this hypothesis, we implemented a new multivariate method that uses machine learning to assess functional lateralization, along with a traditional univariate lateralization method. We related these neural metrics to behavioral indices of holistic orthographic analysis (inversion sensitivity). The multivariate measure successfully detected the lateralization of orthographic processing in the VWFA, and as hypothesized, predicted behavioral differences in holistic orthographic analysis. An exploratory whole brain analysis identified further regions with a relationship between inversion sensitivity and lateralization: one near the junction of the inferior frontal and precentral sulci, and another along the superior temporal gyrus. We conclude that proficient native readers of English exhibit differences in cortical lateralization of the VWFA that have significant implications for reading behavior.
Keywords: MVPA, Orthography, Reading, VWFA, Word-processing, Lateralization
Hemispheric lateralization, the localization of a function predominantly to the left or right hemisphere, has shed light on our understanding of a range of cognitive functions (e.g., Calvo & Beltrán, 2014; Heine, Kuteva, & Kaltenböck, 2014; Schotten et al., 2011). Lateralization describes both laterality of hemispheric dominance and the degree of that dominance (Binder et al., 1996; Hinke et al., 1993). Spoken language processing tends to be lateralized in the left hemisphere (Baciu, Juphard, Cousin, & Bas, 2005; Knecht et al., 2000). In contrast, the lateralization of visual word form (i.e., orthographic) processing exhibits more variability, for reasons that remain poorly understood. In this paper, we test whether differences in the lateralization of the ventral occipitotemporal cortex, termed the “visual word form area” (VWFA), are indicative of individual differences in orthographic analysis. More specifically, we use a new multivariate technique for measuring laterality, and test whether the degree of VWFA lateralization is predicted by sensitivity to visual inversion, which we use as an index of holistic orthographic analysis.
The strongest evidence for variability in VWFA laterality comes from cross-linguistic studies comparing alphabetic versus non-alphabetic writing systems (e.g., English versus Chinese). Typically, readers of alphabetic writing systems exhibit strongly left-lateralized VWFA activation in response to printed words, whereas readers of non-alphabetic writing systems often exhibit bilateral engagement of the left VWFA and its right-hemisphere homologue (rVWFA) (Bolger, Perfetti, & Schneider, 2005; Liu, Dunlap, Fiez, & Perfetti, 2007; Mei et al., 2015, 2013; Mo, Yu, Seger, & Mo, 2015; Nelson, Liu, Fiez, & Perfetti, 2009; Tan et al., 2001). This pattern has even been observed in an artificial orthography study involving native English speakers: learners of an alphabetic orthography exhibited left-lateralized engagement of the VWFA, while learners of a non-alphabetic orthography exhibited bilateral engagement of the VWFA (Hirshorn, Wrencher, Durisko, Moore, & Fiez, 2016).
These cross-linguistic differences in VWFA lateralization may occur because an orthography’s mapping principle – the unit of sound that is represented by its graphemes – influences how a word is visually processed (Hirshorn & Fiez, 2014). Alphabetic orthographies, in which graphemes are mapped onto phonemes in a spoken language, may bias readers toward analytic visual strategies that emphasize the component features of a stimulus (H. K. Pae, Sun-A, Mano, & Kwon, 2017). For example, this could include reading strategies that focus attention on the sequential letters of a visual word form. Non-alphabetic orthographies, in which graphemes map onto larger units of sound (e.g., syllables or morphemes, as in Chinese), may bias readers towards a greater use of holistic visual strategies that emphasize the overall structure of a visual word form (Ben-Yehudah, Hirshorn, Simcox, Perfetti, & Fiez, 2018). For instance, this could include reading strategies that focus attention on the entire shape of the visual word form or the configural relationships between the orthographic subcomponents within a word.
This line of reasoning aligns well with the face processing literature, where engagement of the right visual association cortex has been associated with holistic visual analysis, and engagement of left visual association cortex with analytic or part-based visual analysis (Rossion et al., 2000). This has led reading researchers to test for cross-linguistic differences in holistic versus analytic orthographic analysis by borrowing experimental manipulations from the face recognition literature (Akamatsu, 1999, 2003; Ben-Yehudah et al., 2018; Pae et al., 2017; Pae & Lee, 2015).
For example, in studies of face processing, applying visual-spatial distortions, like inversion, is thought to disrupt holistic visual perception (Maurer, Grand, & Mondloch, 2002; Yovel & Kanwisher, 2008). The resulting slowing of response-times and drop in accuracy after inversion is believed to reflect a switch to the (now, necessary) analytical approach. A strategy switch toward analytical processing is associated with a shift away from the typically right-lateralized fusiform gyrus response to faces (Rossion et al., 2000). Applying this to orthographic analysis, it has been hypothesized that sensitivity to inversion should be linked with relatively greater holistic processing and more bilateral engagement of the VWFA. As predicted by this hypothesis, the reading performance of individuals from non-alphabetic writing systems is more affected by visual distortions than those from alphabetic writing systems (Akamatsu, 1999, 2003; Ben-Yehudah et al., 2018; Pae et al., 2017; Pae & Lee, 2015). Further, visual inversion has a different effect in these groups on the lateralization of the N170, an evoked potential response associated with early visual processing. Visual inversion leads to similar right lateralization of the N170 response to Chinese characters and faces in Chinese readers (M.-Y. Wang, Kuo, & Cheng, 2011), but face and word inversion produces opposite laterality effects in English readers (Rossion, Joyce, Cottrell, & Tarr, 2003). Similar results have been observed with fMRI, with inversion of English words versus faces producing opposite effects on activity patterns in the fusiform gyrus (Sussman, Reddigari, & Newman, 2018). Taken together, there is a strong body of cross-linguistic literature that links sensitivity to visual distortion, holistic orthographic analysis, and right hemisphere engagement.
The current study builds on this prior research by examining VWFA lateralization within a group of native English speakers. Variable engagement of the right VWFA in native readers of an alphabetic orthography has been reported within the literature, though it has received little commentary or systematic investigation (Cohen et al., 2003; Dehaene, Le Clec’H, Poline, Le Bihan, & Cohen, 2002; Reinke, Fernandes, Schwindt, O’Craven, & Grady, 2008; Vigneau, Jobard, Mazoyer, & Tzourio-Mazoyer, 2005). We hypothesize that bilateral engagement of the VWFA is associated with a greater reliance on holistic orthographic analysis, and therefore individual differences in VWFA lateralization should be predicted by inversion sensitivity, a behavioral measure of holistic orthographic analysis.
In measuring VWFA lateralization, the study makes use of both a conventional univariate and new multivariate approach for measuring neural lateralization. For the conventional univariate method, lateralization is examined by comparing the mean response of left and right homologue regions to a task condition (or stimulus). If the mean activation of the two homologues is significantly different, the function being tested is considered to be more lateralized to one hemisphere. If there is no significant difference, the function is considered bilateral. Although the univariate method has advanced our understanding of lateralization, it is restricted to comparing conditions that differentially modulate the overall activation of a voxel or region (Norman, Polyn, Detre, & Haxby, 2006). This limitation inspired the idea of using multivariate techniques to assess lateralization, to yield what we will refer to as a “Multivariate Laterality Index”. Multi-voxel (multivariate) pattern analysis (MVPA) is a technique that – rather than testing a region’s mean activation level – measures the information contained in distributed patterns of neural activity (Haxby et al., 2001), often using machine learning algorithms. This approach typically involves training a classifier to distinguish patterns of activity evoked by conditions of interest. The classifier is then tested on independent data to measure how well conditions or stimuli can be discriminated based on the neural data. The resulting classification performance, or decoding accuracy, reflects the degree to which a condition is represented in the pattern of activity distributed across a region’s multiple voxels. MVPA provides sensitivity to information that cannot be detected in mean activation levels alone (Coutanche, 2013; Tong & Pratte, 2012). Furthermore, participant variation in classifier decoding performance can be a more sensitive biomarker of individual differences than differences in univariate responses (Coutanche, Thompson-Schill, & Schultz, 2011) – a benefit that we also draw on in this work.
In this study we present words, letter-strings, and other stimulus types during an fMRI scan to native English readers who vary in word inversion sensitivity, our index of holistic orthographic analysis. We employ both a traditional univariate approach to measuring lateralization, and a novel multivariate laterality measure that characterizes left and right brain regions based on the information contained in their respective multi-voxel patterns. We hypothesize that individuals with greater word inversion sensitivity (i.e., a bias towards holistic visual processing for words) will employ the VWFA with a greater degree of bilaterality. Furthermore, we hypothesize that the multivariate (pattern-based) laterality technique will be more sensitive than a traditional univariate (activation-based) lateralization measure to this individual difference.
Method
Participants
Data from 22 participants (8 males; 18–22 years old, mean (M) age = 19 years) are analyzed. Two other participants were excluded from the analysis: 1) one participant’s partial brain scan excluded the region of interest, 2) one participant’s head motion exceeded the maximum cutoff of 3.5 mm. The Edinburgh Handedness Inventory was used to determine handedness (Oldfield, 1971). All participants were right-handed with normal or corrected-to-normal vision and native English speakers. Participants completed a prescreening in which they reported no history of hearing or vision problems, learning and reading difficulties, or neurological problems. Participants provided written informed consent and were compensated for their time.
Word Inversion Sensitivity
Prior to scanning, each participant’s sensitivity to visual-spatial distortion (inversion sensitivity) was measured using a lexical decision task (LDT), in order to select participants for the imaging study. In this task participants were presented with both upright and inverted (rotated 180°) letter-strings and instructed to decide as quickly and as accurately as possible whether it is an English word. Participants were shown 20 words and 20 nonwords. Half of the words and nonwords were presented in each orientation. Initially, median RTs for upright words were used to screen out individuals with atypical reading ability. Specifically, of the initial 411 potential participants, participants with RTs outside the 25th to 75th percentile were removed leaving 203. We then used accuracy and response times (excluding RTs for incorrect trials) for analysis. The sensitivity to visual-spatial distortion of each participant was calculated as the ratio of median RT for inverted words and nonwords divided by the median RT for upright words and nonwords, providing a measure we call the lexical decision ratio (LDR). Higher scores on this measure reflect a larger slowing of response-times in response to inverting words/nonwords. The median ratio of the remaining participants was 1.42 with a standard error (SE) of 0.037. To determine the group cutoffs, we used the median ratio ± 2 SE (rounded to 1.35 and 1.5). Thereby, participants with higher sensitivity to visual-spatial distortion (HS) were defined as those individuals with RTs for inverted stimuli that were at least 1.5 times greater than upright stimuli. Participants with lower sensitivity to visual-spatial distortion (LS) were defined as those individuals with RTs for inverted stimuli that were less than 1.35 times that of upright stimuli. Twenty-two participants were successfully grouped to participate in the imaging session.
To ensure group assignment reliability for inversion sensitivity, we also used a second measure, an overt naming task (NT). In this task participants were instructed to name aloud the presented upright and reversed words (FLIGHT → THGILF) as quickly and accurately as possible. The set of stimuli assigned to each orientation condition was matched along each of the dimensions sampled by Graves et al. (2010). Similar to the LDR, to determine the overt naming ratio (ONR), the median reversed orientation RT was divided by the median upright RT. Higher scores again reflect a larger slowing of response-times in response to visual-spatial distortion. A median ratio of 1.16 was used to confirm participants in low and high sensitivity groups, where scores below the median ratio confirmed membership in the group with lower sensitivity to visual-spatial distortion, and scores above the median ratio confirmed membership in the group with higher sensitivity to visual-spatial distortion. Four participants were then excluded from group analysis whose ONR were not consistent with their LDT assignment (these participants are excluded from the group analyses, but included in a continuous analysis examining the relationship between individual lateralization and inversion sensitivity scores). After using both the LDR and ONR, the HS group had 8 participants, the LS group had 10 participants, and 4 participants did not meet the cutoffs of either group.
Participants in both the HS and LS groups were matched in reading sub-skills. Word identification, phonemic decoding, and passage comprehension were assessed using subtests from the Woodcock Reading Mastery Test (Woodcock, 1998). In the word identification (Word ID) subtest, participants read a list of words aloud. The LS group (M = 547, SE = 2.09) did not differ from the HS group (M = 545, SE = 2.92; t(17) = 0.68, p = 0.51) on the Word ID subtest. For phonemic decoding, participants completed a Word Attack task where participants read nonwords out loud. Scores did not differ from the LS group (M = 521, SE = 1.88) and HS group (M = 519, SE = 2.05; t(17) = 0.52, p = 0.61). For the passage comprehension subtest, participants read passages and provided words for missing blanks that correctly fit the context of the passage. The LS group (M = 539, SE = 1.80) and HS groups (M = 534, SE = 2.32) did not differ in scores (t(17) = −1.79, p = 0.09). We used the Comprehensive Test of Phonological Processing (CTOPP) subtests to assess phonological awareness (Wagner, Torgesen, & Rashotte, 1999). LS (M = 102, SE = 3.34) and HS (M = 103, SE = 2.46) groups did not differ in phonological awareness (t(17) = −0.25, p = 0.81). The LS (M = 14.43, SE = 0.47) and HS (M = 13.82, SE = 0.66) groups were matched on vocabulary which was assessed using the Wechsler Adult Intelligence Scale, Fourth Edition Vocabulary test (t(17) = 0.78, p = 0.44; Wechsler, Coalson, & Raiford, 2008). We used the Lexical Knowledge Battery (Perfetti & Hart, 2001) to test spelling, which involved showing participants a list of correctly and incorrectly spelled words and having them determine if the words were spelled properly. The LS (M = 2.06, SE = 0.09) and HS (M = 2.10, SE = 0.13) groups did not differ in performance (t(17) = −0.21, p = 0.83).
fMRI Data Acquisition and Preprocessing
Participants were scanned with a 3-T head only Siemens Allegra magnet and standard radio-frequency coil at the University of Pittsburgh. Prior to functional scanning, whole brain structural images were collected using a T1-weighted echoplanar imaging sequence consisting of 192 axial slices of 1 mm thickness (1 × 1 × 1 mm voxels) parallel to the anterior commissure–posterior commissure (AC–PC) plane. T2-weighted scans recorded blood oxygenation level-dependent (BOLD) response using a one-shot EPI pulse (repetition time (TR) = 1500 ms, echo time (TE) = 25 ms, field of view (FOV) = 200 mm, voxel size = 3.125 × 3.125 × 3.125 mm, 38 slices, flip angle (FA) = 70°).
Imaging data were preprocessed using the Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996) to correct for artifacts and movement. The first 4 volumes of each functional scan were removed to allow for scanner equilibration. All functional images were first slice-time corrected. A motion correction algorithm registered all volumes to a mean functional volume. All participants displayed less than 3.5 mm of head motion. Voxel activation values were scaled to have a mean of 100, with a maximum limit of 200. Structural and functional images were converted to standardized space (Talairach, 1988) for group comparisons.
fMRI Experimental Procedure
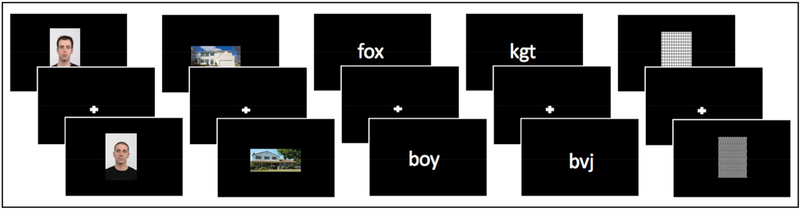
Participants completed a 1-back task, which involves pressing a button if an image repeats, while in the fMRI scanner. Five visual stimuli categories consisting of words, letter-strings, houses, faces, and patterns were presented at the center of the screen (Figure 1). The procedure followed protocols related to previous studies of reading (Fox, Iaria, & Barton, 2009; Rossion, Hanseeuw, & Dricot, 2012). Stimuli were drawn from sets of 40 exemplars for each of the non-orthographic (houses, faces, and patterns) categories, and 157 exemplars for the orthographic (words and letter-strings) categories. The size of the images used in the scanner differed across the five conditions (e.g., stimuli in the “faces” category were all matched in size, but differed from the stimuli matched in size in the “house” category). The letter-strings category consisted of only consonants. Words and letter-strings were matched on length. Participants completed four functional runs lasting six minutes each. Each run consisted of 15 blocks (three of each category, pseudo-randomly ordered). Every block lasted for 15 s and included 15 trials, with the stimulus for each trial displayed for 200 ms followed by an 800 ms fixation cross. A 1-back target was presented for 12.5% of each block. A 9 s baseline condition followed each block. During this baseline, participants attended to a fixation cross at the center of the screen. During each run, the sets of house, face, and pattern stimuli were pseudo-randomly distributed within each of the three blocks for each condition. With the exception of 1-back trials, the words and letter-strings stimuli did not repeat across runs.
Figure 1.
Examples of houses, faces, words, letter-strings, and pattern stimuli presented during the localizer in the fMRI session.
ROI Localization
Within each participant, we localized the VWFA and rVWFA using both univariate and multivariate methods. We defined the VWFA on the middle segment of the left ventral occipitotemporal cortex (vOTC) first using the peak mean activation (univariate), and then decoding accuracy (multivariate). The same methods were then used to localize the rVWFA in the right vOTC.
Localizing using univariate techniques.
We first attempted to localize the VWFA using a univariate contrast of words versus letter-strings because previous research suggests the VWFA is more responsive to words rather than letter-strings (Cohen et al., 2002). The univariate method failed to locate the VWFA and rVWFA using this particular contrast, so a contrast of words and letter-strings versus fixation cross was employed instead. To match the localization approach to multivariate methods (described below) as closely as possible, we conducted a whole brain searchlight approach, in which a spherical mask (3-voxel radius) was sequentially placed around each voxel in the brain to create an ROI (maximum of 123 voxels; Kriegeskorte, Goebel, & Bandettini, 2006). The mean activation for the words and letter-strings versus fixation contrast (i.e., positive values indicate greater activation to words and letter-strings) was assigned to the center voxel of each searchlight. The peak mean activation within the middle segment of the vOTC was identified in each hemisphere of participants to localize the VWFA and rVWFA. The typical location of the univariate localized VWFA is shown in Figure 2B.
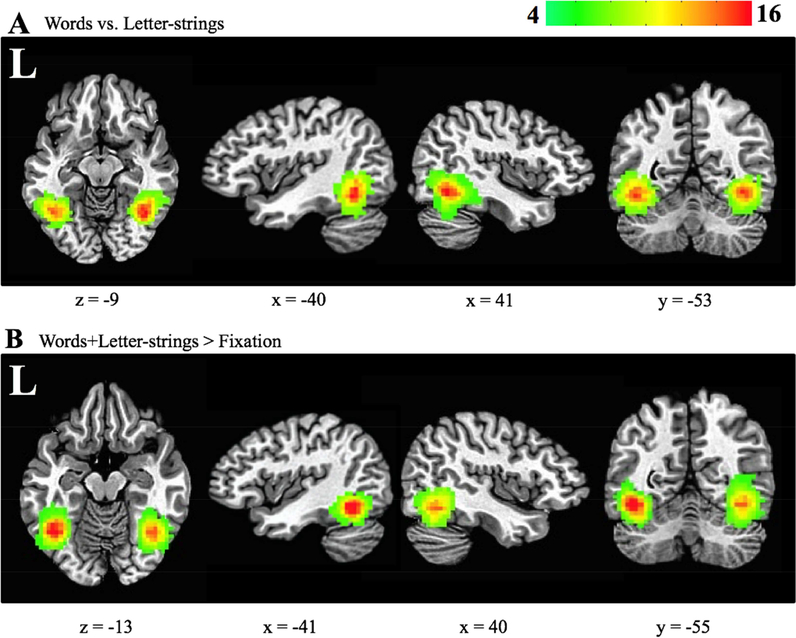
Figure 2.
VWFA and rVWFA subject coverage maps. Axial, left and right sagittal, and coronal views for (A) multivariate-defined (contrast: words versus letter-strings) and (B) univariate-defined (contrast: words+letter-strings > baseline) ROIs. All 22 participants are included in this map. Red represents voxels shared amongst more participants. Green represents voxels shared between fewer participants. The Talairach coordinates for the center of mass of these ROIs were: multivariate-defined VWFA (−40x, −53y, −10z), multivariate-defined rVWFA (41x, −52y, −10z), univariate-defined VWFA (−37x, − 55y, 10z), and univariate-defined rVWFA (39x, −55y, −11z).
Localizing using multivariate techniques.
Multivariate classification was conducted on spatially unsmoothed data within MATLAB using the Princeton Multi-Voxel Pattern Analysis toolbox (Detre et al., 2006). Prior applications of multivariate analysis techniques have shown an ability to distinguish more subtle stimulus distinctions than is possible with a more traditional univariate approach (Haxby et al., 2001). The greater sensitivity of multivariate analysis allowed us to successfully localize the ROIs using a words versus letter-strings contrast, which had not been possible with univariate methods. Similar to the univariate approach to localizing the VWFA and rVWFA, we conducted a whole brain searchlight analysis. Again, this was done by creating a spherical mask with a 3-voxel radius on each voxel in the brain.
Multivariate pattern decoding
For each run, we z-scored each voxel’s timecourse of pre-processed activity values. We shifted the timecourse by two TRs to account for the hemodynamic lag. We then trained a Gaussian Naïve Bayes (GNB) classifier and tested this classifier using activity patterns associated with TRs for words and letter-strings. Decoding was conducted using a leave-one-run-out cross-validation approach. Classification performance from the four iterations was averaged to give a single accuracy value. For each of the four cross-validation iterations, 180 TRs (60 from 3 runs) were used for training, while 60 TRs (in a held-out run) were used for testing (30 word TRs; 30 letter-string TRs).
Mean classifier performance from words versus letter-strings was assigned to the center voxel of each searchlight. The peak decoding accuracy in the left, and then the right, middle segment of the vOTC was used to localize the VWFA and rVWFA, respectively. We note that because a peak searchlight is selected for each hemisphere independently of the other, this ROI selection approach is orthogonal to the question of whether these peaks differ in decoding accuracy (i.e., lateralization). The typical location of the MVPA-localized VWFA is shown in Figure 2A. Additionally, for later comparison with the univariate approach, we also localized the VWFA and rVWFA using a classifier contrast of words and letter-strings versus fixation.
We evaluated the percentage overlap between the univariate and MVPA localized ROIs in each hemisphere by taking the number of shared voxels and dividing this by the total number of voxels for each ROI. The univariate-defined and MVPA-defined VWFA shared a mean overlap of 42% (standard deviation = 17%). The univariate-defined and MVPA-defined rVWFA shared a mean overlap of 32% (standard deviation = 16%).
Principal Components in Mid-Fusiform Cortex
To further examine the generalizability of differences between hemispheres, we also took the left and then right mid-fusiform cortex of each participant, and decomposed the corresponding BOLD signals into statistically uncorrelated components with a principal component analysis (PCA). Data from TRs associated with words and letter-strings (after shifting by 2 TRs to account for the hemodynamic delay) were submitted to a PCA. Components accounting for the top 90% of the variance in each hemisphere’s mid-fusiform cortex (mean number of components = 126.98, SD = 15.46) were used as classifier features for this analysis.
Lateralization analyses
After localizing the regions using both methods (giving univariate ROIs and multivariate ROIs), we extracted univariate and multivariate measures of lateralization from both sets of regions (i.e., univariate and multivariate lateralization measured in univariate-defined ROIs; univariate and multivariate lateralization measured in multivariate-defined ROIs). We used both univariate and multivariate localization techniques in order to eliminate localization bias as a confounding factor in any observed differences across our lateralization methods.
A univariate measure of lateralization of orthographic processing was calculated by subtracting the rVWFA mean activation values for words and letter-strings versus fixation, from VWFA mean activation for words and letter-strings versus fixation. A positive value denotes left lateralization of the VWFA. As each peak is localized independently in each hemisphere, a comparison of these values (to measure lateralization) is an orthogonal analysis. Our new multivariate measure of lateralization was calculated by subtracting the decoding accuracy of the rVWFA from the VWFA. A positive value denotes left multivariate laterality of the VWFA. Participants’ laterality scores were then correlated with their behavioral inversion sensitivity scores.
We also examined temporal signal-to-noise ratio (tSNR) in different regions. The tSNR was computed by dividing the mean signal intensity by the standard deviation of the noise time course for each voxel. We then averaged the tSNR for each univariate-defined and multivariate-defined ROI. TSNR was then compared across ROIs and inversion sensitivity groups to ensure that key group differences were not due to differing levels of noise.
An exploratory approach to investigating whole brain lateralization with a multivariate analysis was also conducted. Each participant’s map of searchlight accuracies (using the contrast of words versus letter-strings) were transformed to standardized space. The data maintained the same resolution as the functional data and remained spatially unsmoothed. Each participant’s searchlight map was then compared with the same searchlight map that was flipped along the inter-hemispheric fissure (so that searchlights originally in the left hemisphere were now projected on the right hemisphere, and vice versa). The searchlight maps were then subtracted from each other, to give a lateralization map, where positive values indicate that a searchlight has stronger decoding performance in a left hemisphere region than its right hemisphere homologue. An exploratory analysis was then conducted using the map, where participants’ searchlight lateralization values were correlated with their inversion sensitivity scores. The r-value from this correlation was allocated to the center of each searchlight. We subsequently verified that these regions contained orthographic information by transforming regions with an identified relationship back to native space to test ‘words versus letter-strings’ decoding. Classification performance was compared to chance performance to test significance.
Results
We examined the lateralization of orthographic processing in our two groups with high versus low inversion sensitivity (LS and HS participants) using a univariate and multivariate approach to lateralization. Before relating lateralization results to word inversion sensitivity, we first examined activation levels and classification performance in the VWFA and rVWFA. We took two approaches to localizing these regions, based on 1) peak activation levels and 2) peak classification performance. This allowed us to compare univariate and multivariate lateralization measures without a confounding effect of how the ROIs were selected. Specifically, it ensured that any differences between univariate and multivariate laterality measures were not because the ROIs were defined in a way that benefits one approach.
Univariate Localized VWFA and rVWFA Results
We first localized the VWFA and rVWFA with a traditional univariate approach, contrasting mean activation for words and letter-strings with fixation. A repeated measures ANOVA was conducted to examine the effect of hemisphere on activation level. There was a significant main effect of hemisphere on activation level, F(1,16) = 38.26, p < 0.001, = 0.69. A simple effects analysis showed the LS group had greater activation in the VWFA (M = 0.33, SD = 0.17) than rVWFA (M = 0.12, SD = 0.17; t(9) = 5.97, p < 0.001, g = 1.18) for words and letter-strings (relative to fixation). The HS group also showed greater VWFA (M = 0.31, SD = 0.13) than rVWFA (M = 0.17, SD = 0.22; t(7) = 3.12, p = 0.017, g = 0.73) activation for this contrast. There was no significant interaction between hemisphere and inversion sensitivity on activation levels, F(1,16) = 1.08, p = 0.315, = 0.02.
We quantified univariate lateralization by subtracting the mean activation in the rVWFA from the mean activation in the VWFA (so that greater values indicate stronger left lateralization). The univariate lateralization measure for the LS group (M = 0.20, SD = 0.11) and HS group (M = 0.15, SD = 0.13) did not differ significantly (t(16) = 1.01, p = 0.32, g = 0.39), suggesting similar univariate lateralization for both groups. These univariate results are shown in Figure 3A.
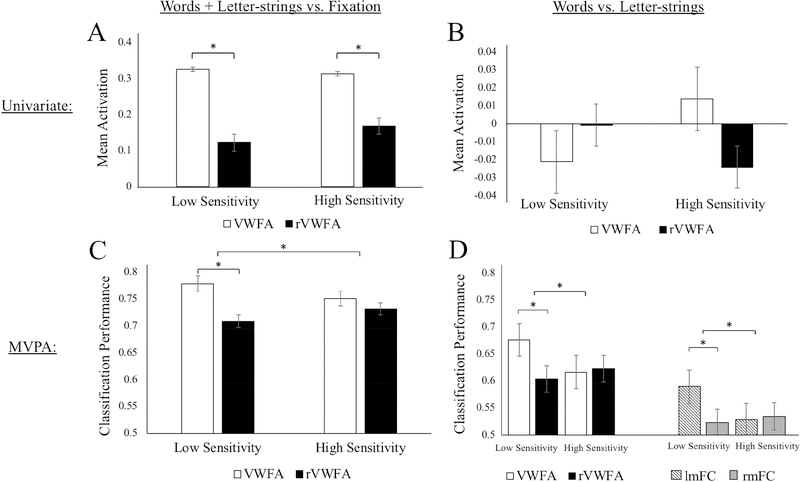
Figure 3.
Mean activation and classification performance for groups with lower, and higher, sensitivity to visual-spatial distortion. The left column (A and C) shows results for words and letter-strings compared to fixation (in univariate localized ROIs). Panel A shows activation levels for words and letter-strings > fixation in each hemisphere. Panel C shows decoding of words and letter-strings versus fixation. Note that the result-of-interest is lateralization (differences between white and black bars), which is orthogonal to ROI selection. The right column (B and D) shows results from the more specific words versus letter-strings contrast (in MVPA localized ROIs). Panel B shows activation levels for words > letter-strings. Panel D shows classification of words versus letter-strings (chance = 0.5) in VWFA and mid-fusiform cortex (following PCA). The significance bar across groups in C and D indicate interactions between hemisphere and inversion group. Error bars show the standard error of the mean.
We next compared classification performance for “words and letter-strings” versus fixation in the univariate localized VWFA and rVWFA regions. We ran a repeated measures ANOVA and found a statistically significant interaction between the effects of hemisphere (lVWFA and rVWFA) and inversion sensitivity (high and low groups) on classification performance F(1,16) = 4.35, p = 0.05, = 0.13. A simple main effects analysis showed that the LS group had significantly stronger decoding for the VWFA (M = 0.78, SD = 0.08) than rVWFA (M = 0.71, SD = 0.10; t(9) = 4.02, p = 0.003, g = 0.74), reflecting greater classification performance in the left region. In contrast, the HS group did not differ in decoding performance in VWFA (M = 0.75, SD = 0.08) and rVWFA (M = 0.73, SD = 0.06; t(7) = 1.19, p = 0.27, g = 0.27). Multivariate lateralization was calculated by subtracting decoding performance in the rVWFA from performance in the VWFA. Multivariate laterality scores were stronger in the LS group (M = 0.07, SD = 0.06) than in the HS group (M = 0.02, SD = 0.05; t(16) = 2.14, p = 0.048, g = 0.85), suggesting that the LS group had a more lateralized VWFA than the HS group. Results are shown in Figure 3C.
MVPA Localized VWFA and rVWFA Results
We next examined laterality based on regions that were localized using the peak words versus letter-strings classification performance, rather than univariate activation, in the vOTC of each hemisphere. A repeated measure ANOVA showed a significant interaction between hemisphere and inversion sensitivity, F(1,16) = 10.07, p = 0.006, = 0.30 on classification performance. Comparing classification performance for words versus letter-strings in these regions across the hemispheres showed that participants in the LS group had significantly stronger words versus letter-strings decoding in the VWFA (M = 0.68, SD = 0.06) than in its right homologue (rVWFA; M = 0.60, SD = 0.06; t(9) = 3.33, p = 0.009, g = 1.28). In contrast, participants in the HS group did not show a significant decoding difference between the VWFA (M = 0.62, SD = 0.03) and rVWFA (M = 0.63, SD = 0.04; t(7) = 1.41, p = 0.20, g = 0.27). The MVPA results for words versus letter-strings within the VWFA and rVWFA are shown in Figure 3D. The LS group (M = 0.07, SD = 0.07) had significantly greater multivariate laterality (t(16) = 3.20, p = 0.006, g = 1.37) than the HS group (M = −0.006, SD = 0.01), indicating that inversion sensitivity was accompanied by less lateralized word decoding.
Using the same multivariate-defined regions, we compared univariate mean activation (a contrast of words versus letter-strings) in the LS and HS groups. There was no significant main effect of hemisphere on activation level, F(1,16) = 0.18, p = 0.68, = 0.01, and no significant difference between VWFA (M = −0.02, SD = 0.1) and rVWFA (M = −0.01, SD = 0.08; t(9) = 0.66, p = 0.52, g = 0.17) in either the LS group or HS group (VWFA: M = 0.01, SD = 0.13; rVWFA: M = −0.02, SD = 0.14; t(7) = 1.37, p = 0.21, g = 0.21). These results are shown in Figure 3B. Using these words versus letter-strings contrast values to assess univariate lateralization revealed no significant difference (t(16) = 1.42, p = 0.18, g = 0.62) between the LS (M = −0.02, SD = 0.1) and HS (M = 0.04, SD = 0.08) groups.
Finally, in order to match the contrast that was necessary for the univariate analysis (words and letter-strings together versus fixation), we also localized the VWFA and rVWFA using classification performance contrasting words and letter-strings versus fixation. There was a significant main effect of hemisphere on classification performance, F(1,16) = 13.60, p = 0.002, = 0.43. Examining these newly localized regions showed that participants in the LS group had significantly stronger words and letter-strings decoding (relative to fixation) in the VWFA (M = 0.84, SD = 0.07) than in its right homologue (the rVWFA; M = 0.78, SD = 0.07; t(9) = 3.61, p = 0.006, g = 0.82). In contrast, participants in the HS group did not show a significant difference of decoding between the VWFA (M = 0.83, SD = 0.05) and rVWFA (M = 0.80, SD = 0.06; t(7) = 1.68, p = 0.137, g = 0.51). In these same multivariate-defined regions, we compared mean activation levels (from a contrast of words and letter-strings versus fixation) in the LS and HS groups. There was a significant main effect of hemisphere on activation levels F(1,16) = 13.30, p = 0.002, = 0.44. The LS group had greater activation in the VWFA (M = 0.13, SD = 0.15) than rVWFA (M = −0.03, SD = 0.14; t(9) = −2.97, p = 0.02, g = 1.06), indicating greater activation in the VWFA than rVWFA for words and letter-strings (relative to fixation). The HS group showed a similar pattern: a significant difference between VWFA (M = 0.09, SD = 0.13) and rVWFA (M = −0.001, SD = 0.18; t(7) = 2.36, p = 0.05, g = 0.55), suggesting greater VWFA mean activation in both groups.
To validate the effects found in the functional ROI approach, we also ran a principal component analysis. We structurally defined the fusiform cortex within the left and right hemispheres, separately running a PCA for data within each region, which should include the VWFA and rVWFA, respectively. We ran a repeated measures ANOVA and found a significant interaction between hemisphere (left and right fusiform cortex) and inversion sensitivity (low and high groups) on classification performance F(1,16) = 5.46, p = 0.03, = 0.22 (Figure 3D). A simple main effects analysis showed that the LS group had higher classification accuracy in the left fusiform cortex (M = 0.59, SD = 0.06) than the right fusiform cortex (M = 0.52, SD = 0.08; t(9) = 3.07, p = 0.013, g = 0.95). The HS group did not differ in decoding performance in the left (M = 0.53, SD = 0.03) and right (M = 0.54, SD = 0.06; t(7) = 0.30, p = 0.775, g = 0.20) fusiform cortex. Multivariate lateralization was calculated by subtracting right hemisphere decoding accuracy from left hemisphere decoding accuracy. There was a significant difference between lateralization in the LS (M = 0.07, SD = 0.07) and HS (M −0.01, SD = 0.06; t(16) = 2.36, p = 0.032, g = 1.16) groups, suggesting that the LS group has more left-lateralized VWFA information than the HS group.
To check whether the above results are due to differences in signal-to-noise ratios, the temporal signal-to-noise ratio for each ROI was evaluated across hemispheres and groups in a repeated-measures ANOVA. There was no significant effect of hemisphere for the univariate-defined regions (left: M = 83.92, SD = 14.05; right: M = 82.86, SD = 21.31; F(1,16) = 0.04, p = 0.85, = 0.002) and no significant interaction between hemisphere and inversion sensitivity, F(1,16) = 0.32, p = 0.58, = 0.02. There was also no effect of hemisphere for the multivariate-defined regions (left: M = 87.64, SD = 19.76; right: M = 86.28, SD = 19.80; F(1,16) = 0.08, p = 0.78, = 0.01) and no significant interaction between hemisphere and inversion sensitivity, F(1,16) = 0.37, p = 0.55, = 0.02. These results suggest that group differences were not due to differing levels of noise.
Relationship Between Lateralization Differences and Inversion Sensitivity Scores
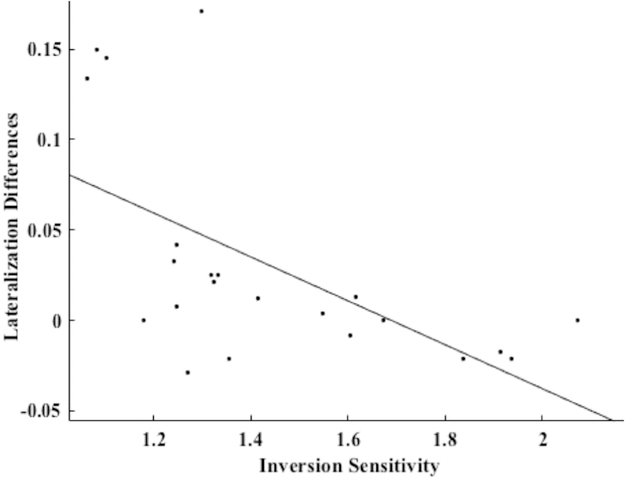
A Pearson’s correlation was computed to assess the continuous relationship between lateralization differences and inversion sensitivity ratios from the lexical decision task (LDRs) of all participants (including the four participants that did not meet group cutoffs). We found that the multivariate laterality measure (VWFA minus rVWFA decoding performance from MVPA-defined searchlights) was significantly negatively correlated with the LDR (r(20) = −0.59, p = 0.004; plotted in figure 4), reflecting weaker left lateralization as inversion-sensitivity increased. Because there were four data points that shared very high lateralization differences in comparison to the other data points, we also ran the correlation excluding them, and still found a significant relationship between lateralization differences and inversion sensitivity (r(16) = −0.49, p = 0.04). Inversion ratios from the naming task (ONR) were also negatively related to lateralization differences (r(20) = −0.46, p = 0.03). To confirm that this relationship was due to lateralization rather than left or right decoding alone, we separately correlated decoding accuracy in the VWFA and rVWFA with inversion sensitivity scores. There was no significant correlation between decoding accuracy in the VWFA and LDR (r(20) = −0.29, p = 0.19). Similarly, correlation between decoding accuracy in the rVWFA alone and LDR was not statistically significant (r(20) = 0.38, p = 0.08). There was also a non-significant correlation between VWFA decoding accuracy and ONR (r(20) = −0.1748., p = 0.44). The ONR did not significantly correlate with decoding accuracy in the rVWFA alone (r(20) = 0.37, p = 0.09).
Figure 4.
Correlation plot showing the relationship between lateralization differences (multivariate laterality index) and inversion sensitivity scores (LDR). There is a significant negative correlation, r(20) = −0.59, p = 0.004. The significant correlation remains after removing the top four points (r(16) = −0.49, p = 0.04).
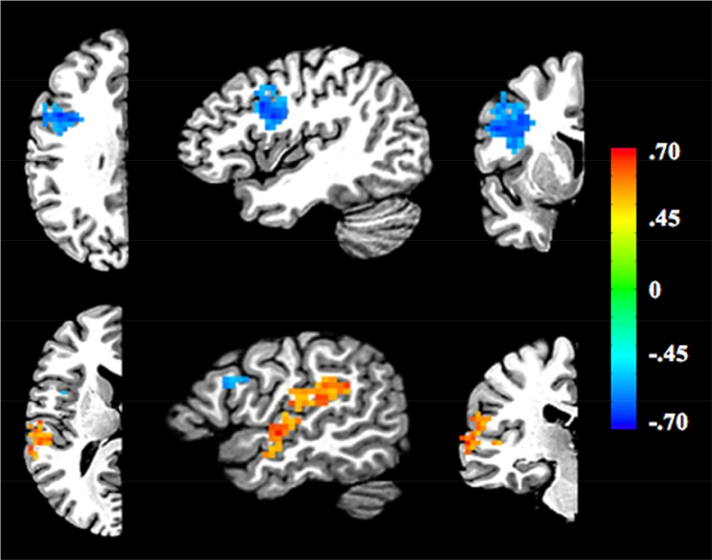
We next took a more exploratory approach. We created multivariate laterality maps for each participant by running a searchlight across their brain and subtracting decoding performance in each right hemisphere searchlight from its associated left hemisphere searchlight. We then ran a correlation between the lateralization maps and participants’ behavioral inversion sensitivity scores. Two clusters were above the corrected threshold of p < 0.05 following cluster-correction. One 136-voxel cluster is located near the junction of the inferior prefrontal and precentral sulci (IFJ; center of mass at −41x, 4y, 29z). This cluster has a negative correlation between lateralization and inversion sensitivity scores, matching the direction of the VWFA’s relationship (stronger left lateralization for lower inversion sensitivity scores). The second 185-voxel cluster runs along the superior temporal gyrus (STG; center of mass at −55x, −37y, 17z). This cluster has the opposite relationship between lateralization differences and inversion sensitivity scores, with greater left lateralization predicting higher inversion sensitivity scores. The locations of the two clusters are shown in Figure 5. To verify that words versus letter-strings decoding was significant in these two regions, the two clusters were transformed from standard to native space for MVPA. Decoding was significant in the lSTG (M = 0.56, SD = 0.06, t(21) = 4.04, p < .001), rSTG (M = 0.54, SD = 0.08, t(21) = 2.37, p = 0.03), lIFJ (M = 0.59, SD = 0.07, t(21) = 5.73, p < .001), and rIFJ (M = 0.58, SD = 0.08, t(21) = 4.67, p < .001) regions. This suggests that all these regions contain orthographic information.
Figure 5.
Results of exploratory lateralization searchlight approach correlating lateralization differences with each person’s inversion sensitivity scores. Location of two clusters were detected above the corrected threshold, one near the junction of the inferior frontal and precentral sulci (IFJ) and superior temporal gyrus (STG). Attention only needs to be given to one half of the brain because of the left - right subtraction employed (i.e., the opposite hemisphere is a reverse mirror image). The blue cluster represents a negative correlation between lateralization differences and inversion sensitivity scores. The red cluster represents a positive correlation between lateralization differences and inversion sensitivity scores. Top row: x = −41, y = 4, z = 29. Bottom row: x = −51, y = 23, z = 13. The different colors on the scale represent r-values.
Discussion
The purpose of this study was to test for an association between inversion sensitivity and VWFA lateralization within a group of native English speakers with normal reading proficiency. A secondary goal was to examine whether a novel multivariate laterality analysis method provides greater sensitivity to the lateralization of some perceptual and cognitive functions. Based on previous work involving native Chinese readers (Kao, Chen, & Chen, 2010; Wang, Kuo, & Cheng, 2011) and bilingual Chinese-English readers (Ben-Yehudah, Hirshorn, Simcox, Perfetti, & Fiez, 2018; Nelson, Liu, Fiez, & Perfetti, 2009) and results from the face processing literature (Rossion, 2000), we predicted that native English readers who are more disrupted by visual distortion would exhibit more bilateral engagement of the VWFA in response to orthographic stimuli. Using an innovative multivariate approach, we observed significantly less multivariate left-lateralization in the VWFA within a group of subjects with high, as compared to low, inversion sensitivity. Additionally, we found that multivariate laterality of the VWFA was negatively correlated with participants’ behavioral word inversion sensitivity scores, such that more bilateral VWFA was associated with greater inversion sensitivity. These results extend previously reported cross-linguistic differences in the lateralization of the VWFA, by showing that similar differences can be observed within a population of native English speakers with normal reading proficiency.
Originally inspired by the face processing literature, a greater degree of word inversion sensitivity has been linked to a processing bias towards holistic visual orthographic analysis (Ben-Yehudah et al., 2018; Kao, Chen, & Chen, 2010; Luo, Chen, & Zhang, 2017; Wang, Koda, & Perfetti, 2003). Given our finding of a significant negative correlation between lateralization in the VWFA and inversion sensitivity, we suggest that native English speakers who exhibit more bilateral engagement of the VWFA and rVWFA have a greater bias towards holistic orthographic coding.
What might give rise to such individual differences in English readers? One possible factor is the method of instruction experienced as early readers. A set of studies using artificial orthographies (Hirshorn, Wrencher, Durisko, Moore, & Fiez, 2016; Mei et al., 2012; Yoncheva, Blau, Maurer, & McCandliss, 2010; Yoncheva, Wise, & McCandliss, 2015) has demonstrated that visual attention to different grain sizes in print-to-speech mapping, ranging from grapheme-phoneme to whole-word relationships, affects the laterality of neural signatures of visual word processing (i.e., N170 and vOTC activation). When participants were instructed to focus on visual units that mapped onto larger phonological units (e.g., whole word versus phoneme; syllable versus phoneme) they exhibited a relatively more bilateral neural signature. Interestingly, English (and other alphabetic writing systems) allow for attention to be focused at different grain sizes, as is demonstrated by different instruction methods across countries (i.e., whole word versus phonics; see Connelly, Thompson, Fletcher-Flinn, & McKay, 2009) and even within the United States (e.g., Baumann, Hoffman, Moon, & Duffy-Hester, 1998; Moats, 2000). Incidentally, our participants were early readers when the ‘reading war’ was still hotly debated (Pearson, 2004). We did not obtain information from our participants on their history of reading instruction, and so future research is needed to assess if and how different methods of reading instruction might lead to individual differences within English readers similar to those observed in the current study.
Individual differences in laterality could also be a result of natural variability in neural organization or orthographic analysis bias, or as a result of developmental differences that affect reading processing, like reading disorders or deafness that affect phonological or sub-word analysis. For example, deaf native signers have been shown to have a larger perceptual span than hearing readers (Bélanger, Slattery, Mayberry, & Rayner, 2012), which could potentially contribute to a greater reliance on holistic orthographic analysis. However, these are unlikely to apply to the current data, as the participants were hearing native English speakers, and high inversion sensitivity readers were matched to the low inversion sensitivity readers in reading sub-skills.
The observed individual differences have broader implications for understanding the processes that support reading. Greater reliance on holistic orthographic analysis has been linked with greater reliance on lexical-level reading procedures (Ben-Yehudah et al., 2018; Hirshorn, Carlos, Durisko, Fiez, Perfetti, & Coutanche, 2018; Pae, Kim, Mano, & Kwon, 2016; Pae & Lee, 2015). Evidence for this comes from comparing the performance of Chinese-English (CE) and Korean-English (KE) bilinguals, whose English reading is influenced by their native language literacy for a non-alphabetic versus alphabetic writing system, respectively. CE have both greater holistic orthographic coding (measured by sensitivity to visual distortion; e.g., inversion sensitivity: Ben-Yehudah et al, 2018; case alternation or inverse fonts: Pae et al, 2015) and a larger bias towards lexical-level processing in word identification. For example, Ben-Yehudah and colleagues found that CE bilinguals’ naming times were biased towards greater reliance on lexical-level measures (e.g., lexical frequency). In contrast, KE naming times were biased towards greater reliance on sublexical-level measures (e.g., consistency of grapheme-phoneme mapping; Ben-Yehudah et al, 2018). Interestingly, similar behavioral patterns have been observed for the participants in the current study, who were invited to participate in a behavioral study of their reading skill (Hirshorn et al., 2018). Individuals with greater inversion sensitivity had relatively greater reliance on lexical-level factors in a naming task, whereas individuals with less inversion sensitivity had relatively greater reliance on sublexical-level factors. Taken together, these clusters of reading profiles provide support for alternate routes to skilled reading in English that can be found across a wide range of reading backgrounds.
It is worth noting that some studies provide evidence in support of neurons within the VWFA being highly selective for whole word processing (holistic/lexical representation; Glezer, Jiang, and Riesenhuber, 2009), which might be seen as contrary to the findings here. However, as discussed earlier, there is also evidence supporting involvement of the VWFA in sublexical processing. Our findings do not have to be seen as incompatible or mutually exclusive from the view that the VWFA contains lexical representations. We suggest that there might be a discrepancy in the relative contributions of lexical and sublexical processing across the hemispheres, with the left contributing relatively more to sublexical/analytic processing in some individuals. We do, however, acknowledge the ongoing debate over the function of the VWFA.
In this study, we found group differences across several ways of defining the VWFA. One interesting aspect of these findings was the similar univariate responses to words and to letter-strings that we observed in the VWFA defined through a “words versus letter-strings” multi-voxel contrast. This is somewhat surprising, because a recent study used a “words vs. consonants” contrast as one of three univariate comparisons to localize a specific sub-region within the left VOTC associated with lexical-level visual word form processing (Lerma-Usabiaga, Carreiras, & Paz-Alonso, 2018). That paper’s reported locus for the words vs. letter-strings contrast was nearly identical to the VWFA locus identified through our multivariate analysis (Talairach: −41, −53, −9 vs. −40, −53, −10 respectively). Notably, however, the size of the BOLD signal difference for their lexical contrasts (real words vs. letter strings, real words vs. pronounceable nonwords, real words vs. false font strings) was substantially smaller than the signal difference observed for perceptual contrasts (real words vs. checkerboards, real words vs. scrambled words, real words vs. phase-scrambled words), and (with 66 participants) that study had considerably more power. Further, positive findings of multi-voxel information without univariate differences have been found in a number of MVPA studies of the visual system (Coutanche, 2013; Harrison and Tong, 2009). Taken together, the differences we observed across analytic methods are consistent with findings that multi-voxel patterns represent finer stimulus distinctions, while broader stimulus distinctions are represented in coarser forms of BOLD organization (Brants, Baeck, Wagemans, & Op de Beeck, 2011).
Multivariate Laterality and Reading Style
As part of our investigation, an exploratory examination of the relationship between multivariate laterality and inversion sensitivity revealed two significant clusters. The results for one cluster, located near the left IFJ, mirrored those seen for the VWFA, with a negative correlation observed between individuals’ multivariate laterality and their inversion sensitivity scores (i.e., the more bilateral, the higher the inversion sensitivity). The results for the second cluster, located in the left STG, showed the opposite pattern, with a positive correlation observed between lateralization and inversion sensitivity (i.e., the more left-lateralized, the higher the inversion sensitivity). Both the IFG and STG have been associated with reading skill and orthographic-phonological processing (Blau, van Atteveldt, Ekkebus, Goebel, & Blomert, 2009; Froyen, Willems, & Blomert, 2011; Taylor, Rastle, & Davis, 2013; Vigneau et al., 2006). The IFJ has been proposed as a component of a speech-based reading pathway in which sublexical orthographic-phonological correspondences are used to “decode” printed words (Taylor et al., 2013; Vigneau et al., 2006), and recent work suggests it may exert a top-down influence over visual processing within the FG (including the VWFA) (Thiebaut de Schotten, Cohen, Amemiya, Braga, & Dehaene, 2014; Vogel, Miezin, Petersen, & Schlaggar, 2012; Zachariou, Klatzky, & Behrmann, 2014). The STG, which houses primary auditory and auditory association cortex, has been implicated in print-sound integration. This has been shown at the letter, syllable, and word levels through differential activation being observed within the STG to bimodal stimuli with congruent versus incongruent constituents (e.g., a visually displayed word presented simultaneously with a matching or mismatching spoken word; Blau et al., 2009; Kronschnabel, Brem, Maurer, & Brandeis, 2014; McNorgan, Awati, Desroches, & Booth, 2014). Speculatively, we propose that greater analytic orthographic coding (as indicated by low inversion sensitivity and left-lateralization of the VWFA) might be associated with greater use of a speech-based pathway that supports sublexical orthographic-phonological mapping; conversely, greater holistic orthographic coding (as indicated by high inversion sensitivity and bilateral engagement of the VWFA and rVWFA) might be associated with more automatic retrieval of acoustic word forms that map onto corresponding visual word forms.
Future directions from this work might include relating other behavioral measures to multivariate laterality. For example, applying this analytical technique could shed light on other lateralization-relevant domains such as face processing or other forms of language processing. More broadly, our multivariate measure of laterality provides opportunities for investigating questions that may have previously led to null findings because of limitations in the sensitivity of univariate lateralization to individual differences. Our multivariate laterality approach will be particularly useful for subtle stimuli contrasts, which require high sensitivity to underlying neural information, such as words versus letter-strings for word processing, or different types of faces for face processing. Such highly specific distinctions are often represented in a region’s multi-voxel patterns (as seen for distinguishing different types of objects from activity in ventral temporal cortex; Haxby et al., 2001). Investigations of cognitive functions that draw on such subtle distinctions in patterns of activity might particularly benefit from applying a measure of lateralization that has access to this multi-voxel information.
Summary
In conclusion, this study demonstrates that multivariate laterality (but not conventional lateralization) of the VWFA during reading predicts an individual’s behavioral sensitivity to word inversion. A bias towards holistic, versus analytical, orthographic processing predicts more bilateral deployment of the VWFA and rVWFA during orthographic processing in native English readers. More generally, multivariate techniques can be used to provide additional insights into how the lateralized –and bilateral– brain achieves perceptual and cognitive functions.
Table 1.
Comparison of HS and LS descriptive and inversion sensitivity statistics.
| High Sensitivity (HS) | Low Sensitivity (LS) | |
|---|---|---|
| Descriptive Statistics | ||
| N | 8 | 10 |
| Gender (N Male) | 5 | 3 |
| Age | 18.88 (0.35) | 19.20 (0.13) |
| Inversion Sensitivity Statistics | ||
| Lexical Decision Ratio | 1.78 (0.19) | 1.22 (0.12) |
| Overt Naming Ratio | 1.28 (0.09) | 1.11 (0.06) |
Note: The mean values are listed against each measure, with the group’s standard deviation in parentheses.
Acknowledgments
A sincere thank you to Chuck Perfetti, members of the Coutanche and Fiez labs for general assistance and helpful discussions related to this project.
Funding
This work was supported by National Institutes of Health grants (1R01HD060388 & T32NS086749). The first author was supported by a Fellowship from the University of Pittsburgh Hot Metal Bridge Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akamatsu N (1999). The effects of first language orthographic features on word recognition processing in English as a second language. Reading and Writing, 11(4), 381–403. [Google Scholar]
- Akamatsu N (2003). The Effects of First Language Orthographic Features on Second Language Reading in Text. Language Learning, 53(2), 207–231. [Google Scholar]
- Baciu M, Juphard A, Cousin E, & Bas JFL (2005). Evaluating fMRI methods for assessing hemispheric language dominance in healthy subjects. European Journal of Radiology, 55(2), 209–218. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Hirshorn EA, Simcox T, Perfetti CA, & Fiez JA (2018). Chinese-English bilinguals transfer L1 lexical reading procedures and holistic orthographic coding to L2 English. Journal of Neurolinguistics. [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, … Haughton VM (1996). Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology, 46(4), 978–984. [DOI] [PubMed] [Google Scholar]
- Blau V, van Atteveldt N, Ekkebus M, Goebel R, & Blomert L (2009). Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Current Biology, 19(6), 503–508. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, & Schneider W (2005). Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Human Brain Mapping, 25(1), 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brants M, Baeck A, Wagemans J, & Op de Beeck HP (2011). Multiple scales of organization for object selectivity in ventral visual cortex. NeuroImage, 56(3), 1372–1381. [DOI] [PubMed] [Google Scholar]
- Calvo MG, & Beltrán D (2014). Brain lateralization of holistic versus analytic processing of emotional facial expressions. NeuroImage, 92, 237–247. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, & Dehaene S (2002). Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain: A Journal of Neurology, 125(Pt 5), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehéricy S, Samson Y, Obadia M, … Dehaene S (2003). Visual Word Recognition in the Left and Right Hemispheres: Anatomical and Functional Correlates of Peripheral Alexias. Cerebral Cortex, 13(12), 1313–1333. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, & Haller M (1993). Models of Reading Aloud: Dual-Route and Parallel-Distributed-Processing Approaches. Psychological Review, 100, 589–608. [Google Scholar]
- Coutanche MN (2013). Distinguishing multi-voxel patterns and mean activation: Why, how, and what does it tell us? Cognitive, Affective, & Behavioral Neuroscience, 13(3), 667–673. [DOI] [PubMed] [Google Scholar]
- Coutanche MN, Thompson-Schill SL, & Schultz RT (2011). Multi-voxel pattern analysis of fMRI data predicts clinical symptom severity. NeuroImage, 57(1), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec’H G, Poline J-B, Le Bihan D, & Cohen L (2002). The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport, 13(3), 321–325. [DOI] [PubMed] [Google Scholar]
- Detre GJ, Polyn SM, Moore CD, Natu VS, Singer BD, Cohen JD, … Norman KA (2006). The multi-voxel pattern analysis (MVPA) toolbox. Presented at the Annual Meeting of the Organization for Human Brain Mapping, Florence, Italy. [Google Scholar]
- Fox CJ, Iaria G, & Barton JJS (2009). Defining the face processing network: optimization of the functional localizer in fMRI. Human Brain Mapping, 30(5), 1637–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froyen D, Willems G, & Blomert L (2011). Evidence for a specific cross-modal association deficit in dyslexia: an electrophysiological study of letter-speech sound processing. Developmental Science, 14(4), 635–648. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, & Riesenhuber M (2009) Evidence for highly selective neuronal tuning to whole words in the “visual word form area.” Neuron 62(2), 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, & Binder JR (2010). Neural systems for reading aloud: a multiparametric approach. Cerebral Cortex, 20(8), 1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, & Tong F (2009). Decoding reveals the contents of visual working memory in early visual areas. Nature, 458(7238), 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, & Pietrini P (2001). Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science, 293(5539), 2425–2430. [DOI] [PubMed] [Google Scholar]
- Heine B, Kuteva T, & Kaltenböck G (2014). Discourse Grammar, the dual process model, and brain lateralization: some correlations. Language and Cognition, 6(1), 146–180. [Google Scholar]
- Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, & Ugurbil K (1993). Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport, 4(6), 675–678. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, & Fiez JA (2014). Using Artificial Orthographies for Studying Cross-Linguistic Differences in the Cognitive and Neural Profiles of Reading. Journal of Neurolinguistics, 31, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn EA, Wrencher A, Durisko C, Moore MW, & Fiez JA (2016). Fusiform Gyrus Laterality in Writing Systems with Different Mapping Principles: An Artificial Orthography Training Study. Journal of Cognitive Neuroscience, 28(6), 882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn EA, Carlos BJ, Durisko C, Fiez JA, Perfetti CA, Coutanche MN (2018). Word inversion sensitivity as a marker of word identification style and visual word form area lateralization. Society for the Neurobiology of Language Annual Meeting Quebec City, Canada. [Google Scholar]
- Kao C-H, Chen D-Y, & Chen C-C (2010). The inversion effect in visual word form processing. Cortex, 46(2), 217–230. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Dräger B, Bobe L, Lohmann H, Ringelstein E, & Henningsen H (2000). Language lateralization in healthy right-handers. Brain: A Journal of Neurology, 123 (Pt 1), 74–81. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, & Bandettini P (2006). Information-based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America, 103(10), 3863–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronschnabel J, Brem S, Maurer U, & Brandeis D (2014). The level of audiovisual print-speech integration deficits in dyslexia. Neuropsychologia, 62, 245–261. [DOI] [PubMed] [Google Scholar]
- Lerma-Usabiaga G, Carreiras M, Paz-Alonso PM (2018) Converging evidence for functional and structural segregation within the left ventral occipitotemporal cortex in reading. Proceedings of the National Academy of Sciences USA 115:E9981–E9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dunlap S, Fiez J, & Perfetti C (2007). Evidence for neural accommodation to a writing system following learning. Human Brain Mapping, 28(11), 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Chen W, & Zhang Y (2017). The Inversion Effect for Chinese Characters is Modulated by Radical Organization. Journal of Psycholinguistic Research, 46(3), 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Grand RL, & Mondloch CJ (2002). The many faces of configural processing. Trends in Cognitive Sciences, 6(6), 255–260. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Awati N, Desroches AS, & Booth JR (2014). Multimodal lexical processing in auditory cortex is literacy skill dependent. Cerebral Cortex, 24(9), 2464–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xue G, Lu Z-L, Chen C, Wei M, He Q, & Dong Q (2015). Long-term experience with Chinese language shapes the fusiform asymmetry of English reading. NeuroImage, 110, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xue G, Lu Z-L, He Q, Zhang M, Xue F, … Dong Q (2013). Orthographic transparency modulates the functional asymmetry in the fusiform cortex: an artificial language training study. Brain and Language, 125(2), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Yu M, Seger C, & Mo L (2015). Holistic neural coding of Chinese character forms in bilateral ventral visual system. Brain and Language, 141, 28–34. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Liu Y, Fiez J, & Perfetti CA (2009). Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping, 30(3), 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, & Haxby JV (2006). Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences, 10(9), 424–430. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Pae HK, & Lee Y-W (2015). The resolution of visual noise in word recognition. Journal of Psycholinguistic Research, 44(3), 337–358. [DOI] [PubMed] [Google Scholar]
- Pae HK, Sun-A K, Mano QR, & Kwon YJ (2017). Sublexical and lexical processing of the English orthography among native speakers of Chinese and Korean. Reading and Writing, 30(1), 1–24. [Google Scholar]
- Pearson PD (2004). The Reading Wars. Educational Policy, 18(1), 216–252. [Google Scholar]
- Perfetti CA, & Hart L (2001). The lexical quality hypothesis In Verhoeven CEPRL (Ed.), Precursors of functional literacy (Vol. 11, pp. 67–86): Amsterdam: John Benjamins. [Google Scholar]
- Reinke K, Fernandes M, Schwindt G, O’Craven K, & Grady CL (2008). Functional specificity of the visual word form area: general activation for words and symbols but specific network activation for words. Brain and Language, 104(2), 180–189. [DOI] [PubMed] [Google Scholar]
- Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, De Gelder B, & Zoontjes R (2000). Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 12(5), 793–802. [DOI] [PubMed] [Google Scholar]
- Rossion B, Hanseeuw B, & Dricot L (2012). Defining face perception areas in the human brain: a large-scale factorial fMRI face localizer analysis. Brain and Cognition, 79(2), 138–157. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, & Tarr MJ (2003). Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. NeuroImage, 20(3), 1609–1624. [DOI] [PubMed] [Google Scholar]
- Schotten M. T. de, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DGM, & Catani M (2011). A lateralized brain network for visuospatial attention. Nature Neuroscience, 14(10), 1245–1246. [DOI] [PubMed] [Google Scholar]
- Sussman BL, Reddigari S, & Newman SD (2018). The impact of inverted text on visual word processing: An fMRI study. Brain and Cognition, 123, 1–9. [DOI] [PubMed] [Google Scholar]
- Talairach J (1988). Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging (1st Edition edition). Stuttgart ; New York: Thieme. [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, & Gao JH (2001). The neural system underlying Chinese logograph reading. NeuroImage, 13(5), 836–846. [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, & Davis MH (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin, 139(4), 766–791. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, & Dehaene S (2014). Learning to read improves the structure of the arcuate fasciculus. Cerebral Cortex, 24(4), 989–995. [DOI] [PubMed] [Google Scholar]
- Tong F, & Pratte MS (2012). Decoding patterns of human brain activity. Annual Review of Psychology, 63, 483–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, … Tzourio-Mazoyer N (2006). Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage, 30(4), 1414–1432. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, & Tzourio-Mazoyer N (2005). Word and nonword reading: what role for the Visual Word Form Area? NeuroImage, 27(3), 694–705. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, & Schlaggar BL (2012). The putative visual word form area is functionally connected to the dorsal attention network. Cerebral Cortex, 22(3), 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, & Rashotte CA (1999). Comprehensive test of phonological processing: CTOPP: ASHA. [Google Scholar]
- Wang M, Koda K, & Perfetti CA (2003). Alphabetic and nonalphabetic L1 effects in English word identification: a comparison of Korean and Chinese English L2 learners. Cognition, 87(2), 129–149. [DOI] [PubMed] [Google Scholar]
- Wang M-Y, Kuo B-C, & Cheng S-K (2011). Chinese characters elicit face-like N170 inversion effects. Brain and Cognition, 77(3), 419–431. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, & Raiford SE (2008). WAIS-IV: Wechsler adult intelligence scale: Pearson; San Antonio, TX. [Google Scholar]
- Woodcock R (1998). Woodcock Reading Mastery Tests - Revised / Normative Update. Manual. USA: AGS. [Google Scholar]
- Yoncheva YN, Blau VC, Maurer U, & McCandliss BD (2010). Attentional focus during learning impacts N170 ERP responses to an artificial script. Developmental Neuropsychology, 35(4), 423–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoncheva YN, Wise J, & McCandliss B (2015). Hemispheric specialization for visual words is shaped by attention to sublexical units during initial learning. Brain and Language, 145–146, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, & Kanwisher N (2008). The representations of spacing and part-based information are associated for upright faces but dissociated for objects: Evidence from individual differences. Psychonomic Bulletin & Review, 15(5), 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Klatzky R, & Behrmann M (2014). Ventral and dorsal visual stream contributions to the perception of object shape and object location. Journal of Cognitive Neuroscience, 26(1), 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]