Abstract
Background:
Interleukin (IL)-6 is elevated in circulation with chronic stress and may contribute to neurobehavioral complications. We have reported that repeated social defeat (RSD) stress in mice caused recruitment of proinflammatory monocytes to the brain and triggered the onset of anxiety-like behavior. Therefore, the purpose of this study was to determine the role of IL-6 signaling in the peripheral immune response, neuroinflammation, and anxiety following stress.
Methods:
WT and IL-6KO mice were subjected to RSD, and immune and behavioral parameters were determined 14 hours later.
Results:
Although monocyte release and recruitment to the brain during stress were maintained in the IL-6KO mice, anxiety and social avoidance were prevented. NanoString analysis of FAC-sorted blood monocytes (CD11b+/Ly6Chi) and brain monocytes (CD11b+/CD45hi) revealed a unique pattern of immune-related gene expression that was dependent on stress and IL-6. For instance, blood monocytes after stress had a transcriptional signature and immune profile consistent with priming, which was attenuated in monocytes from IL-6KO stress mice. Moreover, the monocytes recruited to the brain and associated with the development of anxiety had a transcriptional signature (enhanced IL-1β, CD14, Mmp9, MyD88, Ager, Stat3) that was dependent on IL-6.
Conclusions:
Here, we show the effects of IL-6 on the transcriptional signature of monocytes in circulation and brain after stress. Overall, robust increases in IL-6 after stress induced a primed profile in monocytes that were recruited to the brain and propagated IL-1-mediated inflammation and anxiety.
Keywords: IL-6; stress, monocytes; IL-1β; anxiety; social avoidance
Introduction
The role of inflammatory cytokines in the development and progression of psychiatric illnesses is significant because approximately 20% of the patients fail to respond to treatment and 60% fail to attain the desired response [1, 2]. Furthermore, patients with mood disorders nonresponsive to selective serotonin reuptake inhibitors (SSRIs) showed significantly higher levels of peripheral IL-6 [3, 4]. Similarly, depressed patients non-responsive to anti-depressants with high levels of C-reactive protein (CRP) showed improved symptom outcomes following anti-inflammatory intervention [5]. High levels of CRP and IL-6 were also reported in individuals with anxiety disorders [6, 7]. IL-6 is a consistently elevated biomarker of chronic stress in long-term caregivers [8], early life adversity [9], and depressed individuals that attempted suicide [10]. IL-6 is a pleiotropic cytokine produced by immune and non-immune cells, including T-cells, neutrophils, adipocytes, hepatocytes, and myocytes. IL-6 signaling is complex in that cells normally unresponsive to IL-6 may become responsive via binding of soluble receptors [11]. Peripheral blood cells isolated from stressed individuals show increased production of spontaneous IL-6 and in response to immune challenge [12, 13]. While myriad studies detect higher circulating IL-6 levels in mood disorders, the functional role of IL-6 in mediating neurobehavioral complications is unclear.
Parallel to clinical studies, rodents exposed to stressors (e.g., restraint, footshock and social defeat stress) have increased circulating IL-6 [14–17]. Increase in plasma IL-6 is regulated by the hypothalamic-pituitary-adrenal (HPA) axis. For instance, interfering with HPA activation by adrenalectomy or metyrapone (corticosterone synthesis inhibitor) prevented plasma IL-6 production during stress [15, 17]. Rodent studies have provided some insight into the functional role of IL-6 in behavioral dysregulation. For example, chronic stress and overexpression of central IL-6 triggered depressive-like behavior in rats – an effect unaltered by SSRI, but prevented by treatment with IL-6 antibodies [18]. Notably, IL-6KO mice were resilient to learned helplessness behavior in response to uncontrollable foot-shocks, forced swim, and tail suspension [19]. Furthermore, mice with social avoidance (“susceptible”) following exposure to social defeat stress had higher IL-6 levels compared to those that did not develop social avoidance (“resilient”), and this effect was prevented with IL-6 antibody treatment [16]. Bone marrow transplantation from susceptible mice induced social avoidance in naïve mice after a single sub-threshold exposure to stress [16]. A recent study indicated that peripheral IL-6 may directly enter the brain via increased blood-brain barrier permeability and promote social avoidance following social defeat stress [20]. Taken together, clinical and experimental findings highlight a key role of IL-6 in neurobehavioral deficits following stress.
We have reported that repeated social defeat (RSD) in mice promoted the accumulation of peripheral monocytes in the brain that augmented IL-1β signaling and caused prolonged anxiety-like behavior [21, 22]. RSD also induced a robust increase in plasma IL-6 [17, 23]. Interventions with noradrenergic receptor antagonist or GABAergic agonists prevented RSD-induced IL-6 increase, monocyte recruitment to the brain, and anxiety-like behavior following RSD [24, 25]. Therefore, we aimed to determine the role of IL-6 signaling in the peripheral immune response, neuroinflammation, and anxiety following RSD. Here, we show novel data that IL-6 production during RSD primes peripheral monocytes that traffic to the brain and augment IL-1β signaling that is associated with anxiety-like behavior.
Methods and Materials
Mice
Wildtype male C57BL/6 mice (6–8 weeks old) and CD-1 (12 months old) mice were purchased from Charles River Laboratories. Breeding triads of IL-6KO mice on the C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). Additional information is provided in the supplemental methods. All procedures were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals, and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Repeated Social Defeat (RSD)
Age-matched wildtype and transgenic mice (7–8 weeks old) were exposed to RSD as previously described [21] and outlined in the supplemental methods. In brief, an aggressive CD-1 mouse was introduced into the cage of an established cohort of three resident C57BL/6 mice 2 hours daily for six consecutive days, and resident mice were monitored for submissive behaviors. At the end of the 2 h period, the aggressive mice were placed back into their cages until the next day when the protocol was repeated.
Behavioral analyses
Fourteen hours after the last cycle of stress, mice were tested for anxiety-like behavior in the open field and social avoidance as described in the supplemental methods.
Tissue collection for ELISA,flow cytometry and real time qPCR
Bone marrow, blood, and brain samples were collected following CO2 asphyxiation, fourteen hours after the last cycle of stress, and processed for flow cytometry and ELISA as described in the supplemental methods. From a separate experiment, brain CD11b+ cells were collected immediately after the last cycle of stress for real-time qPCR (supplemental methods).
Immunohistochemistry
Fourteen hours after the last cycle of stress, mice were transcardially perfused and fixed with paraformaldehyde. Brain samples were processed and Iba-1 immunofluorescence was performed as described in the supplemental methods.
NanoString Gene Expression Analysis
Blood monocytes (CD11b+/Ly6Chi) and brain monocytes (CD11b+/CD45hi) were collected and FAC-sorted fourteen hours after the last cycle of stress. RNA copy number was determined for 279 genes using the nCounter Mouse Inflammation v2 Panel Plus (NanoString Technologies, Seattle, WA). Geometric means of positive controls were used to normalize the housekeeping genes, which were then used to normalize the samples. All normalization and data analysis was performed using nSolver Analysis Software 4.0. The cut off for significance was set to p < .05 for differentially expressed genes. Genes differentially expressed following RSD in WT and IL-6KO mice were used for IPA Comparison Analysis of upstream regulators (Ingenuity Pathway Analysis; QIAGEN, Inc.).
ex vivo LPS stimulation of PBMCs
Blood samples were collected 14 hours after the last cycle of stress. Peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll gradient and cultured for 18 hours with LPS.
Statistical Analyses
Data are presented as the average ± standard error of the mean (SEM) for each treatment group. Data were analyzed using two-way (stress × intervention) ANOVA using the IBM SPSS Statistics 25. In the case of main effect of condition (Control versus Stress) or intervention (Wildtype versus IL-6KO) or an interaction, differences between group means were evaluated by an F-protected t test. post hoc analysis results are depicted graphically in figures.
Results
Stress-induced anxiety and social avoidance were prevented in IL-6 deficient mice.
IL-6 is implicated in stress-induced neuropsychiatric deficits, but the functional role of IL-6 is unclear. Here, we aimed to determine the role of IL-6 in the monocyte-mediated response to stress and the development of anxiety. First, anxiety-like behavior and social avoidance were determined in wild-type (WT) and IL-6KO mice 14 hours after the last cycle of repeated social defeat (stress). Representative heat maps of activity for each experimental group are shown in the open field (Fig. 1A). There was a trend for main effect of stress (p=.06) and a significant effect of genotype (p<0.01) on entries into the center of the open field (Fig. 1B). Post hoc analysis confirmed that WT-Stress mice had fewer entries into the center of the open field compared to all other groups (p<0.05). Duration in the center of the open field after stress (Fig. 1C) showed effects of genotype (p<0.05) with a trend towards interaction (stress x genotype, p=.08). Again, post hoc analysis confirmed that WT-Stress mice spent the least amount of time in the center compared to all other groups (p<.05). Locomotor activity was unaffected by stress or genotype (Fig. 1D).
Figure 1. Stress-induced anxiety and social avoidance were prevented in IL-6 deficient mice.
Male WT and IL-6KO C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. Anxiety-like (n=10) and social avoidance (n=8–10) behaviors were determined 14 h later. (A) Representative heat maps of activity in the open field. (B) Number of entries into the center (stress F(1,40)=3.7, p = .06; genotype F(1,40)=11.8, p < .01), (C) duration in center (genotype F(1,40)=4.9, p < .05, stress x genotype, F(1,40)=3.0, p = .09) and (D) Distance traveled in the open field test. Next, social avoidance behavior was determined in the same mice. (E) Schematic diagram of the social avoidance test showing the Social trial. (F) Time spent in the interaction zone during social trial (stress F(1,34)=12.3, p < 01; genotype F(1,34)=4.3, p < .05). (G) Time spent in the corner zone during social trial (stress, F(1,33)=19.5, p < .0001; stress x genotype F(1,33)=4.1, p = .05). Bars represent mean ± SEM. Bars with (*) are significantly different from the controls (post hoc analysis, p < .05).
Social avoidance behavior was also determined after stress. During the empty trial of the social avoidance test, neither stress nor genotype altered the amount of time spent in the interaction and corner zones (data not shown). During the social trial where a CD-1 mouse was present in the cage (Fig. 1E), time spent in the interaction zone (Fig. 1F, stress p<.01; genotype p<.05) and time spent in the corner zone (Fig. 1G, stress p<.0001; interaction p=.05) were both affected by stress and genotype. Post hoc analysis confirmed that WT-Stress mice spent the least amount of time in interaction zone and highest amount of time in corner zone compared to all other groups (p<.05, for each). Collectively, stress-induced anxiety and social avoidance were prevented in the IL-6KO mice.
Stress-induced myeloid cell production and release into circulation were maintained in the IL-6 deficient mice.
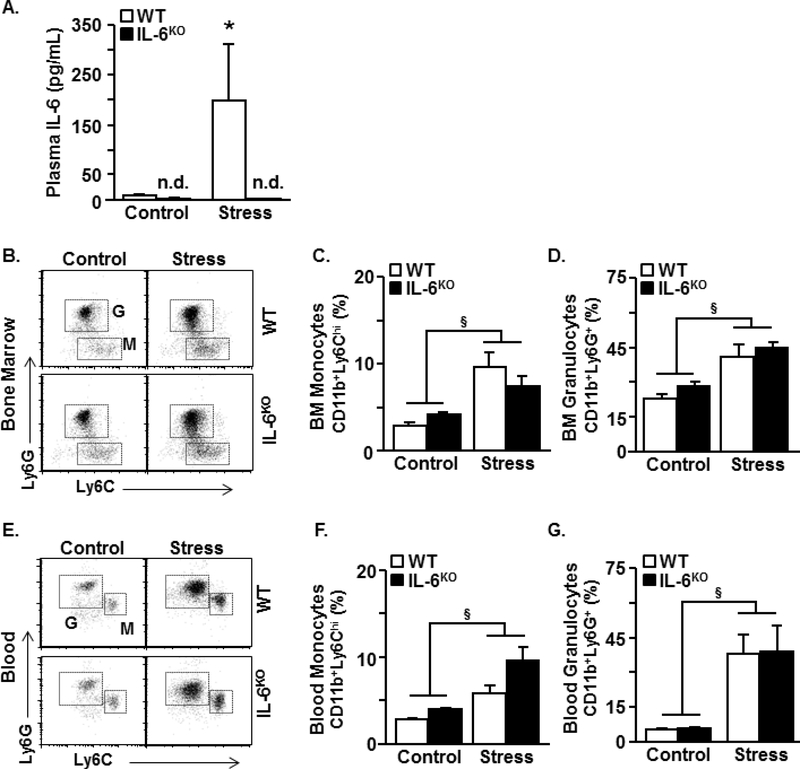
Next, the effect of IL-6 deficiency on the production and release of monocytes and granulocytes after stress was assessed. As a control, IL-6 levels were also determined in the plasma after stress. As expected, stress increased plasma IL-6 (Fig. 2A, p<0.05) and this increase was prevented in the IL-6KO mice (Fig. 2A, stress x genotype, p<.01). Stress also increased the percentage of monocytes (CD11b+/Ly6Chi) and granulocytes (CD11b+/Ly6G+) in the bone marrow (Fig. 2B-D, p<.0001) and circulation (Fig. 2E-G, p<.0001). These immune responses to stress were independent of genotype. Overall, stress induced an increase in the production and release of myeloid cells in both wildtype and IL-6KO mice.
Figure 2. Stress-induced myeloid cell production and release into circulation were maintained IL-6 deficient mice.
Male WT and IL-6KO C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. Several immune parameters were determined 14 h later (n=4–5). (A) IL-6 concentration in the plasma (stress F(1,19)=8.8, p < .01; genotype F(1,19)=9.8, p < .01; stress x genotype F(1,19)=8.9, p < .01). (B) Representative bivariate dot plots of Ly6G and Ly6C labeling in the bone marrow. (C) Percentage of monocytes (CD11b+/Ly6Chi) in the bone marrow (stress F(1,19)=29.3, p < .0001). (D) Percentage of granulocytes (CD11b+/Ly6G+) in the bone marrow (stress (F(1,18)=20.8, p < .0001). (E) Representative bivariate dot plots of Ly6G and Ly6C labeling in the blood. (F) Percentage of monocytes (CD11b+/Ly6Chi) in the blood (stress F(1,10)=50.7, p < .0001; stress x genotype F(1,10)=5.4, p = .05). (G) Percentage of granulocytes (CD11b+/Ly6G+) in the blood (stress F(1,11)=36.3, p < .0001). Bars with (§) indicate significant main effect of stress (p < .05). Bars represent mean ± SEM. Bars with (*) are significantly different from the controls (post hoc analysis, p < .05).
IL-6 deficiency attenuated the stress-induced brain IL-1β expression, but did not affect stress-induced microglia activation and monocyte recruitment to the brain.
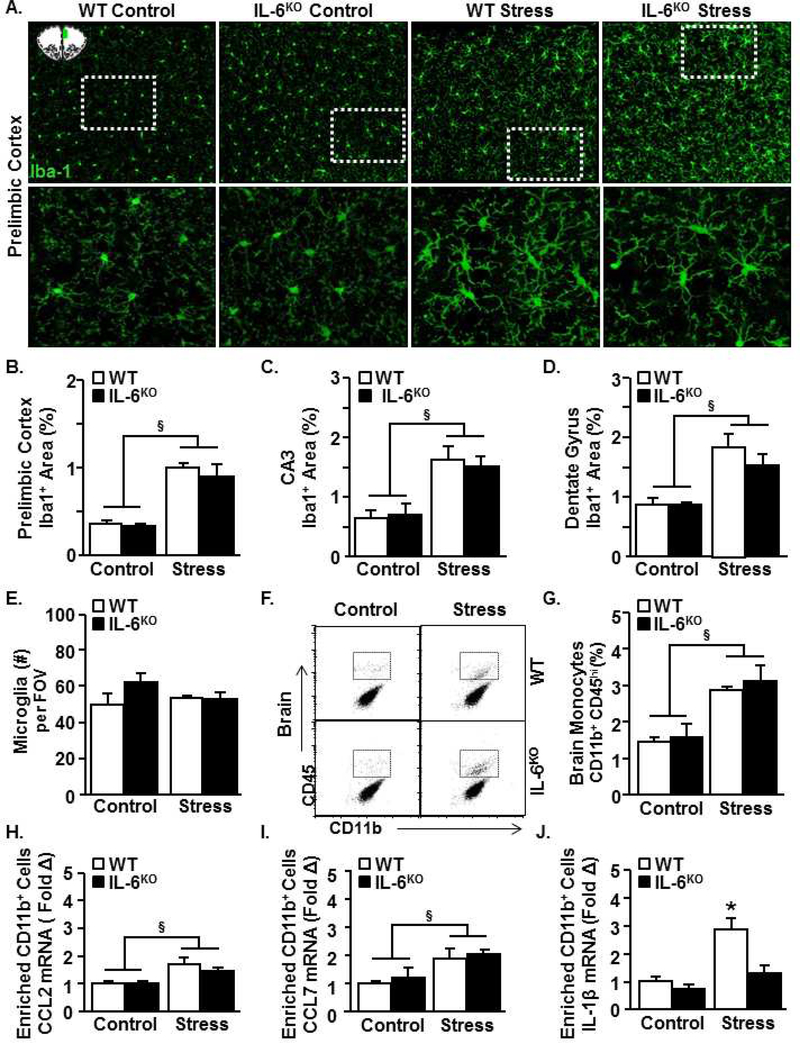
Inflammatory monocytes are recruited to the brain by activated microglia (chemokine-dependent), and provide IL-1β signal to the brain endothelial cells. This immune-to-brain signaling mediated by monocyte-derived IL-1β is critical to the induction of anxiety-like behavior [21]. Therefore, microglial Iba-1 expression was assessed after stress in WT and IL-6KO mice to determine microglial structural activation, which is associated with chemokine induction after stress. Stress increased Iba-1 proportional area in the PrL (Fig. 3A&B, p<.001), CA3 (Fig. 3C, p<.01), and dentate gyrus (Fig. 3D, p<.01). For example, microglia in the stressed brain had larger, elongated cell bodies and thicker processes compared to controls (Fig. 3A). These morphological changes marked by increased Iba-1 are associated with an activated state of microglia [21, 26]. Increased Iba-1 expression occurred in both wildtype and IL-6KO mice. Microglia number did not significantly differ among the groups (Fig. 3E). Thus, microglial morphological activation occurred following stress in an IL-6 independent manner.
Figure 3. IL-6 deficiency attenuated the stress-induced brain IL-1β expression, but did not affect stress-induced microglia activation and monocyte recruitment to the brain.
Male WT and IL-6KO C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. Several neuroimmune parameters were determined 14 h later. (A) Representative images of Iba-1 labeling (n=3) in the prelimbic cortex. The images were taken within the region highlighted in the schematic (top left). Dashed rectangles indicate region of higher magnification images (bottom panel). Percent area of Iba-1 labeling in the (B) prelimbic cortex (stress F(1,13)=27.7, p < .001), (C) CA3 (stress F(1,11)=18.0, p < .01), (D) dentate gyrus (stress F(1,12)=23.1, p < .01). (E) Number of microglia (per field of view) in the prelimbic cortex (stress x genotype F(1,10)=2.6, p =.14). (F) Representative bivariate dot plots of CD11b and CD45 labeling of Percoll-enriched cells from the brain (n=4–5). (G) Percentage of monocytes (CD11b+/CD45hi) in the brain (stress F(1,18)=22.3, p < .001). In a separate study, mice were exposed to stress, and Percoll-enriched CD11b+ cells were isolated from the brain immediately after the final exposure to stress (n=4–5). mRNA levels of (H) CCL2 (stress F(1,15)=10.0, p < .01), (I) CCL7 (stress F(1,13)=12.5, p < .01) and (J) IL-1β (stress F(1,17)=12.5, p < .01; genotype F(1,19)=7.2, p < .05; stress x genotype F(1,17)=3.5, p = .08) were determined. Bars represent mean ± SEM. Bars with (§) indicate significant main effect of stress (p < .05). Bars with (*) are significantly different from the controls (post hoc analysis, p < .05).
Next, monocyte accumulation in the brain and chemokine and IL-1β expression in microglia/monocytes were determined in WT and IL-6KO mice after stress. Stress increased the presence of CD11b+/CD45hi cells in the brain (Fig. 3F&G, p<.001) in a genotype-independent manner. Next, chemokine and cytokine expression were analyzed immediately after stress. This immediate time-point was chosen because CCL2 and CCL7 levels decline at later time-points. Real-time qPCR of enriched CD11b+ cells (microglia/monocytes) showed increased CCL2 and CCL7 levels after stress (Fig.H&I, p<.01 for each). Again, these increases were independent of genotype. Stress increased IL-1β mRNA expression in the enriched CD11b+ cells and this effect tended to be genotype-dependent (Fig. 2J, stress p<.01, interaction p=.08). Post hoc analysis confirmed that WT-Stress mice had the highest level of IL-1β compared to all other groups (p<0.05). Collectively, stress caused microglia activation and monocyte recruitment to the brain independent of genotype, but the corresponding induction of IL-1β was prevented in IL-6KO mice.
A primed immune signature of blood monocytes after stress was dependent on IL-6.
We have shown previously that circulating monocytes recruited to the brain during stress relay an IL-1β signal to the brain, augmenting the neuroinflammatory profile and subsequently inducing anxiety-like behavior in mice [21]. Here, we show that IL-6 plays a role in two key responses to stress: behavior and brain IL-1β expression. Thus, the absence of IL-6 signaling in the IL-6KO mice may alter the activation profile of the monocytes released into circulation during stress. To address this hypothesis, monocytes (CD11b+/Ly6Chi) were isolated from the blood 14 hours after stress, and RNA copy number of 279 immune-related genes was determined using nCounter Nanostring analysis (Fig. 4A).
Figure 4. A primed immune signature of blood monocytes after stress was dependent on IL-6.
Male WT and IL-6KO C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls. (A) 14 h later, monocytes (CD11b+/Ly6Chi) in the blood were collected and FAC-sorted (n=4). Next, RNA was isolated and mRNA copy number of 279 genes was determined using NanoString gene array. Volcano plot (-log10 (p value) vs. fold change) of genes differentially expressed between monocytes from (B) WT stress and WT control groups, C) IL-6KO stress and WT stress groups, and (D) IL-6KO stress and IL-6KO control groups. Genes labeled in red (increased) and blue (decreased) were significantly different. (E) Venn diagram depicts genes significantly altered between WT Stress and WT Control but not between IL-6KO Stress and WT Stress (black background), genes significantly altered between WT Stress and WT Control and between IL-6KO Stress and WT Stress (grey background), and genes significantly altered only between the IL-6KO stress and WT stress (p < .05 for all).
The volcano plot (-log10 (p value) vs. Fold change) in Fig. 4B shows genes differentially expressed in blood monocytes between the WT-Stress and WT-Control mice. Genes increased and decreased in monocytes after stress (p<.05) are labeled in red and blue respectively. Fig. 4C shows genes differentially expressed between IL-6KO-Stress and WT-Stress mice. Fig. 4D shows genes differentially expressed between IL-6KO-Stress and IL-6KO-Control mice. The Venn diagram (Fig. 4E) shows that there were a total of 37 genes (black + grey) significantly altered in blood monocytes after stress. Within this group, 30 genes were different between WT-Stress and WT-Control but not different between IL-6KO-Stress and WT-Stress (black), and 7 of these stress-altered genes were reversed in the IL-6KO-Stress mice (grey; different between IL-6KO-Stress and WT-Stress). Furthermore, there were 12 genes (white) unaltered between the WT-Stress and WT-Controls, but were significantly altered between IL-6KO-Stress and WT-Stress mice. Taken together, stress altered the expression of 37 immune genes in monocytes, of which 7 were reversed in IL-6KO group.
Based on these analyses, a selected list of genes associated with myeloid signature, chemokines/cytokines, immune signaling, and glucocorticoid signaling are shown (Supplemental fig.1). Pairwise comparisons indicated that stress increased (p<.05) the RNA copy number of several key inflammatory (Ly6c, Mmp9, Cxcr2, Alox5, Ccr1) and glucocorticoid sequestration (Fkbp5) genes in blood monocytes. Stress also decreased (p<.05) the genes associated with regulation (Cx3cr1, Tgfbr1), antigen presentation (H2-eb1), and interferon response (Tlr3, Mx1, Mx2, Irf3, Ifi44, Stat2). The stress-associated alterations in Alox5, Ly6C, Cd55, grb2, Mmp9, Tgbr1, and Stat2 were absent in the IL-6KO-Stress mice. Taken together, the primed transcriptional profile of blood monocytes after stress was attenuated in IL-6KO mice.
An IL-6-dependent primed phenotype of blood leukocytes after stress.
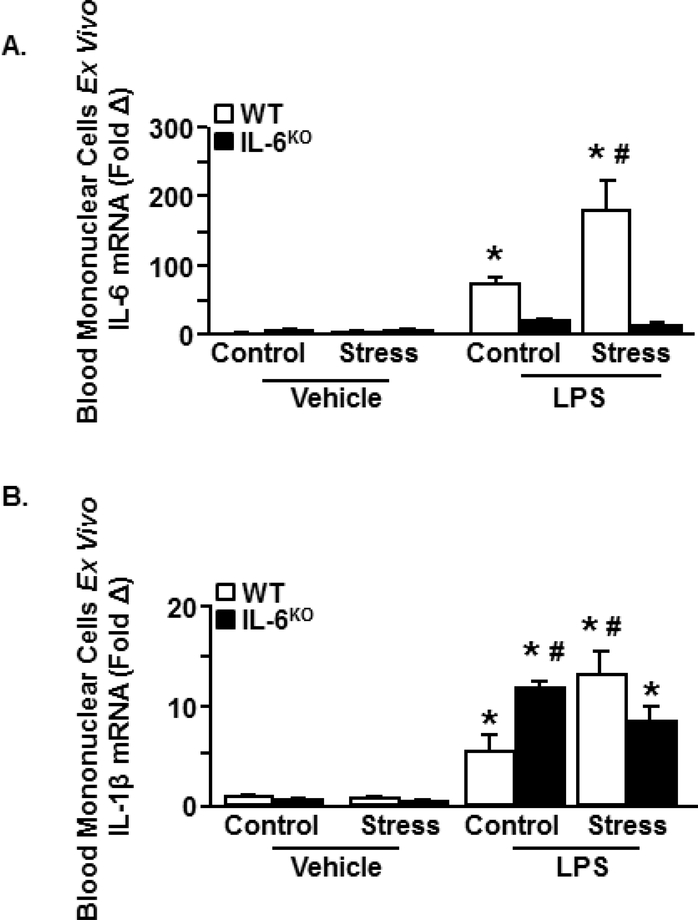
A primed immune profile is functionally defined as an increased cytokine response to innate immune challenge [27]. To assess priming in WT and IL-6KO mice after stress, peripheral blood mononuclear cells (PBMCs) were isolated and stimulated with ex vivo LPS. IL-6 expression in PBMCs was influenced by stress x genotype x LPS (Fig. 5A; p<.05). Following LPS, PBMCs from WT-Stress mice had the highest IL-6 levels compared to all other groups (p<.05). Similarly, IL-1β was also influenced by stress x genotype x LPS (Fig. 5B, p<.01). Compared to WT-Control, PBMCs from WT-Stress mice expressed higher IL-1β after LPS (p<.05). Notably, PBMCs from IL-6KO-Control mice also had high levels of IL-1β after LPS (p<.05). Nevertheless, IL-1β in the IL-6KO-Stress group were not different from that of the WT-Controls after LPS. These findings revealed a primed profile characterized by enhanced IL-6 and IL-1β expression in PBMCs stimulated with LPS after stress. This primed profile was attenuated in the IL-6KO mice.
Figure 5. An IL-6-dependent primed phenotype of blood leukocytes after stress.
Male WT and IL-6KO C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls (n=3). 14 h later, Peripheral Blood Mononuclear Cells (PBMCs) were collected, cultured ex vivo and treated with LPS (0.4 μg/mL). mRNA was collected 18 h later and (A) IL-6 expression (treatment x genotype x stress (F(1,22)=5.9, p < .05) and (B) IL-1β expression (treatment x genotype x stress (F(1,47)=11.2, p < .01) were determined. Bars with (*) are significantly different from the vehicle controls (post hoc analysis, p < .05). Bars with (#) are significantly different from the WT-LPS-control.
The primed and IL-1β signature of monocytes recruited to the brain during stress was dependent on IL-6.
Next, we assessed the transcriptional profile of monocytes (CD11b+/CD45hi) in the brain after stress (Fig. 6A) following a similar protocol as in Fig. 4A. The volcano plot (-log10 (p value) vs. fold change) in Fig. 6B shows genes differentially expressed between WT-Stress and WT-Control mice. Genes increased and decreased in brain monocytes after stress (p<.05) are labeled in red and blue respectively. Fig. 6C shows genes differentially expressed between IL-6KO-Stress and WT-Stress groups. Fig. 6D shows genes differentially expressed between IL-6KO-Stress and IL-6KO-Control mice. IPA analysis showed that stress induced gene expression consistent with increased signaling of IL-6, IL-8, HMGB1, JAK/Stat, and IL-17A pathways in brain monocytes. In contrast, stress in IL-6KO mice reduced expression of genes related to IL-6, macrophage ROS production, HMGB1, JAK/Stat, and IL-17A (Fig. 6E). The Venn diagram (Fig. 6F) shows that there were a total of 46 genes (black + grey) significantly altered in the brain monocytes after stress. Within this group, 34 genes were different between WT-Stress and WT-Control but not different between IL-6KO-Stress and WT-Stress (black), and 12 of these stress-altered genes were reversed in the IL-6KO-Stress mice (grey; different between IL-6KO-Stress and WT-Stress). Furthermore, there were 15 genes (white) unaltered between the WT-Stress and WT-Controls, but significantly altered between IL-6KO-Stress and WT-Stress mice. Taken together, stress altered the expression of 46 immune genes in brain monocytes, of which 12 were dependent on IL-6, including key inflammatory molecules (Il-1β, Ly6C, Mmp9 and Cd14).
Figure 6. The primed and IL-1β signature of monocytes recruited to the brain during stress was dependent on IL-6.
Male WT and IL-6KO C57BL/6 mice were subjected to six repeated cycles of social defeat (Stress) or left undisturbed as controls (n=5). (A) 14 h later, monocytes (CD11b+/CD45hi) in the brain were collected and FAC-sorted. Next, RNA was isolated and mRNA copy number of 279 genes was determined using NanoString gene array. Volcano plot (-log10 (p value) vs. fold change) of genes differentially expressed between brain monocytes from (B) WT stress and WT control groups, (C) IL-6KO stress and WT-stress groups, and (D) IL-6KO stress and IL-6KO control groups. Genes labeled in red (increased) and blue (decreased) were significantly different. (E) Pathways regulated by stress in the wildtype and IL-6KO brain monocytes after stress. (F) Venn diagram depicts genes significantly altered between WT Stress and WT Control but not between IL-6KO Stress and WT Stress (black background), genes significantly altered between WT Stress and WT Control and between IL-6KO Stress and WT Stress (grey background), and genes significantly altered only between the IL-6KO stress and WT stress (p < .05 for all).
Related to these analyses, a selected list of genes associated with myeloid signature, chemokines/cytokines, immune signaling, and glucocorticoid signaling are shown (Supplemental fig. 2). The RNA signature of monocytes in the brain after stress was consistent with the profile of blood monocytes. The progression of monocytes from the blood to the brain during stress was characterized by robust induction of Il-1β, Cd14, and Stat3. Overall, stress increased (p<0.05) expression of several inflammatory-related molecules: Ager, Alox5, Cd14, Ccr1, Cxcl2, IL-1β, Mmp9, Myd88 and Stat3. All of these stress-associated increases were prevented by IL-6 deficiency. Thus, monocytes recruited to the brain during stress were primed in an IL-6dependent manner to express IL-1β.
Discussion
Interleukin-6 (IL-6) is a consistent cytokine biomarker in clinical and experimental reports of chronic stress and is associated with treatment-resistant mood disorders, increased morbidity and mortality [16, 28]. Nonetheless, there is little information on the functional effects of high IL-6 on the physiological, behavioral, and immunological response to stress. Recent studies modeling social stress in mice indicate that peripheral IL-6 can enter the brain and influence the social avoidance response [20]. In addition, mice susceptible or resilient to social avoidance after stress had higher and lower levels of plasma IL-6, respectively [16]. Furthermore, these behavioral effects associated with high IL-6 were prevented by IL-6 antibody treatment. Based on these data, the purpose of this study was to determine the effects of IL-6 on the immune and neuroinflammatory responses to repeated social defeat. Our findings here support the notion that IL-6 is a critical mediator of anxiety-like behavior and social avoidance following social defeat. We provide novel evidence that IL-6 directly and robustly alters the profile of monocytes released from the bone marrow in response to social defeat stress. The molecular signature of these bone marrow-derived monocytes is associated with a primed inflammatory phenotype. Consistent with priming, monocytes from stressed mice had an exaggerated inflammatory response to immune challenge compared to controls. This primed signature of peripheral monocytes took on an IL-6-dependent inflammatory/reactive profile following recruitment to the brain. Thus, IL-6 produced during stress is critical to the generation of primed and pro-inflammatory monocytes that trigger the development of anxiety-like behavior.
A key finding of this study was that stress-induced anxiety-like behavior and social avoidance were prevented in IL-6KO mice. These data are consistent with other studies on social defeat stress. Administration of antibodies targeting IL-6 prevented stress-induced IL-6 production and social avoidance behavior [16]. Conversely, bone marrow transplantation from socially avoidant mice induced social avoidance in naïve mice following a single exposure to stress [16]. It is important to note that social avoidance behavior after repeated social defeat stress is regulated by a mechanism different from anxiety. For example, a single episode of stress does not trigger an immune response that is critical for anxiety, but it is sufficient to induce social avoidance [29]. Because IL-6KO-Stress mice were resistant to social avoidance in our current study, it is likely that IL-6 operates on multiple levels and regulates social avoidance differentially on a neuronal circuitry level. Although the exact mechanisms underlying the effects of IL-6 on neuronal and behavioral regulation are not well understood, studies have implicated the serotonergic system in the brain. For example, increased serotonin transporter (SERT) expression is associated with dampened serotonergic signaling and mood deficits. Therefore, inhibition of SERT activity has been a target for therapy development [30]. One study reported that IL-6KO mice had increased SERT expression at baseline and increased serotonin uptake, however, this was associated with reduced propensity to anhedonic and anxiety-like behavior [31]. This finding is in contrast with studies indicating higher SERT levels as a cause of anxiety-like/depressive-like behaviors [31]. Others showed that peripheral IL-6 produced during stress might directly enter the brain and influence neuronal functions [20]. In fact, this study also detected a monocyte population within the brain vasculature and increased blood-brain barrier permeability after social defeat stress [20]. Taken together, although the specific mechanisms remain unclear, IL-6 and blood-brain barrier alterations may directly alter neurotransmitter systems and social avoidance behavior following stress.
An important finding of this study was that the mRNA signature of monocytes in circulation was consistent with clinical studies on leukocytes from stressed individuals. There is an increased prevalence of circulating CD14+/CD16- monocytes (analogous to the CD11b+/Ly6Chi monocytes in mice) in individuals suffering from chronic stress [12]. Transcriptional analysis of blood monocytes from stressed humans revealed an increased expression of chemotaxis and inflammation-related genes, and a corresponding decrease in regulatory (CX3CR1) genes, viral response genes, and glucocorticoid signaling genes [12, 32]. In PTSD studies, stress-induced increases in inflammatory genes and decreases in antigen-presentation molecules (HLA-DQB1 in humans) and apoptosis-related genes (Birc2) were detected [33]. Furthermore, ex vivo LPS stimulation of peripheral monocytes from stressed individuals triggered an exaggerated production of IL-6 [12]. These clinical reports on blood monocytes are consistent with our current findings on the transcriptional profile of monocytes in circulation and in the brain following stress. Here, we describe a transcriptional signature that is dependent on IL-6 produced during stress. For instance, monocytes released into circulation during stress in the WT mice had lower levels of viral response (Tlr3, Tlr7, Stat2) and regulatory response (Tgfb1, Tgfbr1) genes, and higher levels of myeloid inflammatory response (Ly6C, Mmp9) and glucocorticoid sequestration (Fkbp5) genes. Furthermore, IL-6KO-Stress mice had altered levels of Stat2 and Tgfbr compared to WT-Stress mice. These findings point to the precise transcriptional alterations caused by increased IL-6 during stress, and their implications for stress-induced priming and anxiety.
Consistent with clinical studies of stress, our findings from the peripheral blood mononuclear cells (PBMCs) in WT mice show an exaggerated immune reactivity to ex vivo LPS after stress [12]. The difference in LPS response between wildtype and IL-6KO stress mice shows that IL-6 deficiency is associated with a lack of priming of PBMCs. It is important to highlight that although we assessed the transcriptional profile of monocytes in circulation, the priming of these monocytes may also originate within the bone marrow. It is also relevant to note that IL-6KO-Control PBMCs produced higher levels of IL-1β compared to WT-Control following LPS treatment. This effect has been previously reported in bone marrow-derived cells where myeloid-specific deletion of IL-6 receptor-α caused increased production of IL-1β [34]. In that study, the absence of IL-6rα signaling prevented alternative activation of myeloid cells following LPS, causing enhanced IL-1β production [34]. Nevertheless, in the context of stress, we found that IL6 deficiency during stress reduced the exaggerated inflammatory response to subsequent LPS challenge.
We also extend the clinical findings and show experimental data on the transcriptional signature of monocytes recruited to the brain during stress. Microglia activated during stress produce chemokines to recruit IL-1β-expressing monocytes that propagate IL-1 signaling into the brain via the vascular endothelium [21]. Here, we show that several proinflammatory genes expressed in the brain monocytes were dependent on IL-6. For example, we show that the “primed” peripheral monocytes recruited to the brain during stress acquired a reactive/inflammatory phenotype characterized by elevated levels of Cd14, Ly6C, Mmp9, IL-1β and Myd88. Importantly, these transcripts were significantly reduced in the IL-6KO mice exposed to stress. Notably, Stat3, a key transcription factor in IL-6 signaling was increased in the brain monocytes of the WT-Stress mice but not in the IL-6KO-Stress mice. These data indicate that the transcriptional alterations between the two groups might be mediated by IL-6-Stat3 signaling. Although Stat3 expression was increased in the brain monocytes after stress, this change was not evident in the blood monocytes. This may be related to the more homogenous subpopulation (reactive/inflammatory) of monocytes that accumulate in the brain versus the more diverse monocyte population in the blood. These findings provide an in-depth view of the monocyte phenotype that is critical to the induction of anxiety-like behavior following stress.
The transcriptional signature of monocytes from a “primed” state in circulation to a “reactive/inflammatory” state in the brain is notable. This phenotypic evolution of monocytes indicates that they acquire an activated profile (IL-1β+) following recruitment to the brain and interaction with the vascular microenvironment. Repeated social defeat induces cell adhesion molecule (ICAM-1, VCAM, E-Selectin) and IL-1 receptor-1 expression on the brain vasculature that provide interaction sites for monocytes recruited from the periphery [17, 21, 35]. Notably, IL-6 deficiency did not prevent microglial activation or monocyte recruitment to the brain during stress; however, it blocked anxiety-like behavior. Thus, presence of monocytes in the brain alone does not augment anxiety. The key event is the induction of the pro-inflammatory phenotype in the recruited monocytes, which we demonstrate depends on IL-6.
IL-6 also stimulates corticosterone production following specific inflammatory challenge or exogenous IL-6 administration [36, 37], therefore, the effects of IL-6 deficiency on the HPA/corticosterone response to stress is worth discussing. Although we did not test corticosterone levels in the IL-6KO mice after stress, other studies have reported no change in corticosterone levels in male IL-6KO mice after restraint stress [36, 38]. We have previously shown that corticosterone depletion via adrenalectomy or metyrapone treatment during stress reduces IL-6 production, monocyte release into circulation and the induction of neuroendothelial ICAM-1 during stress [17]. Since, in the current study, IL-6 deficiency was not associated with reduction in circulating monocytes (Fig. 2F) or ICAM-1 (data not shown), the corticosterone response to stress was likely unaltered in the IL-6KO mice.
A limitation of our study is that we do not prove that IL-6 is the direct cause of anxiety-like behavior following stress. Nonetheless, absence of IL-6 signaling in IL-6KO mice or wildtype mice treated with IL-6 antibody also prevented social avoidance in a different model of repeated social defeat stress [16]. Furthermore, bone marrow transplant of “susceptible” monocytes into naïve mice was sufficient to induce susceptibility to social avoidance in the recipient mice [16]. In line with these findings, we show here that IL-6 signaling in monocytes plays a vital role in the induction of anxiety-like behavior after stress. Although the mechanism underlying IL-6-induced priming of monocytes during stress remains unknown, we demonstrate, for the first time, an IL-6-dependent transcriptional signature of monocytes that is critical to the development of anxiety following stress.
In conclusion, we show that IL-6 is vital to the priming of bone marrow-derived peripheral monocytes that are recruited to the brain during repeated social defeat and trigger anxiety-like behavior. In absence of IL-6, monocytes in the brain showed an attenuated inflammatory profile, including reduced IL-1β expression, which was associated with overall reduced inflammatory signaling in the brain and an absence of anxiety-like behavior. These findings provide important insight into the unique monocyte profile induced by the high IL-6 levels. Understanding the transcriptional changes in immune cells during stress may allow for the development of therapeutics against inflammation-associated anxiety disorders.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health (NIMH) grants R01-MH-093473 and R01-MH097243 to J.F.S. and National Institute of Aging grant R01-AG033028 to J.P.G. The authors acknowledge the Genomics Core (Paolo Fadda) and the Analytical Cytometry Core at the Ohio State University for their technical assistance.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H,Kivimaki M (2015): Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 49:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ressler KJ,Mayberg HS (2007): Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 10:1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien SM, Scully P, Fitzgerald P, Scott LV,Dinan TG (2007): Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 41:326–31. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. (1995): Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 34:301–9. [DOI] [PubMed] [Google Scholar]
- 5.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. (2013): A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelzangs N, Beekman AT, de Jonge P,Penninx BW (2013): Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 3:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, et al. (2010): Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 24:1074–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steptoe A, Hamer M, Chida Y (2007): The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 21:901–12. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM,Price LH (2010): Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 35:2617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. (2009): Interleukin6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 66:287–92. [DOI] [PubMed] [Google Scholar]
- 11.Scheller J, Chalaris A, Schmidt-Arras D,Rose-John S (2011): The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 1813:878–88. [DOI] [PubMed] [Google Scholar]
- 12.Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, et al. (2014): Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun. 41:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. (2013): Posttraumatic stress disorder is associated with an enhanced spontaneous production of proinflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. (2013): Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 8:e58488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou D, Kusnecov AW, Shurin MR, DePaoli M,Rabin BS (1993): Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 133:2523–30. [DOI] [PubMed] [Google Scholar]
- 16.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. (2014): Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 111:16136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niraula A, Wang Y, Godbout JP,Sheridan JF (2018): Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance and Neurovascular Adhesion Molecule Expression. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, et al. (2012): Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. 2:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. (2006): IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 23:587–94. [DOI] [PubMed] [Google Scholar]
- 20.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. (2017): Social stress induces neurovascular pathology promoting depression. 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, et al. (2017): Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohleb ES, Powell ND, Godbout JP,Sheridan JF (2013): Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 33:1382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark JL, Avitsur R, Hunzeker J, Padgett DA,Sheridan JF (2002): Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol. 124:9–15. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez K, Niraula A, Sheridan JF (2016): GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 51:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. (2011): β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N,Popovich PG (2009): An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 181:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank MG, Miguel ZD, Watkins LR,Maier SF (2010): Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 24:19–30. [DOI] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB,Glaser R (2003): Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 100:9090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, et al. (2014): Re-establishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taciak PP, Lysenko N, Mazurek AP (2018): Drugs which influence serotonin transporter and serotonergic receptors: Pharmacological and clinical properties in the treatment of depression. Pharmacol Rep. 70:37–46. [DOI] [PubMed] [Google Scholar]
- 31.Kong E, Sucic S, Monje FJ, Savalli G, Diao W, Khan D, et al. (2015): STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci Rep. 5:9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. (2008): A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 64:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N,Shalev AY (2005): Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 10:500–13, 425. [DOI] [PubMed] [Google Scholar]
- 34.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. (2014): Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 15:423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawicki C, McKim D, Wohleb E, Jarrett B, Reader B, Norden D, et al. (2014): Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbull AV, Prehar S, Kennedy AR, Little RA,Hopkins SJ (2003): Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology. 144:1894–906. [DOI] [PubMed] [Google Scholar]
- 37.Silverman MN, Miller AH, Biron CA,Pearce BD (2004): Characterization of an interleukin-6- and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection. Endocrinology. 145:3580–9. [DOI] [PubMed] [Google Scholar]
- 38.Bethin KE, Vogt SK,Muglia LJ (2000): Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 97:9317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.