Abstract
Objective:
To test the efficacy of a hybrid model of the self-help intervention program (hSHIP), which combines a mobile version of SHIP (mSHIP) and personal coaching, to address unique cultural and motivational factors for optimal self-management of type 2 diabetes and prediabetes among Korean Americans (KAs).
Methods:
A single-group feasibility study design was used. The hSHIP utilizes texts and motivational counseling based on well-tested intervention content for KAs. To facilitate the dissemination of hSHIP, we developed a web application adopting the principles of persuasive technology to motivate behavior changes.
Results:
Feasibility assessment found that hSHIP was well accepted by both participants and community health workers who delivered the intervention. An average of 1.3% A1C reduction (from 7.8% to 6.5%) was achieved by KAs with diabetes (n = 165), 51.5% of whom lowered their A1C below 6.5% in 6-months. No one with prediabetes (n = 50) progressed to diabetes. Other clinical outcomes (e.g., weight, depression, and blood pressure) also improved significantly; 41.2% were able to reduce or discontinue antidiabetic drugs.
Conclusion:
The feasibility and initial efficacy of hSHIP were demonstrated.
Practice implication:
This hybrid diabetes self-management model is a viable tool for traditionally underserved groups with diabetes or prediabetes.
Keywords: Persuasive technology, Motivation, Type 2 diabetes, Korean Americans, CHW
1. Introduction
Over the past 12 years, the number of people with diabetes has steadily increased to pandemic levels [1]. In the U.S., among people age 30 years or older, the prevalence of type 2 diabetes (hereafter diabetes) rose from 8.5% in 1999–2000 to 11.3% in 2013–2014, while prediabetes almost doubled (from 16.0% to 27.4%). The total number of prescription medications increased from 1.9 to 2.7 per person, and diabetes-specific medications increased from 1.3 to 1.8. Yet despite aggressive pharmacotherapy, the levels of diabetes-related biomarkers (hemoglobin A1C, blood plasma insulin, and figlucose) also rose, even though overall sugar consumption at the population level has declined (according to the National Health and Nutrition Examination Survey, 1999–2014).
This sharp increase in the prevalence of diabetes and prediabetes is of concern as the population grows older and becomes more diverse [2]. As the population ages, so do its needs for care: the frequency and burden of chronic diseases are increasing, and many subgroups within the increasingly diverse U.S. population are experiencing health disparities despite great efforts to redress such gaps during the last decade [2,3].
Diabetes self-management education and support (DSMES) is an essential component of the nation’s diabetes management and control [4], but there remains ample room for improvement. Current strategies to sustain long-term changes in diabetes self-management are insufficient [5], and diabetes support for linguistically or culturally isolated communities is inadequate [6]. Outcome data on the effectiveness of current DSMES vary widely. The outcomes of therapeutic lifestyle change (TLC) interventions, a major component of DSMES, for example, have been statistically and clinically significant in reducing A1C, but on average, these reductions have been modest, from 0.3% to 0.7% [7,8]. They are less than the reductions obtained with pharmacotherapy [9], and data indicating long-term sustainability of such changes are largely unavailable. Given the chronic nature of diabetes, lifestyle changes to manage the disease require a life-long commitment—a mobilization of personal, financial, and social resources. The maintenance of personal motivation is critical. But most interventions provide short-term and intermittent education only.
At the same time, many linguistically isolated community groups are systemically excluded from research or demonstration projects. The Diabetes Prevention Program (DPP) has been effective in diabetes prevention [10], and in recent years the CDC has made concerted efforts to expand the DPP curriculum in various community settings. Yet although some new interventions using the DPP have been expanded to ethnic minority communities, few community programs using the DPP have been validated among Asian Americans, including Korean Americans (KAs). Moreover, although the beneficial effect of weight loss on the reduction of A1C in people with diabetes is evident [11], the DPP may not even be directly applicable to the KA community: KAs are in general leaner than their White or Black counterparts [12]. In our prediabetes and diabetes sample of KAs, only 4.7% were obese (BMI ≥ 30) and 10.2% were overweight (BMI ≥ 25).
In addition to weight loss, to be effective for underserved ethnic or linguistic minority populations, diabetes management and control must address (a) cultural needs, including ethnic language and diet, cultural beliefs, knowledge about the disease and attitudes toward it, and (b) community and personal resources. KAs, as members of a predominantly first-generation immigrant community, have limited resources, including a stiff language barrier, insufficient numbers of Korean-speaking care providers, and a lack of culturally and linguistically accommodated self-management programs. Research has consistently shown that overwhelming numbers of KAs suffer from uncontrolled diabetes with resulting serious consequences, as well as from low self-confidence and social isolation resulting from linguistic and cultural barriers [13–15]. Social isolation and limited health literacy have long been identified as major barriers to successful management of chronic diseases in the general population and are even more problematic for many KAs. This new immigrant group is also known to have persistent problems with access to basic care and information, and even those with access to care (i.e., seniors with Medicare) often utilize it inadequately [16]. In addition, gaps exist in the application of health technology to healthcare research on ethnic/linguistic minority populations including KAs. Specifically, there is a lack of culturally tailored, health literacy-accommodated and/or technology-assisted interventions incorporating minority languages and cultures, due to methodological complexity and resource demands on research teams (time and effort).
To address these scientific and practice gaps in implementing TLC in ethnic minority populations, we designed a program that combines scalable health technology with TLC protocols for diabetes management and tested it in several clinical trials in the KA community. Our original program, “the Self-Help Intervention Program for KAs with DM (SHIP),” was designed to provide first-generation KA immigrants with diabetes with culturally tailored diabetes self-management enhancement services delivered by bilingual Korean community health workers (CHWs) and a bilingual nurse team [17–19].
Despite well-documented successful outcomes, the SHIP can be difficult to disseminate in its current format because it requires considerable human resources and financial costs. Mobile phone-based health platforms (mHealth) can be more cost-effective for reaching a large population of individuals, including linguistic minority populations. To optimize the SHIP’s innovations (e.g., its culturally tailored curriculum and counseling protocols, training protocols, and well-trained CHWs) without diluting the program’s potency, we developed a new mHealth version of the SHIP by incorporating the principles of persuasive technology [20]. In addition, to address the relatively slow “technology readiness” of our target population, we incorporated human interaction into the intervention using CHWs as facilitators.
This hybrid intervention, hSHIP, which combines digital and human touch, was inspired and influenced by the collective work of B. J. Fogg, who coined the term “persuasive computing “(later broadened to “persuasive technology”), and his colleagues at the Stanford Persuasive Technology Laboratory. Persuasive technology is a new, evolving branch of implementation science that acknowledges the ubiquitous yet invisible influences of technology on behavioral change. Fogg postulates seven primary task support principles that, when incorporated into systems, applications, and technologies, support and enable behavior change without coercion [20,21].
For our hSHIP, we developed a chronic disease management system (CDMS) that combines all processes of project management (recruitment and enrollment, monitoring, questionnaires, messaging, reporting, etc.) in real time and delivers the intervention’s components (education and training, monitoring and counseling, messaging, goal setting, etc.) into a web application. Research nurses and CHWs communicate with program participants in real time using smartphone modules for SMS and notifications in the CDMS.
In this article, we report the results of a feasibility study and pilot test of the hSHIP, the new iteration of our TLC program aided by CDMS and CHWs, for the management of prediabetes and diabetes in the KA community.
2. Research design and methods
2.1. Design
A single-group feasibility study design with repeated measures was used to determine the feasibility and initial efficacy of the hSHIP intervention. Participants in the hSHIP group received the intervention with a structured psycho-behavioral diabetes educational component delivered in Korean by assigned CHWs through personalized text messages, home glucose monitoring, and digital counseling. The interactive digital component of the intervention is structured according to principles of persuasive system design, such as personalization, self-monitoring, tailoring, linking, and strategic use of praise [20].
2.2. Theoretical framework
This pilot study was guided by the information, motivation, and behavioral skills model of self-care (IMB) [22]. The IMB model is a good fit for diabetes self-management interventions because it focuses on a set of information (e.g., diabetes self-care-related knowledge), motivation (e.g., ongoing counseling using motivational interview techniques), and behavioral skills (e.g., diabetes self-care activities) that are conceptually and empirically associated with clinical outcomes (e.g., glucose control). The IMB model has been successfully applied to diabetes self-care [23]. We incorporated elements of persuasive technology including interaction [20], dialog, system credibility, and social support [21], into the IMB to enable system-wide interactive feedback.
In practice, people are likely to change their behaviors if they comprehend a balance between the costs and benefits of those changes in their own terms in their own information-resource gradients. To sustain the changes, constant and positive feedback among knowledge, belief, actions, and rewards is essential. The pathways of behavioral changes in our hSHIP are schematically depicted in Fig. 1.
Fig. 1.
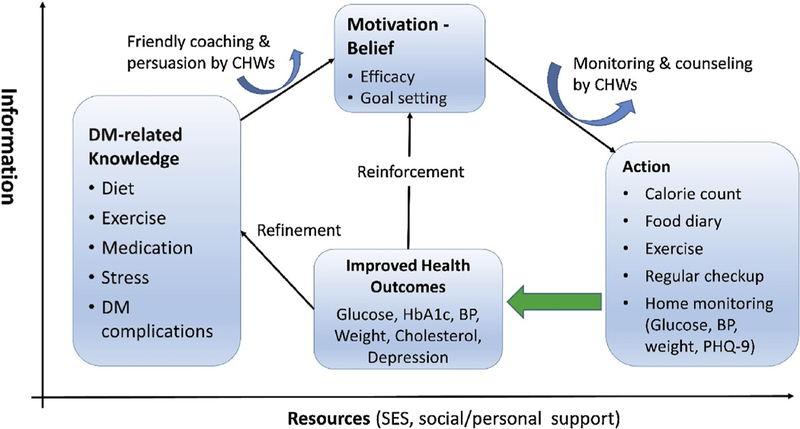
Community-based self-help care model of T2DM management. TLCs is a process, not an event and it continues in a feedback loop. Community health workers (CHWs) assist participants to set short- and long-term goals using the SMART (Specific, Measurable, Achievable, Relevant, Time-bound) principle and to achieve the goals by training skills. They communicate online (using the CDMS and smartphone) and offline (e.g., face-to-face interview and counseling).
2.3. Recruitment
During 6 months from March 1, 2017 to August 31, 2017, a total of 311 KAs responded to our program announcements, which we advertised through local ethnic newspapers, social media outlets, and e-mails to the KA community in the Baltimore-Washington metropolitan area. Eligible participants were community-dwelling KAs who (a) were diagnosed with prediabetes or diabetes but without kidney failure, (b) were able to read and understand Korean, (c) used a smartphone, and (d) could visit the study site and provide evaluation data three times (at baseline and at 3 and 6 months).
A total of 247 KAs with prediabetes or diabetes met the eligibility criteria and were enrolled in our program. They were put into one of three groups: (a) prediabetes (A1C 5.7%–6.4%), (b) moderate diabetes (A1C 6.5%–7.9%), or (c) severe diabetes (A1C ≥ 8.0%). Of those who were enrolled, 32 dropped out, yielding a final retention rate of 88.3%. The reasons for drop included difficulties in using a smartphone and the web app (n = 6), schedule conflicts (n = 7), the burden of traveling too far for data collection visits (n = 2), or difficulty following the TLC recommendations (n = 5).
2.4. CHWs as primary interventionists
To build community infrastructure, the primary interventionists for the hSHIP were CHWs. As the primary contact for study participants, the CHWs manage the CDMS, including recruitment, enrollment, scheduling, monitoring, communication, case management, counseling, and reporting. Most of the CHWs’ functions and protocols are embedded in the CDMS. When a situation arose that was not covered by scripted protocols, the CHWs were aided by a team of bilingual nurses and project directors.
2.5. Intervention
The interventions had three major components: (a) goal setting, (b) skills training, and (c) home monitoring. All components were administered concurrently the CDMS.
2.5.1. Goal setting
Initially, goal setting was an iterative process that followed motivational interviewing techniques [24], because most participants were not familiar with goal setting. Many did recognize the term A1C, albeit with varying degrees of familiarity; but the majority were not clear about the role of A1C in diabetes or about the relationship between A1C and self-care activities. As part of the goal-setting process, we shared the most well-established evidence-based TLC recommendations from various programs sponsored by public agencies, including the DPP [10,25], the DPP Outcomes Study [26], and Look AHEAD [27]. In addition, we highlighted the quantitative results from our previous studies as well as compelling testimonials from participants who participated our traditional TLC intervention programs [17–19]. Table 1 details the goals for each group in this study.
Table 1.
hSHIP objectives and goals.
| A1C (at enrollment) | Prediabetes (<6.5%) |
Moderate Diabetes (A1C: 6.5%–7.9%) |
Severe Diabetes (A1C ≥ 8.0%) |
|---|---|---|---|
| Short-term (3 months) | −0.5% | −1.0% | −1.5% |
| Mid-term (6 months) | <5.5% | <6.5% | −0.5% |
| Long-term (12 month) | Maintain | Maintain | <7.0% |
| Goalsa | No DM medication | Stop/Reduce DM medication | Stop insulin/Reduce DM medication |
Participants consulted with healthcare providers when initiating, changing, or stopping medication.
2.5.2. Skills training
Skills training was provided in two ways: through online training modules for essential diabetes management knowledge and skills, and through offline one-on-one interviews and coaching sessions using motivational interviewing [24]. During the 6-month project, CHWs met each participant at least three times for coaching on (a) how to achieve the participant’s personal goals by practicing healthy diet and exercise, (b) reinforcing the principles and methods of healthy diet such as monitoring calorie intake and glycemic index, (c) how to use home monitoring devices and record their data on the participant’s homepage, (d) medication review, (d) stress management, and (d) ways to improve therapeutic communication with care providers and CHWs.
In the first counseling session, participants could envision their pursuit of a healthy life with a clear goal and several basic skills. In the subsequent interview sessions, both CHWs and participants together reviewed the participants’ progress reports for the last 3 months, assessed their performance against the goals, refined the goals, and discussed ways to improve. The first session took 30–60 min per person, and the subsequent sessions took less than 30 min.
2.5.2.1. Motivation-based reinforcement.
Motivation-based reinforcement was provided by (a) daily SMS notifications (except on holidays and weekends) via the smartphone for 6 months; these were short text messages (25 words or less) announcing that a health message had been delivered to the participant’s personal homepage; and (b) in-depth health messages (500–1000 words) delivered to the participant’s personal homepage daily (except on holidays and weekends) on the etiology of diabetes, TLC, the role of diet and exercise in diabetes management, foods, pros and cons of certain pharmacotherapies, and testimonies. True to the principles of persuasive design [17], the message addressed all aspects of diabetes management in laypersons’ language, using culturally relevant infographic illustrations and data visualizations. Each message could be read in 5 min. The goal of these messages was to sustain engagement in self-management. A total of 120 messages were delivered during the project period for each participant.
2.5.2.2. Personalized intervention.
With insights gained from self-care behavioral data from each individual through CDMS interactive features, the CHWs were able to give personalized recommendations through one-on-one counseling with participants. Using the CDMS’s messenger module, the CHWs and participants could communicate directly regarding schedules and appointments, notifications, and questions about all issues related to the program. These communications occurred strictly between CHWs and individual participants in order to personalize the intervention and provide personal recommendations. For example, when participants’ uploaded data, the CHW would correspond accordingly. Outliers to normal values would trigger a notification or alarm to CHWs, case managers, and participants using both SMS and the messenger module. CHWs could then call the participant. Alerts were also sent using SMS and the messenger module to those who did not upload home monitoring data or who had not read health messages for more than 2 weeks. CHWs called any participant who did not respond to alerts or notifications.
2.5.3. Home monitoring
At enrollment, participants received a set of home monitoring devices including monitors for blood sugar (along with strips and lancets), blood pressure (BP), and activity, as well as a weight scale. We asked participants to measure weight, glucose level, BP, exercise, food intake, and sleep twice a week and to record these on their homepage. Measurement and recording took less than 10 min. All devices were Bluetooth-enabled to send data automatically via smartphone, but we disabled this function because of the target population’s general “technological readiness.” In addition, the benefit of manual recording outweighed that of convenience, since recording by fingertips on multisensory devices enhances both short-term (i.e., knowledge) and long-term (i.e., muscle memory or skills) retention of intervention contents by increasing self-engagement and commitment [28,29].
2.6. Data collection
After obtaining informed consent, we collected the participants’ demographic information at baseline, and diabetes-related efficacy [30], diabetes knowledge [31], and depression using the Patient Health Questionnaire-Korean version (PHQ-9K) [32] at baseline and at 3 and 6 months, using the built-in questionnaire module in our CDMS. We also measured BP and weight at each follow-up point. All blood-related tests were conducted using point-of-care products such as A1C Now+ for hemoglobin A1C and total LipidPlus for total cholesterol.
3. Results
3.1. Feasibility
Our process evaluation indicated that implementing the hSHIP in the KA community was quite feasible. During the 6-month recruitment period, we were able to recruit 311 KAs with diagnoses of diabetes or prediabetes. Of those, 247 KAs with prediabetes or diabetes met the eligibility criteria and enrolled in the program, and 215 completed the intervention and follow-up outcome evaluation with 630 counseling sessions. Only 6 participants missed the counseling session at 3 months and 9 at 6 months and the attendance rate was 96.5%. Although the majority of the participants were older adults, they were able to accept the technology-assisted intervention components relatively easily, given that the culturally tailored intervention was coordinated by bilingual Korean CHWs, the technology’s interface was translated into Korean, and participants could text interactively in Korean; they accept the digital touch, as long as it is aided by essential human touch.
3.2. Sample characteristics
The mean age was 60.9 years. The majority were married (86.2%), worked full or part-time (64.6%), had lived in the U.S. for more than 20 years (63.7%), and had college or advanced degrees (68.5%). Almost two thirds (70.7%) owned their house, and slightly over half (54.6%) reported that they were somewhat comfortable with their current income. However, as a population with limited English proficiency, they expressed frustration associated with that language barrier. Many felt that their spoken English was poor (66.1%), that they needed translators for documents in English (70.5%), and that they needed interpreters when they visited English-speaking healthcare providers (72.0%). These demographic characteristics were similar among the prediabetes, diabetes I (A1C ≥ 6.5%–7.9%), and diabetes II (A1C 8.0%) groups (Table 2).
Table 2.
Sample characteristics.
| Indicator/Group | Prediabetes (n = 50) |
Moderate Diabetes (n = 114) |
Severe Diabetes (n = 51) |
Total (n = 215) |
|---|---|---|---|---|
| Gender | 25 (50.0%) | 55 (48.3%) | 31 (60.8%) | 111 (51.6%) |
| Male, n (%) | ||||
| Age, mean (SD) | 59.3 (7.2) | 61.4 (6.7) | 61.2 (8.6) | 60.9 (7.3) |
| Marital status | 43 (86.0%) | 99 (86.8%) | 43 (84.3%) | 185 (86.1%) |
| Married, n (%) | ||||
| Working, n (%) | 39 (78.0) | 69 (60.5%) | 31 (60.7%) | 139 (64.6%) |
| Years in USA, n (%) | ||||
| ≤10 years | 5 (10.0%) | 8 (7.0%) | 6 (11.8%) | 19 (8.8%) |
| 11–20 years | 13 (29.0%) | 31 (27.2%) | 15 (29.4%) | 59 (27.4%) |
| 21–30 years | 15 (30.0%) | 19 (16.7%) | 11 (21.6%) | 45 (20.9%) |
| >30 years | 17 (34.0%) | 56 (49.1%) | 19 (37.3%) | 92 (42.8%) |
| Education, n (%) | ||||
| ≤High school graduate | 16 (32.7%) | 38 (33.6%) | 14 (25.0%) | 67 (31.5%) |
| ≤College graduate | 27 (55.1%) | 59 (52.2%) | 28 (54.9%) | 114 (53.5%) |
| Advanced degree | 6 (12.2%) | 16 (14.2%) | 10 (19.6%) | 32 (15.0%) |
| Own housing | 38 (76.0%) | 81 (71.1%) | 33 (64.7%) | 152 (70.7%) |
| Living arrangement, n (%) | ||||
| Living alone | 4 (8.0%) | 7 (6.1%) | 3 (6.0%) | 14 (6.5%) |
| With spouse | 26 (52.0%) | 41 (40.0%) | 31 (62.0%) | 98 (45.8%) |
| With spouse + children | 20 (40.0%) | 66 (57.9%) | 16 (32.0%) | 102 (47.7%) |
| Comfortability of living with current income, n (%) | ||||
| Uncomfortable | 20 (41.7%) | 55 (51.9%) | 17 (34.7%) | 92 (45.3%) |
| Alright | 16 (33.3%) | 25 (23.6%) | 21 (42.9%) | 62 (30.5%) |
| Comfortable | 12 (25.0%) | 26 (24.5%) | 11 (22.5%) | 49 (24.1%) |
| Speaking English, n (%) | ||||
| Good/Excellent | 14 (28.0%) | 37 (33.0%) | 21 (41.2%) | 72 (33.8%) |
| Poor/Very Poor | 36 (72.0%) | 75 (67.0%) | 30 (58.8%) | 141 (66.1%) |
| Need a translator when reading English documents, n (%) | ||||
| Rarely | 14 (28.0%) | 31 (28.2%) | 17 (34.0%) | 62 (29.5%) |
| Always | 36 (72.0%) | 79 (71.8%) | 33 (66.0%) | 148 (70.5%) |
| Need an interpreter when visiting English-speaking medical provider, n (%) | ||||
| Rarely | 10 (20.0%) | 34 (30.6%) | 15 (28.9%) | 59 (28.0%) |
| Always | 40 (80.0) | 77 (69.4%) | 35 (70.0%) | 152 (72.0%) |
| Diagnosed in the past, n (%) | ||||
| Prediabetes | 17 (34.0%) | 1 (0.9%) | 0 (0.0%) | 18 (8.4%) |
| Type 2 diabetes | 4 (8.0%) | 83 (72.8%) | 47 (88.7%) | 134 (61.8%) |
| High blood pressure | 18 (36.0%) | 62 (54.4%) | 30 (58.8%) | 110 (51.2%) |
| High cholesterol | 18 (36.0%) | 76 (66.7%) | 34 (66.7%) | 128 (59.5%) |
| Year since diagnosis, mean years (SD) | ||||
| Prediabetes | 2.4 (1.2) | 1.0 (0.0) | – | 2.3 (1.2) |
| Type 2 diabetes | 1.3 (0.5) | 6.2 (4.7) | 10.0 (6.9) | 7.3 (5.9) |
| High blood pressure | 9.8 (9.1) | 9.5 (7.6) | 10.6 (7.0) | 9.8 (7.6) |
| High cholesterol | 4.1 (4.1) | 5.9 (4.2) | 7.0 (5.4) | 6.0 (4.6) |
| Reported Medication, n (%) | ||||
| Prediabetes | 1 (2.0%) | – | – | 1 (0.9%) |
| Diabetes | 76 (66.7%) | 42 (82.4%) | 118 (54.9%) | |
| High blood pressure | 15 (30.0%) | 60 (52.6%) | 28 (54.9%) | 103 (47.9%) |
| High cholesterol | 11 (22.0%) | 68 (59.7%) | 29 (56.9%) | 108 (50.2%) |
Note: 11 people were diagnosed with prediabetes before or at enrollment; 18 people were diagnosed diabetes just before or at enrollment.
It should be noted that 11 participants (22.0%) were diagnosed with prediabetes and 18 (10.9%) with diabetes either before or at enrollment. In addition, about half reported that they were on medications for diabetes (54.9%), hypertension (47.9%), and high cholesterol (50.2%). The proportions of those taking medication were higher in the diabetes groups than in the prediabetes group (Table 2).
3.3. A1C changes and achievements of benchmark of TLC program
The prediabetes group reduced A1C by −0.4% at 3 months and −0.6% at 6 months, and slightly less than two thirds (63.6%) of this group successfully lowered A1C below 5.7%. About a quarter (22.8%) of the moderate diabetes group (A1C 6.5%–7.9% at baseline) achieved reduction of A1C by more than 1.0% at 3 months, and two thirds (66.7%) were successful in lowering A1C below 6.5% at 6 months. In addition, the mean A1C of the severe diabetes group (A1C ≥ 8.0%) was reduced by 1.8% at 3 months, with about half of the severe diabetes group (49.0%) having achieved their objective of lowering A1C more than 1.5%. The same group further lowered A1C by 2.3% at 6 months and about half (47.1%) successfully achieved their goal of lowering A1C below 7.0%. The A1C of both diabetes groups combined was reduced by 0.9% at 3 months and 1.3% at 6 months, respectively; overall, half (51.5%) at 6 months lowered A1C below 6.5%. Using paired t-tests, all A1C reductions at 3 and 6 months from baseline were statically significant at p < 0.001 (Table 3).
Table 3.
Performance by indicators and changes over time (% goal achieved).
| Indicator/Group | Baseline | 3 Months | Δ (%1) | 6 Months | Δ (%2) |
|---|---|---|---|---|---|
| Hemoglobin A1C, mean (SD/%) | |||||
| Prediabetes (n = 50) | 6.2 (0.2) | 5.8 (0.5) | −0.4c (26.0%) | 5.6 (0.4) | −0.5c (56.0%) |
| Moderate (I; n = 114) | 7.0 (0.5) | 6.5 (0.6) | −0.5c (22.8% | 6.2 (0.6) | −0.8c (66.7%) |
| Severe (II; n = 51) | 9.5 (1.3) | 7.7 (1.2) | −1.8c (49.0%) | 7.2 (1.1) | −2.3c (47.1%) |
| Total (I & II) (n = 165) | 7.8 (1.4) | 6.9 (1.0) | −0.9c (37.6%3) | 6.5 (0.9) | −1.3c (51.5%3) |
p < 0.05
p < 0.01
p < 0.001 using paired t-test (two-tailed) compared to baseline.
Proportion of objective achieved at 3 months (A1C reduction <−0.5% for prediabetes, <−1.0% for moderate diabetes group and <−1.5% for severe diabetes group).
Proportion of objective achieved at 6 months (A1C <5.7% for prediabetes group, <6.5% for moderate diabetes group and <7.0% for severe diabetes group).
Proportion of A1C reduction <−1.0% at 3 months and A1C < 6.5% at 6 months.
3.4. Changes in biophysical and psychosocial indicators during the program
3.4.1. Weight
The average weight reduction in all groups and at all periods ranged from −0.4 kg (0.9 lbs) to −1.6 kg (3.5 lbs), with 1.2 kg (2.6 lbs) as a total mean. All reductions from baseline were statistically significant except in the prediabetes group at 6 months (p = 0.362)
3.4.2. BP
All groups lowered both systolic and diastolic BP, and the reductions of systolic BP ranged on average between −3.4 and −7.6 mm/Hg, with a mean of −6.3 mm/Hg at 3 months and −4.0 mm/Hg at 6 months. All reductions of systolic BP were statistically significant at 3 months but only that of the moderate diabetes group was significant at 6 months (p = 0.008). All groups also lowered their diastolic BP in a similar manner, and the reductions at each period were statistically significant except in the prediabetes group at 6 months (p = 0.073). Therefore, the proportions of total people with BP controlled (SBP/DBP < 130/ 80 mm/Hg) significantly increased by 16.3% (p < 0.001) and 11.2% (p = 0.021) at 3 and at 6 months, respectively.
3.4.3. Total cholesterol
The mean total cholesterol in all groups was 153.9 mg/dL at baseline, with significant reduction (−4.6 mg/dL) at 3 months, but it increased (+3.6 mg/dL) at 6 months. Changes over time in total cholesterol in each group were also mixed, with a range between −6.8 mg/dL and −5.4 mg/dL and +7.3 mg/dL; no change was statistically significant except in the moderate dibabetes group at 3 months (p = 0.044).
3.4.4. Depression
The total mean depression score was 5.1 points at baseline, with significant improvement at 3 months (−0.4 points, p = 0.035) and again at 6 months (−0.9 points, p < 0.001). Furthermore, the proportion of people with the optimal depression score (<5) increased by 3.3% at 3 months and 23.9% at 6 months. Although the number of people with the optimal depression score increased from baseline in all groups at both 3 and 6 months, only the 6-month data was statistically significant (p = 0.008).
3.4.5. Diabetes-related efficacy
All groups improved efficacy by 1.1–2.2 points (out of a total 8–32 points), which were statistically significant except in the prediabetes group at 6 months (p = 0.108).
3.4.6. Diabetes knowledge
All groups improved diabetes knowledge at each interval. All increases ranged between 1.7 and 3.3 points (out of a total 22 points) and were statistically significant (Table 4).
Table 4.
Physiological indicator changes over time by group.
| Indicator/Group | Baseline | 3 Months | Δ (3–0) | 6 Months | Δ (6–0) |
|---|---|---|---|---|---|
| Body Weight (kg), mean (SE) | |||||
| Prediabetes (n = 50) | 66.9 (1.7) | 65.7 (1.8) | −1.2 (0.2)c | 66.4 (1.9) | −0.4 (0.4) |
| Moderate (n = 114) | 65.7 (1.1) | 64.3 (1.1) | −1.4 (0.2)c | 64.1 (1.1) | −1.6 (0.2)c |
| Severe (n = 51) | 67.2 (1.6) | 66.2 (1.6) | −1.0 (0.3)c | 66.0 (1.6) | −1.2 (0.4)b |
| Total (n = 215) | 66.3 (0.8) | 65.1 (0.8) | −1.2 (0.1)c | 65.1 (0.8) | −1.2 (0.2)c |
| Systolic Blood Pressure (mm/Hg), mean (SE) | |||||
| Prediabetes (n = 50) | 127.8 (2.2) | 121.5 (2.0) | −6.3 (1.8)c | 124.4 (2.4) | −3.4 (2.1) |
| Moderate (n = 114) | 128.2 (1.6) | 122.5 (1.4) | −5.8 (1.2)c | 124.5 (1.4) | −3.7 (1.4)b |
| Severe (n = 51) | 130.7 (2.2) | 123.1 (3.2) | −7.6 (3.5)a | 125.8 (3.7) | −5.0 (3.9) |
| Total (n = 215) | 128.7 (1.1) | 122.4 (1.2) | −6.3 (1.1)c | 124.8 (1.3) | −4.0 (1.3)b |
| Diastolic Blood Pressure (mm/Hg), mean (SE) | |||||
| Prediabetes (n = 50) | 79.9 (1.4 | 73.7 (1.4) | −6.3 (1.1)c | 76.7 (1.8) | −3.2 (1.8) |
| Moderate (n = 114) | 77.4 (1.0) | 71.7 (1.0) | −5.8 (0.8)c | 72.6 (0.9) | −4.8 (1.0)c |
| Severe (n = 51) | 80.0 (1.5) | 73.8 (2.1) | −6.2 (2.1)b | 74.3 (2.3) | −5.7 (2.2)a |
| Total (n = 215) | 78.6 (0.7) | 72.6 (0.8) | −6.0 (0.7)c | 74.0 (0.8) | −4.6 (0.8)c |
| Blood Pressure Controlled (SBP/DBP: <130/80 mm/Hg), mean (SE)* | |||||
| Prediabetes (n = 50) | 25 (50.0% | 30 (60.0%) | +5 (10.0%) | 25 (50.0%) | 0 (0.0%) |
| Moderate (n = 114) | 52 (45.6%) | 71 (62.3%) | +19 (16.7%)b | 69 (60.5%) | +17 (14.9%)a |
| Severe (n = 51) | 15 (29.4%) | 26 (51.0%) | +11 (21.6%)a | 22 (43.1%) | +7 (13.7%) |
| Total (n = 215) | 92 (42.8%) | 127 (59.1%) | +35 (16.3%)c | 116 (54.0%) | +24 (11.2%)a |
| Total Cholesterol (mg/dL), mean (SE)** | |||||
| Prediabetes (n = 45) | 161.3 (4.8) | 159.2 (3.4) | −2.1 (4.2) | 168.5 (5.2) | +7.3 (5.0) |
| Moderate (n = 100) | 151.3 (3.6) | 145.9 (3.1) | −5.4 (2.7)a | 154.3 (3.6) | −3.0 (3.3) |
| Severe (n = 39) | 152.0 (4.4) | 146.7 (4.4) | −5.3 (3.9) | 152.9 (4.8) | +0.9 (4.8) |
| Total (n = 184) | 153.9 (2.5) | 149.3 (2.1) | −4.6 (2.0)a | 157.5 (2.5) | +3.6 (2.4) |
| Depression (PHQ-9), mean (SE) | |||||
| Prediabetes (n = 50) | 5.1 (0.6) | 4.4 (0.7) | −0.7 (0.5) | 4.3 (0.6) | −0.8 (0.5) |
| Moderate (n = 113) | 4.9 (0.3) | 4.6 (0.3) | −0.4 (0.2) | 4.3 (0.3) | −0.6 (0.3)a |
| Severe (n = 50) | 5.4 (0.6) | 5.2 (0.6) | −0.2 (0.3) | 4.0 (0.5) | −1.4 (0.4)c |
| Total (n = 215) | 5.1 (0.3) | 4.7 (0.3) | +2 (4.0%) | 4.2 (0.2) | −0.9 (0.2)c |
| Depression, n (%) optimal (PHQ-9 < 5)* | |||||
| Prediabetes (n = 50) | 29 (58.0%) | 31 (62.0%) | +2 (4.0%) | 33 (66.0%) | +4 (8.0%) |
| Moderate (n = 113) | 58 (50.9%) | 62 (54.9%) | +4 (3.5%) | 72 (63.7%) | +14 (12.4%) |
| Severe (n = 50) | 24 (47.1%) | 25 (50.0%) | 1 (2.0%) | 33 (66.0%) | +9 (18.0%) |
| Total (n = 213) | 111 (51.6%) | 118 (55.4%) | +7 (3.3%) | 138 (64.8%) | +27 (23.9%)b |
| Diabetes-Related Efficacy (8–40 points), mean (SE) | |||||
| Prediabetes (n = 50) | 25.9 (0.8) | 27.6 (0.7) | +1.6 (0.8)a | 27.0 (0.7) | +1.1 (0.7) |
| Moderate (n = 114) | 26.2 (0.4) | 27.6 (0.4) | +1.4 (0.4)c | 27.9 (0.4) | +1.7 (0.4)c |
| Severe (n = 51) | 25.4 (0.6) | 27.6 (0.6) | +2.2 (0.7)b | 27.3 (0.7) | +2.0 (0.8)a |
| Total (n = 215) | 25.9 (0.3) | 27.6 (0.3) | +1.7 (0.3)c | 27.5 (0.3) | +1.6 (0.3)c |
| Diabetes-Related Knowledge (0–22 points), mean (SE) | |||||
| Prediabetes (n = 50) | 13.0 (0.6) | 16.1 (0.5) | +3.1 (0.5)c | 16.3 (0.5) | +3.3 (0.5)c |
| Moderate (n = 114) | 14.8 (0.4) | 16.5 (0.3) | +1.7 (0.3)c | 16.9 (0.3) | +2.1 (0.3)c |
| Severe (n = 50) | 14.8 (0.6) | 16.5 (0.5) | +1.7 (0.4)c | 16.6 (0.4) | +1.9 (0.5)c |
| Total (n = 193) | 14.4 (0.3) | 16.4 (0.2) | +2.0 (0.2)c | 16.7 (0.2) | +2.4 (0.2)c |
p < 0.05
p < 0.01
p < 0.001 using paired t-test (two-tailed) compared to baseline.
Test of proportion (two-tailed) compared to baseline.
Excluding too low values.
3.5. Changes in antidiabetic medication regimen during intervention
At enrollment, a total of 131 participants (79.4%) were on antidiabetic drugs including insulin (n = 11). At 3 months, 54 (41.2%) were able to either stop or reduce the number or the dosage of their medications after review of the A1C test results in consultation with their primary care doctors. It should be noted that 5 (45.5%) of 11 insulin users stopped insulin completely and that the glucose control status between those who continued and those who stopped insulin was comparable at each period (A1C 9.7% vs. 9.5&, diff = 0.12%, p = 0.870 at baseline using paired t-test; 8.5% vs. 7.8%, diff = 0.72%, p = 0.367 at 3 months; 7.6% vs. 7.7%, diff = 0.14%, p = 0.874). The A1C reductions in the stop/reduce-medication group and the medication group were comparable in each period. However, in terms of achieving the program goals (i.e., A1C reduction to levels of A1C below 7.0%), the stop/reduce-medication group (75.9%) outperformed the medication group (71.4%) (Table 5).
Table 5.
Hemoglobin A1C mean (SE/%) changes over time by antidiabetic medication status (Diabetes Group only).
| Medication/Group | Baseline | 3 Mon | Δ (%1) | 6 Months | Δ (%2) |
|---|---|---|---|---|---|
| None (n = 34) | 7.1 (0.1) | 6.5 (0.1) | −0.6(23.5%) | 6.1 (0.1) | −0.9 (88.2%) |
| Continue (n = 77) | 8.0 (1.3) | 7.1 (0.1) | −0.9 (37.7%) | 6.6 (0.1) | −1.4 (71.4%) |
| Stop/Reduce (n = 54) | 7.8 (0.2) | 6.7 (0.1) | −1.1 (46.3%) | 6.7 (0.1) | −1.3 (75.9%) |
Proportion of A1C reduction of < 1.0% at 3 months.
Proportion of A1C < 7.0% at 6 months.
4. Discussion and conclusion
4.1. Discussion
Our findings demonstrate that the hSHIP program, a TLC intervention with persuasive technology combining human and digital instruction, was working in both the prevention and management of diabetes. In the prediabetes group, no one developed diabetes in 6 months and more than half (56.0%) successfully lowered their A1C to normal (below 5.7%), with an A1C reduction of 0.5% at 6 months. These findings were equivalent to or better than those reported in other studies with a web version of the DPP [33]. Furthermore, the A1C reduction of 1.3% in the diabetes group at 6 months in our hSHIP was comparable to reduction with antidiabetic drugs [34], and better than improvement with some third-generation antidiabetic drugs: −0.6% with dipeptidyl peptidase-4 [35] and −0.8% to −1.0% with the sodium-glucose co-transporter 2 [36]. In particular, the A1C reduction of 2.3% at 6 months in the severe diabetes group (A1C ≥ 8.0%) should be noted. Finally, two thirds (66.7%) of those in the moderate diabetes group and half (47.1%) in the severe diabetes group lowered their A1C below 6.5%, for a combined average of 51.5%.
We attribute the effectiveness of our program to several factors. First, the hSHIP supported by CDMS incorporating persuasive technology was effective in systematically motivating participants to actively engage in self-management activities. The hSHIP assisted their goal-setting and provided skills training to achieve those goals. Second, there was strong rapport between CHWs and participants through constant feedback from CHWs (i.e., human touch), real-time data monitoring, and text messages (digital touch).
In addition, many participants were able to stop or reduce diabetes medications when they successfully lowered A1C at 3 months after consulting with their primary care doctors. Although stopping/reducing antidiabetic drugs was a long-term goal of our program, such a quick change was unanticipated. Furthermore, the diabetes control status (A1C < 7.0%) of participants who stopped or reduced diabetic medications was better than those for participants who continued their medications.
This finding also indicates the high level of effective patient– provider communication in a group of participants who traditionally maintain a rather passive style of communication with providers [37]. At enrollment, for example, almost all participants could not recall their last A1C test result or the names of their medications, consequently, resulting in underreporting. Instead, they reported categorically, “I was told that my A1C was high,” or “I’m taking diabetes drugs,” even though some pharmacists had provided their medications’ names handwritten in Korean. At 3 months, most recalled their last A1C value and the names of their medications, if any, correctly. The hSHIP intervention enabled participants to improve their self-efficacy and understand the relationship between A1C, blood glucose, and antidiabetic medications; as a result, they could initiate conversations on the possibility of stopping/reducing medications. The home monitoring reports of blood glucose, weight, BP, diet, and exercise could help family doctors who might otherwise have been reluctant to reduce antidiabetic medication.
Our findings demonstrate the effectiveness of TLC interventions and support the CDC’s recommendation that diet and exercise should be the primary intervention for type 2 diabetes management. The side effects of diabetes medications are well documented in the CDC-approved medication packaging labels (e.g., weight gain and hypoglycemia, especially for those on sulfonylurea and/or insulin) [38,39], and there are long-term side effects as well (e.g., increased risk of cardiovascular-related mortality) [40,41]. A well-designed TLC program can result in better outcomes than from pharmacotherapy use. In our hSHIP program, 76.4% achieved A1C < 7.0%, which was much higher than those of 25.9%–63.2% from pharmacotherapy [42]. Furthermore, the frequency was higher in the stop/reduce-medication group (75.9%) than in the continue-medication group (71.4%), which indicates that reducing antidiabetic medication when patients’ target blood glucose control is met (A1C < 7.0%) may be beneficial to long-term diabetes management.
4.1.1. Limitations
This study has some limitations. This was a trial done at a single center with a single ethnic minority sample. Certain sample (population) characteristics, such as the participants’ high adherence to the program (e.g., high retention rate) might not be transferable to other settings, because the study center has remained within the community for many years, serving KAs who are, as first-generation immigrants, eager for health education in Korean [19].
4.2. Conclusion
Despite the limitations, the findings of this study have strong clinical implications. Our findings suggest that it is possible to sustain motivation to engage in self-care behaviors over the long term, so that those behaviors will translate into optimal clinical outcomes. The key to sustaining motivation is constant and immediate feedback through a combination of digital and personal touch, because positive, real-time feedback helps to eliminate uncertainties in self-care behaviors. Furthermore, utilizing the most innovative technology in an accessible, personalized self-help intervention will proactively reduce potential heath disparity gaps, and is consistent with the movement toward precision medicine/health.
4.3. Practice implications
A hybrid self-help intervention program (hSHIP) that combines the digital instruction through persuasive technology and the rapport of CHWs with participants is a viable intervention to improve diabetes control and reduce social isolation among underserved minority groups in the U.S.
Acknowledgements
We would like to acknowledge editorial support with manuscript development provided by the Cain Center for Nursing Research and the Center for Transdisciplinary Collaborative Research in Self-Management Science (P30, NR015335) at The University of Texas at Austin School of Nursing.
Funding source and disclosure
This work was supported by the Technology Innovation and the Industrial Strategic Technology Development Program (10053704, Development of Smart Healthcare System and Pilot Project for Military Personnel and Global Healthcare) funded by the Korean Ministry of Trade, Industry, and Energy (MOTIE).
Footnotes
Declaration of interest toward
The authors confirm that there is no conflict of interest.
Ethical approval
All patient/personal identifiers have been removed or disguised so that the patient/person(s) described are not identifiable and cannot be identified through the details of the text.
References
- [1].Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF, Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence, Popul. Health Metr 8 (2010) 29, doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, Global estimates of diabetes prevalence for 2013 and projections for 2035, Diabetes Res. Clin. Pract 103 (2014) 137–149, doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- [3].Fried LP, Begg MD, Bayer R, Galea S, MPH education for the 21st century: motivation, rationale, and key principles for the new Columbia public health curriculum, Am. J. Public Health 104 (2014) 23–30, doi: 10.2105/AJPH.2013.301399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Condon JE, Cypress M, Faulkner P, Fischl AH, Francis T, Kolb LE, Lavin-Tompkins JM, MacLeod J, Maryniuk J, Mensing C, Orzeck EA, Pope DD, Pulizzi JL, Reed AA, Rhinehart AS, Siminerio L, Wang J, National standards for diabetes self-management education and support, Diabetes Care 44 (2017) 1409–1419, doi: 10.2337/dci17-0025. [DOI] [PubMed] [Google Scholar]
- [5].Venditti EM, Bray GA, Carrion-petersen M, Delahanty LM, Edelstein SL, Hamman RF, Hoskin MA, Knowler WC, Ma Y, First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes, Int. J. Obes 32 (2008) 1537–1544, doi: 10.1038/ijo.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI, Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions, Diabetes Care 35 (2012) 2681–2689, doi: 10.2337/dc11-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].King GL, Marguerite J. McNeely, Lorna E. Thorpe, Marjorie L.M. Mau, Jocelyn Ko, Liu LL, Sun A, Hsu WC, Chow EA, Understanding and addressing unique needs of diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders, Diabetes Care 35 (2012) 1181–1188, doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cradock KA, ÓLaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KA, Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta-analysis, Int. J. Behav. Nutr. Phys. Act 14 (2017) 18, doi: 10.1186/s12966-016-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham CK, Lohani GP, Mirza W, Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management, Front. Endocrinol 8 (2017) 6, doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Center for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation, National Diabetes Prevention Program, (2018). (Accessed 12 February 2018) https://www.cdc.gov/diabetes/prevention/pdf/curriculum.pdf/.
- [11].Gummesson A, Nyman E, Knutsson M, Karpefors M, Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes, Diabetes Obes. Metab 19 (2017) 1295–1305, doi: 10.1111/dom.12971. [DOI] [PubMed] [Google Scholar]
- [12].Hsu WC, Boyko EJ, Fujimoto WY, Kanaya A, Karmally W, Karter A, Arakaki R, Pathophysiologic differences among asians, native hawaiians, and other pacific islanders and treatment implications, Diabetes Care 35 (2012) 1189–1198, doi: 10.2337/dc12-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kang JH, Han HR, Kim KB, Kim MT, Barriers to care and control of high blood pressure in Korean American elderly, Ethn. Dis 16 (2006) 145–151 PMID: 16599363. [PubMed] [Google Scholar]
- [14].Chung J, Seo JY, Lee J, Using the socioecological model to explore factors affecting health-seeking behaviors of older Korean immigrants, Int. J. Older People Nurs 13 (2018) e12179, doi: 10.1111/opn.12179. [DOI] [PubMed] [Google Scholar]
- [15].Song HJ, Han HR, Lee JE, Kim JY, Kim KB, Ryu JP, Kim MT, Does access to care still affect health care utilization by immigrants? Testing of an empirical explanatory model of health care utilization by Korean American immigrants with high blood pressure, J. Immigr. Minor. Health 12 (2009) 513–519, doi: 10.1007/s10903-009-9276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shin HS, Kim MT, Juon HS, Kim KB, Patterns and factors associated with health care utilization among Korean American elderly, Asian Am. Pac. Isl. J. Health 8 (2000) 116–129 . [PubMed] [Google Scholar]
- [17].Kim KB, Kim MT, Lee HB, Nguyen T, Bone LR, Levine D, Community health workers versus nurses as counselors or case managers in a self-help diabetes management program, Am. J. Public Health 106 (2016) 1052–1058, doi: 10.2105/AJPH.2016.303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim MT, Kim KB, Huh B, Nguyen T, Han HR, Bone LR, Levine D, The effect of a community-based self-help intervention: Korean Americans with type 2 diabetes, Am. J. Prev. Med 49 (2015) 726–737, doi: 10.1016/j.amepre.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim MT, Kim KB, Ko J, Jang Y, Levine D, Lee HB, Role of depression in diabetes management in an ethnic minority population: a case of Korean Americans with type 2 diabetes, Br. Med. J. Open Diabetes Res. Care 5 (2017) e000337 https://drc.bmj.com/content/5/1/e000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fogg BJ, Persuasive Technology: Using Computers to Change What We Think and Do, Morgan Kaufmann Publishers, San Francisco, 2003. [Google Scholar]
- [21].Orji R, Vassileva J, Mandryk RL, Modeling the efficacy of persuasive strategies for different gamer types in serious games for health, User Model. User-Adapt. Interact 24 (2014) 453–498, doi: 10.1007/s11257-014-9149-8. [DOI] [Google Scholar]
- [22].Fisher WA, Fisher JD, Harman JJ, The information-motivation-behavioral skills model: a general social psychological approach to understanding and promoting health behavior, in: Suls J, Wallston KA (Eds.), Social Psychological Foundations of Health and Illness, Blackwell Publishing, Malden, MA, 2003, pp. 82–106, doi: 10.1002/9780470753552.ch4. [DOI] [Google Scholar]
- [23].Meunier S, Coulombe S, Beaulieu MD, Côté J, Lespérance F, Chiasson JL, Bherer L, Lambert J, Houle J, Longitudinal testing of the information-motivation-behavioral skills model of self-care among adults with type 2 diabetes, Patient Educ. Couns 99 (2016) 1830–1836, doi: 10.1016/j.pec.2016.06.011. [DOI] [PubMed] [Google Scholar]
- [24].Rollnick S, Miller WR, Butler CC, Motivational Interviewing in Health Care: Helping Patients Change Behavior, Guilford Press, New York, 2008. [Google Scholar]
- [25].National Institute of Diabetes and Digestive and Kidney Diseases, Diabetes Preventive Program (DPP), (2018). (Accessed September 2018) https://www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp.
- [26].National Institute of Diabetes and Digestive and Kidney Diseases, Diabetes Prevention Program Outcomes Study (DPPOS), (2018). (Accessed September 2018) https://www.niddk.nih.gov/news/for-reporters/diabetes-prevention-program-outcomes-study.
- [27].National Institute of Diabetes and Digestive and Kidney Diseases, Look AHEAD: Action for Health in Diabetes, (2018). (Accessed September 2018 https://www.niddk.nih.gov/news/for-reporters/look-ahead.
- [28].Freeman S, Eddy SL, McDonough M, Smith MK, Okoroafor N, Jordt H, Wenderoth MP, Active learning increases student performance in science, engineering, and mathematics, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 8410– 8415, doi: 10.1073/pnas.1319030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shusterman R, Muscle memory and the somaesthetic pathologies of everyday life, Hum. Mov 12 (2011) 4–15, doi: 10.2478/v10038-011-0001-2. [DOI] [Google Scholar]
- [30].Lorig K, Ritter PL, Villa FJ, Armas J, Community-based peer-led diabetes self-management: a randomized trial, Diabetes Educ 35 (2009) 641–651, doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- [31].Fitzgerald JT, Funnell MM, Hess GE, Barr PA, Anderson RM, Hiss RG, Davis WK, The reliability and validity of a brief diabetes knowledge test, Diabetes Care 21 (1998) 706–710, doi: 10.2337/diacare.21.5.706. [DOI] [PubMed] [Google Scholar]
- [32].Han C, Jo SA, Kwak JH, Pae CU, Steffens D, Jo I, Park MH, Validation of the Patient Health Questionnaire-9 Korean version in the elderly population: the Ansan Geriatric Study, Compr. Psychiatry 49 (2008) 218–223, doi: 10.1016/j.comppsych.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [33].Sepah SC, Jiang L, Ellis RJ, McDermott K, Peters AL, Engagement and outcomes in a digital diabetes prevention program: 3-year update, BMJ Open Diabetes Res. Care 5 (2017) e000422 https://drc.bmj.com/content/5/1/e000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC, The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis, Diabetes Care 33 (2010) 1859–1864, doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Food and Drug Administration, Januvia (Sitagliptin) Tablets, (2006). (Accessed 12 February 2018) https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021995s019lbl.pdf.
- [36].Food and Drug Administration, INVOKANA (Canagliflozin) Tablets, for Oral Use, (2013). (Accessed 12 February 2018) https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204042s000lbl.pdf.
- [37].Cooper LA, Roter DL, Patient-provider communication: the effect of race and ethnicity on process and outcomes of healthcare, in: Smedley BD, Stith AY, Nelson AR (Eds.), Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care , National Academies Press, Washington, DC, 2003, pp. 552–593. https://www.ncbi.nlm.nih.gov/books/NBK220354/. [PubMed] [Google Scholar]
- [38].Phung OJ, Scholle JM, Talwar M, Coleman CI, Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes, JAMA 303 (2010) 1410–1418, doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- [39].Pontiroli AE, Miele L, Morabito A, Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis, Diabetes Obes. Metab 13 (2011) 1008–1019, doi: 10.1111/j.1463-1326.2011.01433.x. [DOI] [PubMed] [Google Scholar]
- [40].Gamble JM, Simpson SH, Eurich DT, Majumdar SR, Johnson JA, Insulin use and increased risk of mortality in type 2 diabetes: a cohort study, Diabetes Obes. Metab 12 (2010) 47–53, doi: 10.1111/j.1463-1326.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- [41].Ou HT, Chang KC, Li CY, Wu JS, Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: a nation-wide longitudinal study, Cardiovasc. Diabetol 15 (2016) 41, doi: 10.1186/s12933-016-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D, Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients, Diabetes Obes. Metab 14 (2012) 228–233, doi: 10.1111/j.1463-1326.2011.01512.x. [DOI] [PubMed] [Google Scholar]


