Abstract
Aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1) is a photoreceptor-specific chaperone of phosphodiesterase 6 (PDE6), a key effector enzyme in the phototransduction cascade. It contains an N-terminal FK506-binding protein (FKBP) domain and a C-terminal tetratricopeptide repeat (TPR) domain. Mutations in AIPL1, including many missense mutations in both FKBP and TPR domains, have been associated with Leber congenital amaurosis (LCA), a severe inherited retinopathy that causes blindness. TPR-domain containing proteins are known to interact with HSP90. However, the structure of AIPL1-TPR domain is presently not determined and little is known about the contribution of the TPR domain to the chaperone function of AIPL1. Here, we report the backbone and sidechain assignments of the TPR domain of AIPL1. These assignments reveal that AIPL1-TPR is an α-helical protein containing 7 α-helices connected via short loops. Peak broadening or structural disorder is observed for a cluster of hydrophobic residues of W218, W222 and L223. Therefore, these assignments provide a framework for further structural determination of AIPL1-TPR domain and its interactions with various binding partners for elucidation of the mechanism of TPR contribution to the chaperone function of AIPL1.
Keywords: TPR, AIPL1, chaperone, phosphodiesterase 6, tetratricopeptide repeat
Biological context
The AIPL1 gene encoding aryl hydrocarbon receptor (AhR)-interacting protein-like 1 (AIPL1) is linked to Leber congenital amaurosis (LCA), a severe, early-onset, inherited retinopathy that leads to early onset blindness (Sohocki et al. 2000). The name AIPL1 originates from the protein homology to aryl hydrocarbon receptor-interacting protein (AIP) (Sohocki et al. 2000). AIP is a ubiquitous HSP90 co-chaperone of aryl hydrocarbon receptor and other nuclear receptors (Trivellin and Korbonits 2011). The sequences of AIPL1 and AIP are ~50% identical. Both proteins are composed of an N-terminal FK506-binding protein (FKBP) domain and a C-terminal tetratricopeptide repeat (TPR) domain with three tetratricopeptide repeats (Das et al. 1998; Sohocki et al. 2000).
AIPL1 is a photoreceptor-specific protein, and in rods and cones it serves as a specialized chaperone for cGMP-specific phosphodiesterase-6 (PDE6), a key effector enzyme in the phototransduction cascade (Gopalakrishna et al. 2016; Liu et al. 2004; Ramamurthy et al. 2004). LCA-causing mutations have been identified in either FKBP or TPR-domain of AIPL1. It has been demonstrated that mutant AIPL1 proteins fail to chaperone PDE6 (Gopalakrishna et al. 2016), and akin mutations in PDE6 itself, also cause retinal degeneration subsequent to elevation of intracellular cGMP (Bowes et al. 1990; Farber and Lolley 1974; Gopalakrishna et al. 2017; Pittler and Baehr 1991). The FKBP domain of AIPL1 facilitates the folding of PDE6 by interacting with the C-terminal prenyl lipid modifications of the enzyme (Majumder et al. 2013; Yadav et al. 2015). The recent atomic structure of the AIPL1-FKBP domain revealed the structural basis for the unique binding of the prenyl moieties to AIPL1-FKBP (Yadav et al. 2017; Yu et al. 2017). In contrast, little is known about the contribution of the TPR domain to the chaperone function of AIPL1 (Yadav and Artemyev 2017). TPR-domain containing proteins are known to interact with HSP90 (Taipale et al. 2010). The TPR domains of AIPL1 and AIP interact with HSP90 as well (Hidalgo-de-Quintana et al. 2008). Several LCA-associated mutations within the TPR domain of AIPL1 impacted its interaction with HSP90 (Hidalgo-de-Quintana et al. 2008). However, chimeric protein containing AIPL1-FKBP linked to the AIP-TPR domain failed to chaperone PDE6 (Gopalakrishna et al. 2016). This suggests that the TPR domain of AIPL1 plays a unique role in chaperoning PDE6 that is distinct from “generic” binding of HSP90 to conventional TPR domains.
Despite the critical significance of the AIPL1-TPR domain for the function of AIPL1 and the health of photoreceptors, to date, there is no structural information on this protein and its interaction with potential partners in the chaperone-client complex. NMR assignments for the AIPL1-TPR domain are a first step in understanding its structural features and interactions with its client during folding and/or assembly of PDE6.
Methods and experiments
Cloning, protein expression, and purification
DNA sequence encoding the AIPL1-TPR (aa 171–316) was PCR-amplified from pET15b vector harboring human AIPL1 gene (Yadav et al. 2015) using 5’ primer with NcoI site and 6 His tag and 3’ primer with NdeI site. The PCR product was then cloned into the pET15b vector using NcoI/NdeI site. The construct used in this study contains a total of 155 amino acids consisting of a N-terminal His tag (9 residues, MGHHHHHHG) and AIPL1-TPR domain (146 residues from residue 171 to residue 316).
The N-terminal His-tagged AIPL1-TPR protein was expressed in BL21 (DE3) E. coli cells. To obtain uniformly 15N and 13C-labeled AIPL1-TPR, E. coli BL21 (DE3) cells were adapted to a minimal medium containing 15NH4Cl and 13C6-glucose. 0.5 mL of adapted BL21 (DE3) cells were then inoculated in 50 mL of minimal media containing 15NH4Cl (1 g/L), 13C6-glucose (3 g/L) and 100 µg/mL of ampicillin and incubated at 37 °C with shaking until OD600 reached ~0.8–1.0. This culture was then added to 1 liter of minimal media containing 15NH4Cl, 13C6-glucose and 100 µg/mL of ampicillin, and cells were grown at 37 °C to OD600 of ~0.6. Then protein expression was induced by adding 250 µM of IPTG, and cells were grown overnight at 18 °C. The cell pellets were sonicated on ice (five 30s pulses) in a buffer containing 50 mM Tris-HCl (pH 7.5), 400 mM NaCl, and 10 mM dithiothreitol (DTT) (buffer A), and protease inhibitor mixture (Roche Applied Science, Indianapolis, IN, USA). AIPL1-TPR was purified over Ni-NTA resin (EMD Millipore, Billerica, MA, USA) using buffer A containing 250 mM imidazole for elution. AIPL1-TPR was further purified by ion-exchange chromatography on a HiTrap SP FF (GE Healthcare, Pittsburgh, PA, USA) and gel filtration chromatography on a HiLoad 16/600 Superdex 75 column (GE Healthcare, Pittsburgh, PA, USA) equilibrated with 25 mM Na2HPO4 (pH 7.5), 400 mM NaCl and 10 mM of DTT.
NMR spectroscopy
NMR spectra were acquired on a Bruker Neo 600 MHz NMR spectrometer at 35 °C using 0.5 mM uniformly [13C,15N]-labeled AIPL1-TPR protein in a buffer containing 20 mM sodium phosphate (pH 7.5), 700 mM NaCl, and 6 mM DTT in 90% H2O / 10% D2O. A suite of triple resonance NMR experiments including HNCACB, HN(CO)CACB, HNCO, and HN(CA)CO experiments (Yamazaki et al. 1994) were collected for backbone assignments of AIPL1-TPR. Side-chain assignments were obtained by acquiring C(CO)NH, H(CCO)NH, HBHA(CO)NH, and HCCH-TOCSY spectra (Clore and Gronenborn 1994). The collected data were processed using NMRPipe (Delaglio et al. 1995) and analyzed using NMRView (Johnson and Blevins 1994).
Resonance assignments and data deposition
The AIPL1-TPR protein aggregates with time at a salt concentration of < 400 mM NaCl. Therefore, we have used a buffer containing a higher salt concentration of 700 mM NaCl to keep the protein stable during NMR data acquisition. The 15N/1H HSQC spectrum of AIPL1-TPR is shown in Figure 1. Clearly, the cross-peaks in the HSQC spectrum are well dispersed, indicating a well-folded protein. All backbone amide cross-peaks in the 15N/1H HSQC spectrum are assigned except for two weak peaks (at 118.45/8.85 and 113.58/6.84 ppm) which did not show any cross-peak in the triple resonance experiments. The assigned backbone amides are labeled in the 15N/1H HSQC spectrum using the wild type protein sequence numbering (Fig. 1). All backbone amides of AIPL1-TPR are assigned except for E195, E196, W218, W222, L223, E282, K299, and N-terminal 5 residues. Therefore, excluding the His tag (9 residues) at the N-terminus, 94% (134 out of 142) of non-proline backbone amides, 94% (137 out of 146) of Cα, 89% (130 out of 146) of Cβ, 94% (137 out of 146) of CO, and 86% (125 out of 146) of Hα are assigned. The 15N chemical shifts of V264 and Q316 backbone amides resonate at a lower field, thus they are folded in the acquired spectrum (red peaks, Fig. 1). The assigned chemical shifts of AIPL1-TPR were deposited in BioMagResBank (http://www.bmrb.wisc.edu) under the accession number 27614.
Fig. 1.
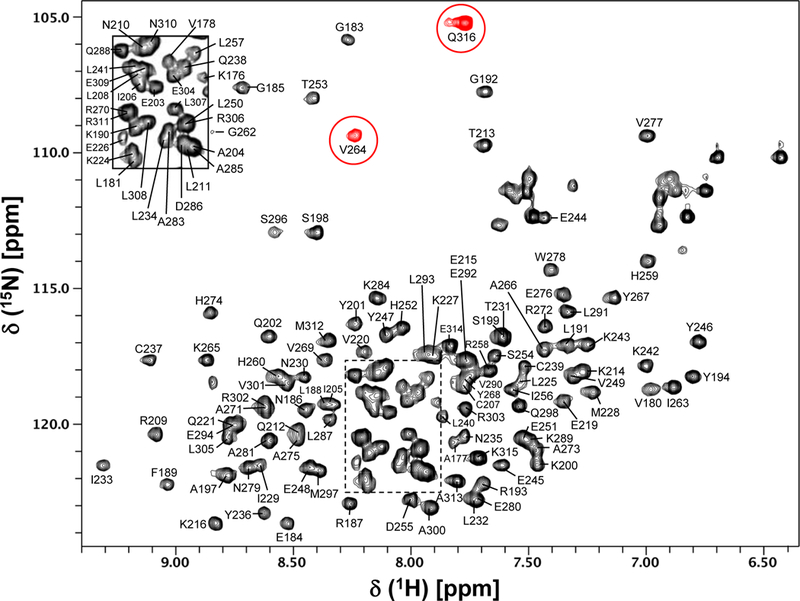
15N/1H HSQC spectrum of human AIPL1-TPR domain. The assigned backbone amides are labeled using the wild type protein sequence numbering. The folded peaks are colored in red. The inset shows the peak labelling of a crowded region indicated by the dotted rectangular box.
Figure 2 shows the plot of predicted probability of α-helical and β-sheet secondary structures as a function of the residue number of AIPL1-TPR. The secondary structures of AIPL1-TPR obtained from the analysis of the assigned backbone atoms (HN, N, Cα, Cβ, CO, Hα) using TALOS+ (Shen et al. 2009) are labeled (Fig. 2). Clearly, AIPL1-TPR is an α-helical protein consisting of 7 α-helices without any β-strands. These α-helices are connected via short loops. Interestingly, this determined secondary structure arrangement of AIPL1-TPR in terms of number of α-helices and helix lengths is very similar to that reported for AIP-TPR domain (Morgan et al. 2012) and is also analogous to that of the TPR domain of FKBP51 and FKBP52 except that the TPR domain of FKBP51/52 has a much longer α7-helix since it contains a calmodulin-binding site near the C-terminus (Sinars et al. 2003; Wu et al. 2004). We observe in this study that the backbone amides of W218 (located in the loop connecting α2 and α3) and W222 and L223 (located in the beginning of α3) are broad beyond detection in the triple resonance experiments, suggesting that this cluster of exposed hydrophobic residues may be disordered or involved in intermolecular interaction.
Fig. 2.
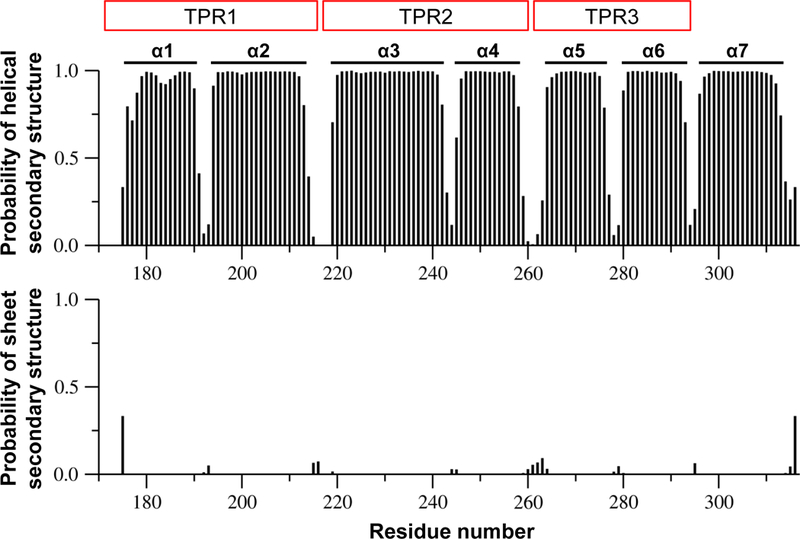
Plot of predicted probability of α-helical and β-sheet secondary structures as a function of residue number of AIPL1-TPR domain. Wild type human AIPL1-TPR protein sequence umbering is used here. These predicted probability values were obtained from the TALOS+ analysis of the assigned backbone atoms (HN, N, Cα, Cβ, CO, Hα). The secondary structures obtained from these analyses of the assigned backbone of AIPL1-TPR are labeled in the figure. Clearly, this is an α-helical protein consisting of 7 α-helices connected via short loops, and this protein does not contain any β-strands. The 3 TPR repeats of AIPL1-TPR are also labeled as TPR1 to TPR3.
With these AIPL1-TPR assignments, we will start in the next studies to investigate what proteins AIPL1-TPR interacts with and how AIPL1-TPR binds and recognizes its binding partners during folding and assembly of PDE6 for the understanding of photoreceptor function and regulation.
Acknowledgments
This work was supported by the National Institutes of Health grant EY-10843 to N.O.A and Pediatric Ophthalmology Career Starter Research Grant from the Knights Templar Eye Foundation to R. P. Y.
Footnotes
Conflict of interest: the authors declare that they have no conflicts of interest.
Ethical standards: all of our experiments comply with accepted ethical standards.
References
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB (1990) Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 347:677–680 doi: 10.1038/347677a0 [DOI] [PubMed] [Google Scholar]
- Clore GM, Gronenborn AM (1994) Multidimensional heteronuclear nuclear magnetic resonance of proteins. Methods in enzymology 239:349–363 [DOI] [PubMed] [Google Scholar]
- Das AK, Cohen PW, Barford D (1998) The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. The EMBO journal 17:1192–1199 doi: 10.1093/emboj/17.5.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293 [DOI] [PubMed] [Google Scholar]
- Farber DB, Lolley RN (1974) Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 186:449–451 [DOI] [PubMed] [Google Scholar]
- Gopalakrishna KN, Boyd K, Artemyev NO (2017) Mechanisms of mutant PDE6 proteins underlying retinal diseases. Cell Signal 37:74–80 doi: 10.1016/j.cellsig.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna KN, Boyd K, Yadav RP, Artemyev NO (2016) Aryl hydrocarbon receptor-interacting protein-like 1 is an obligate chaperone of phosphodiesterase 6 and is assisted by the gamma-subunit of its client. J Biol Chem 291:16282–16291 doi: 10.1074/jbc.M116.737593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-de-Quintana J, Evans RJ, Cheetham ME, van der Spuy J (2008) The Leber congenital amaurosis protein AIPL1 functions as part of a chaperone heterocomplex. Investigative ophthalmology & visual science 49:2878–2887 doi: 10.1167/iovs.07-1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA (1994) NMR View: A computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614 doi: 10.1007/BF00404272 [DOI] [PubMed] [Google Scholar]
- Liu X et al. (2004) AIPL1, the protein that is defective in Leber congenital amaurosis, is essential for the biosynthesis of retinal rod cGMP phosphodiesterase. PNAS USA 101:13903–13908 doi: 10.1073/pnas.0405160101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Gopalakrishna KN, Cheguru P, Gakhar L, Artemyev NO (2013) Interaction of aryl hydrocarbon receptor-interacting protein-like 1 with the farnesyl moiety. J Biol Chem 288:21320–21328 doi: 10.1074/jbc.M113.476242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RML, Hernández-Ramírez LC, Trivellin G, Zhou L, Roe SM, Korbonits M, Prodromou C (2012) Structure of the TPR domain of AIP: Lack of client protein interaction with the C-terminal α−7 helix of the TPR domain of AIP is sufficient for pituitary adenoma predisposition. PLOS ONE 7:e53339 doi: 10.1371/journal.pone.0053339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler SJ, Baehr W (1991) Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. PNAS USA 88:8322–8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy V, Niemi GA, Reh TA, Hurley JB (2004) Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. PNAS USA 101:13897–13902 doi: 10.1073/pnas.0404197101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223 doi: 10.1007/s10858-009-9333-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J (2003) Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. PNAS USA 100:868–873 doi: 10.1073/pnas.0231020100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohocki MM et al. (2000) Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nature genetics 24:79–83 doi: 10.1038/71732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nature reviews Molecular cell biology 11:515–528 doi: 10.1038/nrm2918 [DOI] [PubMed] [Google Scholar]
- Trivellin G, Korbonits M (2011) AIP and its interacting partners. The Journal of endocrinology 210:137–155 doi: 10.1530/JOE-11-0054 [DOI] [PubMed] [Google Scholar]
- Wu B et al. (2004) 3D structure of human FK506-binding protein 52: Implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. PNAS USA 101:8348–8353 doi: 10.1073/pnas.0305969101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RP, Artemyev NO (2017) AIPL1: A specialized chaperone for the phototransduction effector. Cell Signal 40:183–189 doi: 10.1016/j.cellsig.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RP, Gakhar L, Yu L, Artemyev NO (2017) Unique structural features of the AIPL1-FKBP domain that support prenyl lipid binding and underlie protein malfunction in blindness. PNAS USA 114:E6536–E6545 doi: 10.1073/pnas.1704782114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RP, Majumder A, Gakhar L, Artemyev NO (2015) Extended conformation of the proline-rich domain of human aryl hydrocarbon receptor-interacting protein-like 1: implications for retina disease. J Neurochem 135:165–175 doi: 10.1111/jnc.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Lee W, Arrowsmith CH, Muhandiram DR, Kay LE (1994) A suite of triple-resonance NMR experiments for the backbone assignment of 15N, 13C, 2H-labeled proteins with high sensitivity. J Am Chem Soc 116:11655–11666 [Google Scholar]
- Yu L, Yadav RP, Artemyev NO (2017) NMR resonance assignments of the FKBP domain of human aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1) in complex with a farnesyl ligand. Biomol NMR Assign 11:111–115 doi: 10.1007/s12104-017-9730-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


