Abstract
Biomonitoring of chemical concentrations in humans is important for detecting, monitoring, and addressing a wide range of health threats. However, it is virtually absent across many African nations, including Ethiopia. This study aims to determine urinary concentrations for metals and trace elements in populations living in the central Ethiopian Rift Valley. The region is unindustralized, rural, and characterized by unique geologic rifting and volcanic activities that have produced vast pyroclastic materials, forming its aquifer and fertile agricultural soils. Millions of people in the region rely on wells for drinking water and are engaged in cereal-based subsistence agriculture. We enrolled a total of 386 residents aged 10-50 years old (201 females and 185 males). The levels of 23 elements except F− were quantified in water and urine samples by ICP-MS. Mean concentrations of B, F−, Ca, and Mg were measured in mg/L levels, while concentrations of Mo, Zn, Sr, Rb, and Li ranged between 100 and 700μg/L. Mean concentrations between 5 and 15μg/L were found for Ni, Cu, and Mn, while Ag, Be, Cd, Co, Pb, Sb, Th, TI, and U were all below 5μg/L. Arsenic and Al had mean concentrations between 30 and 50μg/L. Mean urinary concentrations of Ca, Cu, Mg, Pb, Sr, and Zn were significantly higher in males than females, whereas Co and Mn were higher in females. Finally, younger individuals (10 to 30 years) had significantly higher mean concentrations of B, Cd, Co, Mg, Mo, and Pb than those between 31 to 50 years, whereas only Ca was higher in the older age group. The concentration ranges of B, Mo, Mn, TI, Li, Zn, and in particular F−(0.44-44.6mg/L) and As (2.2-164−g/L) in urine were higher than the reference ranges reported in healthy unexposed North American and European populations, while those for the remaining 16 elements were comparable to published reference ranges from such settings. The established concentration ranges are important to monitor future changes in exposure, and risk factors for disease, that might stem from the economic growth and industrialization that is currently underway in the region.
Keywords: Trace elements, human biomonitoring, concentration ranges, children and adults, volcanic area
1. Introduction
Biomonitoring of biological matrices has proven to be a useful tool to evaluate exposure to environmental chemicals (Haines et al., 2017; CDC, 2015; Goullé et al., 2013; Woodruff et al., 2011). Biomarkers measured in blood, urine, or other tissues are widely used as indicators of exposure in the general population—both in occupational and environmental settings that more closely reflect total exposure from all possible sources and routes (Angerer et a;., 2007; Sexton et al., 2004). While a large number of biomonitoring studies have been conducted in developed countries (e.g., Saravanabhavan et al., 2017; Coelho et al., 2013; CDC, 2012; Alimonti et al., 2005; Wilhelm et al., 2005; Paschal, et al., 1998), similar studies are rare in most developing nations and especially in Africa. There are no current large-scale human biomonitoring initiatives on the continent, even at the country level to establish a background level of environmental chemical exposures. Yet, limited studies are available in certain African subpopulations that focus on specific metals and trace elements in blood (Benin: Yedomon et al. 2017; Egypt: Moawad et al., 2016; Congo: Tuakuila et al., 2015; Tunisia: Khlifi et al., 2014; Morocco: Laamech et al., 2014).
In many countries, where surface water is lacking or polluted, people increasingly depend on groundwater wells as their primary source of drinking water. The Ethiopian Rift Valley (ERV), an arid and semi-arid climate, where fresh surface water resources are scarce or inaccessible to households for most of the year due to the scattered nature of the communities (Paul et al., 2018). The region is extensively covered with volcanic rocks and sediments primarily composed of pyroclastic ashes that release both toxic and essential elements into groundwater and agricultural soils (Chorowicz, 2005; Riemann et al., 2003; Rango et al., 2012, 2014, 2017). There is thus an apparent long-term exposure from naturally-occurring toxic and essential elements in water and food sources. By simultaneously measuring multiple elements in urine using ICPMS (Inductively Coupled Plasma Mass Spectrometer), we can estimate current exposures and determine the concentration ranges for these elements in this study population. This is important to allow monitoring of changes in future exposures and related human health risks, as it is routinely done in many countries in North America (Saravanabhavan et al., 2017; CDC, 2012; Ting et al., 1999; Paschal, et al., 1998), and Europe (Ganzleben et al., 2017; Pérez-Gómez et al., 2013; Černá et al., 2012; Fréry et al., 2012; Levy et al., 2007; Reis et al., 2004; Schulz et al., 2012). Such biomonitoring studies from different countries are important because reference concentrations reflect a variety of different factors, including geography, ethnicity, background levels of pollution, lifestyle, and diet.
To the best of our knowledge, no biomonitoring data currently exist to establish reference values in Ethiopia. In this study, we measured the concentrations of a range of potentially toxic and essential elements in drinking water and in urine. Measurement of these elements in water is relevant in the region as the communities primarily use well water for drinking and cooking. Measurement in urine, meanwhile, is useful for biomonitoring as many chemicals are excreted via urine. These biomarkers will provide more accurate exposure information and help to generate concentration values of chemicals from non-occupationally-exposed populations in the region. In addition, we compare these concentrations with those established in biomonitoring studies from other countries, where sampling has been conducted in populations that are generally believed to be unexposed to major anthropogenic sources of pollution, although the possibility of low levels of exposure in such studies cannot be excluded entirely. This comparison provides perspective on the sources and levels of trace elements in urine that could pose health risks or benefits among Ethiopians and informs targeting of future health-related studies in the region.
2. Materials and methods
2.1. Study population
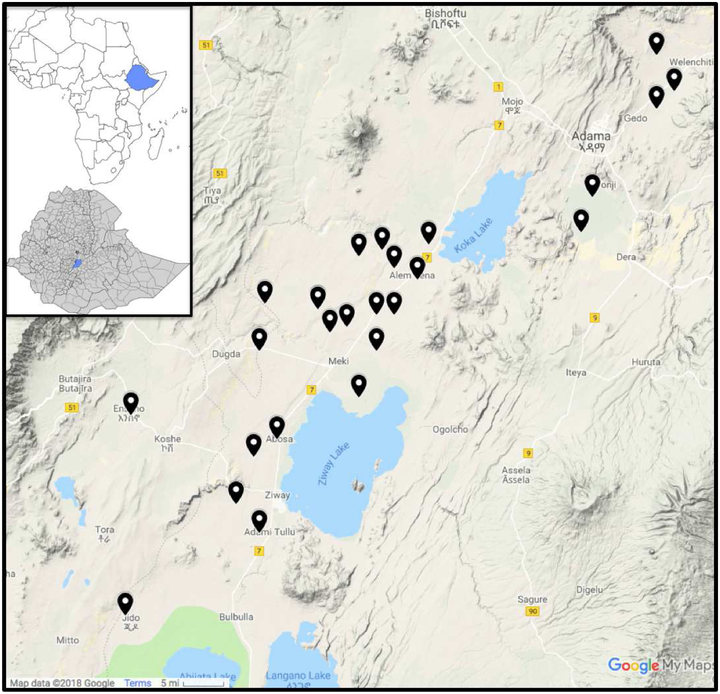
A total of 386 individuals (10 to 50 years old) were enrolled from 27 rural communities during two sampling periods (June 2015 and May 2016) (Figure 1). Based on extensive prior work conducted to characterize the range of F− levels in community water sources in the Ethiopian Rift Valley region, communities covering the range of F− exposures were selected for the study. Given the high correlation in F− and other exposures in this location (Rango et al., 2009; 2014), it likely represents most of the range of potential exposures in the Rift population. Individuals in the sample communities are primarily engaged in cereal based agricultural activities. In each sample community, households were selected by counting off every n households (where n was selected based on target sample sizes from each community), and 1 to 2 subjects from each household were enrolled in the study. Study inclusion criteria for selected individuals were: Age between 10 and 50 years that approximately match the length of consumption of well water. In each community, we attempted to select individuals from the randomly sampled households so as to get equal representation across different ages and sexes (e.g., 10–20, 20–30, 30–40, and 40–50 years). Thus, at least 2 subjects from each age group were enrolled in each community, with a nearly equal proportion of males to females. We also collected survey data on anthropometric measures of weight and height for deriving body mass index (BMI), using digital weighing scales to capture individuals’ weight, and measuring tapes for their height (Table 1). Included in the questionnaire were questions on smoking habits and use of tobacco products. Field enumerators (graduate students and nurses/medical doctors) were trained on the content of the questionnaire. The study received ethical approval (Protocol No. C0863) from the Institutional Review Board (IRB) at Duke University. All subjects provided consent, and parents/guardians gave permission for children to participate in addition to children giving their own assent.
Figure 1.
Groundwater and 12-hour urine sampling sites in the central Ethiopian Rift Valley.
Table 1.
Statistical descriptions of the characteristics of study population
| Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean± SD | Min | 5th | 25th | 50th | 75th | 95th | Max | |
| Age (years) | 386 | 24.6± 11.1 | 10.0 | 11.0 | 15.0 | 22.0 | 34.0 | 45.0 | 50.0 |
| Weight (kg) | 382 | 50.4± 11.8 | 21.0 | 27.7 | 43.8 | 52.0 | 58.5 | 67.1 | 82.9 |
| Height (m) | 382 | 1.59± 0.11 | 1.24 | 1.36 | 1.54 | 1.60 | 1.67 | 1.77 | 1.86 |
| BMI (kg/m2) | 382 | 19.6± 3.17 | 8.7 | 14.7 | 17.7 | 19.4 | 21.3 | 25.4 | 30.7 |
Regarding communicating results of our project findings, the communities are generally aware of health issues linked to the drinking water, though they are not aware of the type and specific concentrations of different contaminants in their water. Given the range of on-going studies in the region, some are becoming more familiar with the term 'fluoride' and in its role in causing health problems. Typical complaints among populations in the region concern the lack of access to alternative water resources, and pains from teeth and bone disorders. In prior and ongoing work, we have been informing local leaders and participants of the findings from our research on F− levels in their drinking water, and of the location of alternative low F− sources or dietary choices that may buffer the toxicity of F−. Based on our new measurements, we will now include new information about As and other elements in future communications with these populations.
2.2. Water, and urine sampling and analytical methods
Water samples were collected from 27 community wells, each of which served as the primary sources for drinking and cooking purposes used by households in the study communities. The samples were collected from active pumping wells after being allowed to flow for a few minutes prior to sampling. The water samples were then filtered in the field using disposable 60mL volume luer lock syringes and 0.45μm Mixed Cellulose Ester membrane filters directly into 60mL polyethylene bottles pre-cleaned with trace metal grade ~1N HCl and ~1N HNO3, and then rinsed with deionized water of resistivity >18MΩ/cm. Twelve-hour urine samples (overnight, 6pm-6am) were collected from 386 individuals. Urine samples were collected in acid pre-cleaned disposable urine collection plastic beakers (each with a capacity of one liter) that were provided to each individual and immediately transferred to 60mL acid-washed polyethylene bottles. Participants were instructed to carefully collect urine samples and shown how to best avoid possible contamination. The water and urine samples were acidified with high-purity HNO3 (Fisher Optima) that was prepared adding it in the bottles before samplings. Water and urine samples were then kept in zero-degree freezer, and properly packed, stored and transported to the laboratory in the United States. The first author has been trained in ‘Shipping Biological Materials’ and has extensive experience on proper handling of biological samples including sampling, packing, and shipping procedures.
The concentration of metals and trace elements in drinking water, and 12-hour urine samples were determined using inductively coupled plasma mass spectrometer (ICP-MS). The F− concentrations in drinking water and 12-hour urine samples were determined electrochemically using the Thermo Scientific Orion Ion-Selective Electrode (ISE). Samples were buffered with an equal volume of total ionic strength adjustment buffer (TISAB II) solution of pH 5–5.5, which allows for optimal analysis of F−. Calibration standards (1, 10, and 20 mg/L of F−) were prepared from a 100 mg/L stock solution. The range of electrode calibration slope for a 10-fold change in F− concentration was −57 to −60 mV, which is within the acceptable range.
Quality control was conducted using freeze-dried urine reference material (Seronorm LOT 0511545; LGC Standards). The accuracy of ICP-MS trace element measurements in urine was within the acceptable range of 82% to 115% relative to the LGC standard. The accuracy of ISE’s F− measurements for both urine and water standards ranged from 98% to 102.5% relative to the LGC standard. Repeatability of the trace element measurements in urine was also tested in a second lab at the Research Triangle Institute (RTI) International (Research Triangle Park, United States) for 25 samples, and results were found to be consistent with our measurements. For example, the repeatability of As, Pb, and Mo was 103.8%, 95%, and 102.7%, respectively. The recovery for As, Pb, and Mo in urine samples with respect to the NIST SRM 2668 low standard was 105%, 94.4%, and 98% respectively. The concentrations below detection limits (DLs) were measured (in μg/L) for silver (0.01), Al (1.7), Be (0.04), Co (0.02), Cd (0.04), Cu (0.57), Mn (0.33), Ni (0.56), Pb (0.06), Sb (0.04), Th (0.05), TI (0.01), and U (0.02). In this work, we did not collect field blanks and field controls, however, we have taken the necessary precaution to avoid any contamination in water and urine samples including proper collection, storage and transportation of sampling materials such as pre-cleaned polyethylene bottles and beakers, and syringes/filters.
2.3. Statistical Analysis
Descriptive analyses were carried out using percentiles, means, and standard deviations for continuous variables. The data were not normally distributed and thus required log-transformation prior to statistical analysis. The relationships between trace elements concentrations in water, and urine were evaluated using Pearson’s correlation coefficient. Comparison of urinary metals and trace element concentrations in the subjects based on sex, age, and BMI was performed using t-tests. For the biomarker data falling below the detection limit, we recorded concentrations as DLs/2, prior to log-transformation. If the proportion of biomonitoring results below the DL was greater than 40%, geometric means (GM) were not calculated. Biomonitoring percentiles that are less than the DL are reported as bd. The statistical significance was set at p<0.05.
3. Results and discussion
3.1. Characteristics of the study population
The average age of study individuals was 24.6±11 years old. Females represented 52% (n=201) of the respondents (Table 1). The majority of the population in the study region is engaged in cereal-based subsistence farming, and most respondents have low education and socio-economic status (e.g., Paul et al., 2018). The mean BMI of the participants was 19.6±3.2kg/m2 (range: 8.7-30.7kg/m2). Based on the WHO classification, most participants were categorized as normal weight (56%; n=215) or underweight (39%; n=148). Five percent (5%; n=19) of respondents were overweight.
3.2. Distribution of trace elements in drinking water
The distribution of metals and trace elements measured in the 27 sampled community wells is presented in Table 2. The highest concentrations (relative to levels normally considered safe) were for F− and As, and these exceeded the WHO recommended limits of 1.5mg/L and 10μg/L, respectively. Arsenic in the drinking water wells ranged from 0.16 to 54.9μg/L; 26% of samples contained As concentrations above 10μg/L. Fluoride concentration ranged from 0.6 to 15 mg/L, with 93% of samples exceeding the WHO recommended limit. In addition, the concentration of Mn was elevated in three of the wells (163, 211, and 422μg/L), exceeding the 50μg/L USEPA secondary standard. Only one sample (25.5 μg/L) had a concentration of U that exceeded the 15μg/L WHO drinking water standard. Overall, the concentrations of toxic metals (such as B, Cd, Li, Pb, Sb, Sr, Ni, Co, Cu, Be, Zn, and TI) was below the lowest of these three agencies drinking water standards (Table 2). Due to Rift aquifer geochemistry, the water samples contained very low levels of Ca (mean:15.8mg/L) and Mg (mean: 3.8mg/L), compared to levels found in groundwater in the Ethiopian highlands (Rango et al., 2009). The alkaline pH of the Rift groundwater allows the release of F− and other oxyanion forming elements such as As, B, and Mo to occur at elevated levels. Yet, this high pH limits the occurrence of metals such as Mn, Al, Cu, Pb, Zn, and Ni in the Rift groundwater (Rango et al, 2014).
Table 2:
Summary of EC, TDS, and major and trace elements concentrations in 27 community water samples considered in this study.
| Min | Max | Percentiles | Mean | Water quality standards | Samples Exceeding lowest standard (% of villages) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | 75 | 95 | WHO | U.S. EPA |
EU | |||||
| Major elements (mg/L) | ||||||||||||
| F− | 0.55 | 15 | 1.41 | 2.53 | 5.59 | 9.88 | 13.7 | 6.48 | 1.5 | 4.0 | 1.5 | 25 (96%) |
| Ca2+ | 0.36 | 40.7 | 0.61 | 5.47 | 15.2 | 23.5 | 38.77 | 15.8 | - | - | - | - |
| Mg2+ | bd | 13.8 | 0.02 | 1.06 | 3.66 | 5.11 | 11.63 | 3.84 | - | - | - | - |
| Trace elements (μg/L) | ||||||||||||
| As | 0.16 | 54.9 | 0.59 | 1.98 | 4.9 | 17.7 | 54.9 | 10.2 | 10 | 10 | 10 | 7 (26%) |
| Cd | bd | 0.08 | bd | bd | 0.01 | 0.02 | 0.08 | 0.02 | 3 | 5 | 5 | 0 (0%) |
| Pb | bd | 1.59 | bd | bd | 0.04 | 0.34 | 0.73 | 0.2 | 10 | 15 | 10 | 0 (0%) |
| U | bd | 25.5 | 0.5 | 1.23 | 2.96 | 6.25 | 25.5 | 4.67 | 15 | 30 | - | 1 (4%) |
| Ag | bd | 0.04 | bd | bd | bd | bd | bd | bd | - | 100a | - | 0 (0%) |
| Al | 2.3 | 310 | 2.74 | 5.9 | 18.2 | 52.2 | 200 | 43.6 | - | 200a | - | 2 (7%) |
| B | 9.0 | 638 | 18.9 | 54.7 | 111 | 289 | 556 | 185 | 2400 | - | 1000 | 0 (0%) |
| Be | bd | 0.17 | bd | bd | bd | 0.07 | 0.15 | 0.04 | - | 4 | - | 0 (0%) |
| Co | bd | 0.44 | bd | 0.01 | 0.02 | 0.07 | 0.12 | 0.05 | 2000 | 13000 | 2000 | 0 (0%) |
| Cu | 0.03 | 4.91 | 0.03 | 0.11 | 0.36 | 0.61 | 3.89 | 0.9 | 2000a | 1300 | - | 0 (0%) |
| Li | 3.6 | 156 | 5 | 12.0 | 32.6 | 57.9 | 143 | 41.8 | - | 700 | - | 0 (0%) |
| Mn | bd | 422 | bd | 0.86 | 3.41 | 28.3 | 211 | 37.8 | 400 | 50a | - | 3 (11%) |
| Mo | 0.34 | 73.7 | 0.93 | 4.24 | 7.16 | 12.5 | 73.7 | 13.4 | - | - | - | - |
| Ni | bd | 0.99 | bd | bd | 0.05 | 0.45 | 0.72 | 0.22 | 70 | - | 20 | 0 (0%) |
| Rb | 0.84 | 52.2 | 1.31 | 4.44 | 8.59 | 15.5 | 35.7 | 11.5 | - | - | - | - |
| Sb | bd | 0.74 | bd | bd | 0.01 | 0.13 | 0.74 | 0.11 | 20 | 6 | 5 | 0 (0%) |
| Sr | 3.9 | 227 | 4.16 | 32.9 | 86.8 | 136 | 223 | 91.4 | - | 4000 | - | 0 (0%) |
| Th | bd | 0.16 | bd | 0.01 | 0.01 | 0.02 | 0.06 | 0.02 | - | - | - | - |
| Tl | bd | 0.01 | bd | bd | bd | bd | bd | bd | - | 2 | - | 0 (0%) |
| Zn | 1.24 | 143.4 | 1.85 | 7.8 | 16.1 | 41.3 | 88.3 | 28.5 | 5000a | - | - | 0 (0%) |
Notes: bd=below detection;
Secondary standard.
Regulatory limits for inorganic chemicals in drinking water established by the WHO (2011), the U.S. EPA (2009), and the EU Council (1998).
The health effects of F− in the region have been studied (Rango et al., 2012, 2017); however, the health concerns from low- to moderate-concentrations of As in the region require further investigation. Many epidemiological studies suggest risk of cancer, cardiovascular disease, respiratory illness, and diabetes from As exposures in water below the EPA maximum contamination level of 10μg/L (Moon et al., 2013; Garcia-Esquinas et al., 2013; Leonardi et al., 2012; Navas-Acien et al., 2008). This suggests there may be no safe threshold for As exposure (Schmidt, 2014). It is therefore reasonable to consider that the level of As exposure found in our study may be associated with adverse health effects. Overall, the very low levels of metals measured in drinking water suggest no immediate health risks for conditions such as kidney disease (e.g., from U and Cd), or neurodevelopment disorders (e.g., Pb) in this population.
3.3. Distribution of metals and trace elements in urine
Table 3 illustrates the statistical distribution (percentile, min, max, and means) of metals and trace elements in urine samples of the study population, as well as the reference ranges and 95th percentiles reported in other studies from developed countries. We found that mean concentrations of B, F−, Ca, and Mg were measured in mg/L levels, whereas concentrations for Mo, Zn, Sr, Rb, and Li fell between 100 and 700μg/L. Mean concentrations between 5 and 15μg/L were found for Ni, Cu, and Mn, and below 5μg/L were observed for Ag, Be, Cd, Co, Pb, Sb, Th, TI, and U. Arsenic and Al had mean concentrations between 30 and 50μg/L. Since no biomonitoring studies in urine have been carried out in Ethiopia or many other African countries, we mainly depended on established reference concentration ranges from North American and European populations for comparison. While these comparisons relate to populations with very different characteristics and environmental exposures, they do provide a benchmark for consideration of the observed elemental levels. The concentrations of Ag, Al, Be, Ca, Cu, Pb, Rb, Sb, Th, and U were found to fall within the reference ranges. Cadmium, Mg, Co, Sr, and Zn were slightly higher, but even these were within the 95th percentile of the ranges found in studies of healthy unexposed populations (Table 3). One of the elements with exceptionally high concentration ranges was F−. Levels of As, B, Mo, Mn, TI, Li and Zn were also found to exceed the comparison ranges. All of these elements were significantly positively correlation with the respective elements in the drinking water, except for Mn and Zn. Here we discuss these and other elements in additional detail.
Table 3.
Summary of metals and trace elements concentrations in urine samples of individuals in the study area
| Min | Max | Percentiles | Mean | Geometric Mean |
Reference range in healthy and unexposed population in studies in other countries | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic elements |
5 | 25 | 50 | 75 | 95 | *not reported for those >40% bd |
a,b,c,d Range | e Range | 95% Percentile | Geometric Mean | ||||||
| Ag | bd | 0.20 | bd | bd | bd | 0.01 | 0.04 | 0.01 | * | 0.04-0.88(c) | <0.01-0.02 | 0.03(i) | ||||
| Al | bd | 1851 | 4.0 | 15.0 | 23.8 | 37.4 | 94 | 45.8 | 25.0 | 2.4-19.5(c); 2.3-110(d) | 0.6-5.1 | 9.27(J) | 2.15(J) | |||
| As | 2.15 | 164 | 5.54 | 11.4 | 18.9 | 38.9 | 102 | 30.7 | 20.9 | <0.5–48.2(a); 15(b) | 5.3-11.7 | 27(f) | 48.8(J) | 46(k) | 8.63(k) | 8.63(J) |
| B | 227 | 8216 | 591 | 1152 | 1723 | 2579 | 4737 | 2019 | 1677 | 490-3290(c) | 380-3600 | |||||
| Be | bd | 0.77 | bd | bd | bd | 0.04 | 0.31 | 0.05 | * | 0.04-0.76(c) | <0.01-0.018 | 0.79(h) | <0.007(J) | <0.072(k) | <0.072(k) | |
| Ca | 1.73 | 382 | 3.3 | 10.6 | 26.1 | 55.4 | 155 | 43.5 | 24.3 | Mean: ~120 mg/L(d) | - | |||||
| Cd | bd | 3.35 | bd | 0.27 | 0.61 | 1.05 | 2.28 | 0.76 | 0.61 | 0.05-1.64(a); 0.8(b) | 0.005-0.46 | 1.3(f); 0.42(i) | 1.06(J) | 0.842(k) | 0.124(k) | 0.228(J) |
| Co | bd | 9.44 | bd | 0.52 | 1.13 | 1.93 | 3.93 | 1.44 | 1.05 | <0.12-2.05(a) | 0.04-0.81 | 8.3(h); 1.53(i) | 1.003(J) | 1.35(k) | 0.391(k) | 0.153(J) |
| Cu | bd | 47.8 | bd | 2.21 | 5.59 | 9.07 | 17.4 | 6.5 | 5.21 | 4.6-40.4(a) | 1.9-15.9 | 25(f); 13(i) | 19.6(J) | 6.94(J) | ||
| F− | 0.44 | 44.6 | 2.2 | 4.6 | 8.5 | 13.4 | 23.9 | 10.1 | 7.76 | 0.3-1(d) | - | 1.4(f) | ||||
| Li | 5.0 | 589 | 24.6 | 66 | 110 | 164 | 279 | 126 | 100 | Mean: ~0.5(d) | 5.2-23 | 115(i) | 75(J) | 22.3(J) | ||
| Mg | 0.57 | 611 | 28.4 | 67 | 105 | 164 | 275 | 125 | 97.1 | ~90mg/L(d) | - | |||||
| Mn | bd | 298 | bd | bd | 0.51 | 2.95 | 31 | 8.4 | * | <0.09-7.8(a) | <0.05-0.24 | 3.33(h); 0.21 (i) | 0.355(J) | 0.37(k) | 0.123(k) | |
| Mo | 11.7 | 2884 | 64 | 197 | 367 | 614 | 386 | 484 | 335 | 2.8-288(a) | 12-108 | 170(f); 168(h); 94(i) | 116(J) | 139(k) | 33.9(k) | 23(J) |
| Ni | bd | 61.0 | bd | 3.49 | 7.4 | 11.6 | 21.5 | 8.63 | 6.95 | <0.3-59(a) | 0.24-2.7 | 4.4(f); 2.5(i) | 4.73(J) | 1.73(J) | ||
| Pb | bd | 3.00 | bd | bd | 0.14 | 0.36 | 0.88 | 0.26 | 0.21 | 0.3-30(a) | 0.12-2.9 | 1.9(f); 6.4(h); 2.1(i) | 2.81(J) | 1.17(k) | 0.277(k) | 0.74(J) |
| Rb | 83 | 4186 | 143 | 321 | 502 | 777 | 1671 | 637 | 494 | 284-4096(c) | 500-5500 | 2424(i) | ||||
| Sb | bd | 2.0 | bd | bd | bd | bd | 0.05 | 0.02 | * | 0.19-1.1 (c) | 0.022-0.104 | 0.17(f);4.17(h);0.18(i) | 0.236(J) | 0.043 (k) | 0.043(k) | 0.029(J) |
| Sr | 6.5 | 788 | 17 | 38.4 | 84.1 | 162 | 303 | 117 | 79.4 | Mean: ~220(d) | 18-260 | 444(i) | 299(k) | 81.2(k) | ||
| Th | bd | 3.3 | bd | bd | bd | bd | 0.3 | 0.07 | * | 0.01-0.28(d) | 0.0005-0.005 | 0.61(f), 0.0031(g) | ||||
| Tl | bd | 11.9 | 0.15 | 0.65 | 1.47 | 2.72 | 6.25 | 2.06 | 1.44 | 0.07-0.7(c) | 0.05-0.54 | 1.18(h); 0.43(i) | 0.5(J) | 0.421(k) | 0.141 (k) | 0.168(J) |
| U | bd | 4.3 | bd | bd | bd | bd | 0.05 | 0.03 | * | 0.01-0.35(d) | 0.0007-0.019 | 0.0345(g); 0.01(i) | 0.03(J) | 0.039(k) | 0.005(k) | |
| Zn | 26.7 | 2983 | 66 | 167.3 | 287 | 502 | 1066 | 386 | 283 | 266-846(c); 27-850(d) | 40-430 | 1100(f); 692(i) | 1048(J) | 227(J) | ||
Notes: bd=below detection. All concentrations are in μg/L, except Ca, Mg, and F− in mg/L. For each element, the range 95th percentiles and geometric mean of reference values reported for healthy populations in different geographical regions is also presented.
White and Sabbionia, 1998 – UK population;
Wilhelm et al., 2004 – German population;
Minoia et al., 1990 – Italian population;
Caroli et al., 1994, and
Rodushkin et al., 2000–Sweden population;
Saravanabhavan et al., 2016 – Canada population;
Paschal, et al., 1998 – United States population;
Heitland and Köster, 2006 – German population;
Hoet et al., 2012– Belgium population;
CDC, 2018 – United States population;
GMs were not calculated if > 40% of samples contain bd.
Arsenic concentrations in urine of studied individuals were found to range from 2.2 to 164μg/L (mean: 30.7±29μg/L). The majority (82%; n=317) of individuals urine samples had As concentrations below 50μg/L and 19.4% (n=75) of individuals had As below 10μg/L. Only 5% of individuals had As between 100 and 164μg/L. The range of As levels was higher than those in other populations—United Kingdom (<0.5-48μgL), Sweden (5.3-11.7μg/L), Belgium (95th percentile; 48.8μg/L), and Canada (95th percentile; 27μg/L). More than 60% of individuals had As level exceeding the reference value of 15μg/L reported in German studies (Wilhelm et al., 2004, 2005). The 30.7μg/L observed in our study was also higher than the mean As concentrations of 16.7μg/L in the urine of individuals from uncontaminated areas of Lombardy, Italy (Reimann and de Caritat, 1998). The strong correlation of As in drinking water and urine in our study population indicates the As in urine mainly comes from drinking water or non-fish dietary elements, as sea food consumption is uncommon in the region. One of the major factors determining the toxicity of As is its metabolism, since As is excreted in urine as organic arsenical species (monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA)), as well as unmetabolized inorganic As (Aposhian and Aposhian, 2006). The inorganic As species, arsenate (As-V) and arsenite (As-III), are more toxic and carcinogenic than MMA and DMA, and As-III is more toxic than As-V (Heinrich-Ramm et al, 2002; Aposhian and Aposhian, 2006). A study of As speciation in water, food, and urine is needed to more fully characterize the inorganic and organic components of As.
Fluoride concentrations fell between 0.44 and 44.6mg/L (mean:10.1±7.1 mg/L). More than 96% of the urine samples contained F− at levels higher than the Biological Exposure Index (BEI) value of 2mg/L established by industrial hygienists as a guideline for occupational exposure (ACGIH, 2012). The maximum of 44.6mg/L is much higher than reported levels seen in other studies including from endemic areas (e.g., Susheela et al., 2002). We also compared our data with Biomonitoring Equivalents (BEs), which refer to the concentration or range of concentrations of chemicals in a biological medium that is consistent with an existing health-based exposure guideline. The F− range we have obtained in our study is far beyond the established range for BEs (1.1-2.1mg/L) (Aylward et al., 2015), indicating high potential health risks to the Rift population.
Boron concentrations were found to range between 227 and 8216μg/L (mean: 2018±1287μg/L), with a 95th percentile of 4737μg/L. These values are higher than reported reference values of 490-3290μg/L and 380-3600μg/L in other studies (Minoia et al., 1990; Rodushkin et al., 2000). Optimal intake of B has been shown to be essential for healthy bones, joints and cartilage, interact with Ca, Mg, and vitamin D and to play a vital role in the metabolism of bone (Khaliq et al., 2018; Nielsen and Stoecker, 2009; Newnham, 1994; Meacham 1994).
The highest Mo value encountered was 2884μg/L. The Mo ranges were much higher than the reference ranges in other studies (White and Sabbionia, 1998: 2.8-288 mg/L; Rodushkin et al., 2000: 12-108μg/L) and the 95th percentile (170μg/L) value reported by Saravanabhavan et al. (2017). The 95th percentile (386μg/L) value was higher than the 116μg/L reported by Hoet et al. (2012) and 94μg/L reported by Heitland and Koster, (2016). The Mo concentrations in drinking water (0.34-74μg/L) were well below the levels in urine, indicating that diet is the main source of Mo exposure. The urine Mo concentration obtained in our study had a range of 11.7-2884μg/L, which overlaps with the established BEs for Mo (200-7500 μg/L; Hays, et al., 2016), indicating increased health risks to the Rift population.
Manganese concentrations varied from below the detection limit to 298 μg/L, with a 95th percentile of 31μg/L, which significantly exceeds the 95th percentile of 0.37μg/L (CDC, 2018) and ranges (<0.09-7.8 μg/L and <0.05-0.24 μg/L; White and Sabbionia, 1998; Rodushkin et al., 2000) observed in reference populations. About 38% of individuals had Mn exceeding the maximum value of 7.8μg/L reported for reference populations (White and Sabbionia, 1998). Only twelve of the individuals had Mn between 90μg/L and the maximum value 286μg/L measured in our study. The concentration range of 1-8μg/L is considered normal (ATSDR, 2012), which correlates with what was observed in the reference populations in Table 3. While Mn is essential at low levels, it is neurotoxic and carcinogenic at higher concentrations (Bouchard et al., 2007).
Thallium concentrations varied from below the detection limit to 11.9μg/L, which is higher than the reported ranges of 0.07-0.7μg/L (Caroli et al., 1994) and 0.05-0.54μg/L (Rodushkin et al., 2000) in reference populations. The 95th percentile (6.25μg/L) value was also higher than the 95th percentiles of 0.42, 0.5, and 1.18μg/L reported in several other studies (CDC, 2018; Hoet et al., 2012; Paschal, et al., 1998). The German human biomonitoring (HBM) Commission established HBM I concentration values of 5μg/L for TI, for the concentration at and below which there is no risk of adverse health effects for the general population (Apel et al., 2017). In our study subjects, 92% (n=355) had TI concentration below 5μg/L. Current knowledge on the health effects of TI is limited, but it may be associated with headaches, anorexia, and pain in the arms, thighs, and abdomen (ATSDR, 2013; Peter and Viraraghavan, 2005).
Lithium concentrations were found between 5 and 589μg/L, which is higher than the reported ranges of 5.2-23μg/L (Caroli et al., 1994) and 0.8-40.5μ/L (Abou-Shakara et al., 1989). The 95th percentile (179μg/L) value was higher than the 75μg/L reported by Hoet et al. (2012) and 115μg/L reported by Heitland and Köster, (2016). Chronic high-dose Li medication is well known to cause health effects such as gastrointestinal pain, diarrhea, weight gain, edema, hypothyroidism, and renal tubular damage (Aral et al., 2008). On the other hand, studies have showed Li in drinking water to be associated with lower suicide rates in Texas (Schrauzer and Shrestha,1990), Japan (Ohgami et al., 2009), and Austria (Kapusta et al., 2011). The Li level (3.6-156 μg/L) found in drinking water in our study matches the Li ranges in the Texas study (<1-160μg/L).
The Zinc range (26.7-2983μg/L) was higher than the reference intervals reported by (Rodushkin et al., 2000: 40-430μg/L; Croli et al., 1994: 27-850μg/L). The 95th percentile (1066μg/L) value was comparable to the 1100 and 1048μg/L reported by Saravanabhavan et al. 2017 and Hoet et al. (2012).
The concentrations of other elements such as Pb, Cd, U, Ni, Co, Cu, and Ag were generally low and within the reference ranges found in healthy populations (Table 3). The 95th percentiles were (in μg/L) for Pb (0.88), Cd (2.28), U (0.05), Ni (21.5), Co (3.9), Cu (17.4), Ag (0.04), Al (94), Be (0.31), Rb (1671), Sb (0.05), Sr (303), Th (0.3), and TI (0.05); and (in mg/L) for Ca (155) and Mg (275). Lead, Cd and U are well known nephrotoxic contaminants (Skerfving et al., 1998; Nordberg et al., 2007; Åkesson et al., 2005; Kurttio et al., 2002). Cadmium concentrations ranged between below DL and 3.4μg/L (mean: 0.76±0.69μg/L). Most individuals (73.4%; n=285) had urinary Cd below 1μg/L. The Cd concentration in urine is influenced by smoking habits and age. As a few male subjects (n=10) were smokers, there may not be a major influence of smoking on Cd levels in this population. A study in Germany established a reference value of 0.8μg/L for non-smoking adults. Yet this value is lower than the 95th percentile of Cd (2.3μg/L) in our study. The German HBM Commission established HBM I and HBM II values for urinary Cd as guidelines for determining health risks for children and adults (Apel et al., 2017). In our study, 38.3% (n=59) of the children (10-18 years old) had urinary values below 0.5μg/L (the HBM I level) with no risk of adverse health effects, whereas 8.4% (n=13) of them had values exceeding the HBM level II value of 2μg/L, above which adverse health effects are possible. For adults (18-50 years old), 76.3% (n=177) had value below the HBMI level of 1μg/L, and none had values above the HBM II level of 4μg/L. The maximum urinary Cd concentration obtained in our study was 3.4μg/L, well above the BE range for Cd (1.1-1.5μg/L), indicating potentially heightened health risks.
Calcium and Mg concentrations ranged between 1.7 and 382mg/L, and 0.57 and 611mg/L, respectively. The mean values for Ca (43.5mg/L) and Mg (124.4mg/L) were lower and higher than what is measured for Ca—145.7mg/L and Mg—78 mg/L in other research (e.g., Dlugaszek et al, 2011). Caroli et al. (1994) found a mean Ca concentration of 90mg/L, which is also higher than the mean Ca level measured in our study. The low level of Ca measured among our study individuals demands further investigation, particularly to identify the possible effect chronic F− exposure on Ca status and its urinary excretion.
We observed age, sex and BMI differences in the levels of the elements. Individuals in younger age groups (10 to 30 years) had significantly higher mean concentrations of B, Cd, Co, Mg, Mo, and Pb than older ones between 31 to 50 years, whereas only Ca was significantly higher in the older age group (Table 4a). Mean urinary concentrations of Ca, Cu, Mg, Pb, Sr, and Zn were significantly higher in males than females, whereas only Co and Mn were higher in females. In total, mean values of 14 of the 23 elements (Ag, As, B, Ca, Cd, Cu, F, Li, Mg, Mo, Pb, Sb, Sr, and Zn) were higher in males than females (Table 4b). Individuals with normal or overweight BMI (>18.5kg/m2) had significantly higher urinary Ca and lower Mn concentrations than underweight (<18.5kg/m2) individuals (Table 4c). While several studies (e.g., Aprea et al., 2018; Heitland and Köster, 2006; Hoet et al., 2012) have documented age and sex dependence for biomonitoring levels of metals and trace elements, we are unsure at this time of the physiological reasons for such dependence; this issue therefore warrants further investigation.
Table 4.
Difference in log urine concentrations by Age, Sex, and BMI
| a) T-Test by Age | b) T-Test by Sex | c) T-Test by BMI status | ||||
|---|---|---|---|---|---|---|
| (Age<30 – Age>30) | (Female – Male) | (normal BMI – underweight BMI) | ||||
| Ag | 0.11 | (1.28) | −0.08 | (−1.05) | 0.03 | (0.41) |
| Al | 0.14 | (1.21) | 0.12 | (1.13) | −0.06 | (−0.52) |
| As | 0.06 | (0.64) | −0.05 | (−0.60) | −0.07 | (−0.71) |
| B | 0.14* | (1.99) | −0.06 | (−0.91) | −0.06 | (−0.92) |
| Be | 0.14 | (1.36) | 0.03 | (0.30) | 0.00 | (0.01) |
| Ca | −0.30* | (−2.37) | −0.37** | (−3.19) | 0.40*** | (3.35) |
| Cd | 0.38* | (2.17) | 0.00 | (0.01) | −0.15 | (−0.90) |
| Co | 0.80*** | (4.9) | 0.34* | (2.21) | −0.26 | (−1.64) |
| Cu | 0.25 | (1.75) | −0.29* | (−2.25) | 0.05 | (0.40) |
| F− | −0.05 | (−0.57) | −0.02 | (−0.27) | 0.13 | (1.64) |
| Li | 0.07 | (0.91) | −0.01 | (−0.10) | 0.10 | (1.30) |
| Mg | 0.28** | (3.02) | −0.30*** | (−3.56) | −0.06 | (−0.70) |
| Mn | 0.34 | (1.63) | 0.47** | (2.64) | −0.60** | (−3.13) |
| Mo | 0.24* | (2.32) | −0.18 | (−1.88) | −0.13 | (−1.37) |
| Ni | 0.09 | (0.69) | 0.00 | (−0.00) | −0.07 | (−0.55) |
| Pb | 0.42** | (3.17) | −0.30* | (−2.42) | −0.17 | (−1.30) |
| Rb | −0.03 | (−0.43) | 0.12 | (1.64) | 0.08 | (1.09) |
| Sb | 0.05 | (0.80) | −0.02 | (−0.30) | −0.04 | (−0.79) |
| Sr | 0.10 | (0.95) | −0.24* | (−2.50) | 0.07 | (0.75) |
| Th | 0.02 | (0.18) | 0.12 | (1.33) | −0.01 | (−0.09) |
| Tl | 0.14 | (0.89) | 0.15 | (1.03) | −0.10 | (−0.68) |
| U | 0.03 | (0.42) | 0.11 | (1.48) | −0.01 | (−0.19) |
| Zn | 0.04 | (0.46) | −0.48*** | (−5.98) | −0.11 | (−1.30) |
t statistics in parentheses:
p<0.05;
p<0.01;
p<0.001
We observed significant positive correlation between As, B, Cd, F−, Li, Mo, Rb, and U in drinking water and urine, indicating the important role of drinking water as the source of exposure to these elements (Table 5). Conversely, we found significant negative correlation between Ag, Ca, Co, Mg, Mn, Sr, TI, and Zn in drinking water and urine. No correlation was observed between the rest of the elements in drinking water and urine.
Table 5.
Pairwise correlation between log urine and water concentrations
| Pearson's correlation coefficient |
Significance level |
|
|---|---|---|
| Ag | −0.12 | 0.0218* |
| Al | −0.09 | 0.0787 |
| As | 0.55 | 0.0000** |
| B | 0.26 | 0.0000** |
| Be | −0.01 | 0.8914 |
| Ca | −0.11 | 0.0243* |
| Cd | 0.15 | 0.0025** |
| Co | −0.33 | 0.0000** |
| Cu | 0.09 | 0.0881 |
| F | 0.70 | 0.0000** |
| Li | 0.44 | 0.0000** |
| Mg | −0.18 | 0.0004** |
| Mn | −0.25 | 0.0000** |
| Mo | 0.11 | 0.0245* |
| Ni | −0.05 | 0.3563 |
| Pb | 0.02 | 0.7515 |
| Rb | 0.14 | 0.0071** |
| Sb | −0.07 | 0.2016 |
| Sr | −0.11 | 0.0333* |
| Th | −0.01 | 0.7855 |
| Tl | −0.11 | 0.0316* |
| U | 0.15 | 0.0029** |
| Zn | −0.19 | 0.0002** |
=p<0.05;
=p<0.01
Limitations of the study: Since biomonitoring data in other regions of Ethiopia is absent, it was not possible to compare levels in the Rift population relative to individuals in other regions of the country. Due to the unique geologic setting of the Rift, the concentration values primarily serve as baseline concentration ranges for these sample populations. We also did not measure creatinine or the specific gravity of urine, which would have allowed normalization for dilution. However, many similar biomonitoring studies report concentration values as we do, allowing comparison with our findings. In addition, our study collected 12-hour urine, which captures a more representative variation of elemental concentrations, unlike spot samples that are likely to vary over time reflecting short-term changes in diet, lifestyle, or activities (e.g., Smolders et al. 2014). We also anticipate that the relatively uniform diet, water sourcing, and lifestyle of individuals in the Rift would yield relatively stable correlations between individuals’ spot and 12-hour urine. Nonetheless, it would be worthwhile to study variation in the urinary excretion of elements in both spot and longer collection times.
4. Conclusion
The established concentration ranges are relevant in providing baseline levels of exposure in this study population, which resides in a central Ethiopian Rift Valley known for its unique geology and geography. This unique location features vast volcanic pyroclastic materials that play a major role in the chemical composition of water, soil, and therefore food sources and biomarkers. We found urinary concentrations of As, F−, B, Mo, Mn, Li, and Zn that exceeded reference values from healthy unexposed populations in other regions, whereas other elements were within or close to the ranges of reference populations. While it is important to note that this comparison provides a picture of concentrations, it is not necessarily indicative of deficient, optimal, or toxicity thresholds above which pathological states would be expected in our populations. Several factors (including lifestyle, nutritional status, ethnicity, sex, age, and interaction of the elements) besides chemical exposures determine health and disease. In fact, the health effect of excess F− has already been well-recognized in the region, but the health risk of low-to-moderate levels of As in Rift water and urine remains unknown. Meanwhile, the established baseline values in the Rift population may reflect those in other countries in the Great African Rift Valley with similar geologic settings. Overall, there is a need to initiate comprehensive biomonitoring studies in Ethiopia and other African countries to establish reference ranges that allow better monitoring of environmental and other changes that might result from ongoing economic development and industrialization. We stress that the presented concentration ranges can be integrated into future studies of the toxicity or deficiency of the measured elements. This will help to define normal or pathological ranges and will inform establishment of public health policies.
Highlights.
Concentrations of 23 elements were established in urine of Ethiopian Rift populations.
As, F−,B, Mo, Mn, Tl, Li, and Zn inurine were higher than in the reference populations.
Elevated levels of elements such as As call for speciation and health studies.
The study highlights the need for more biomonitoring studies in African nations to protect public health.
Acknowledgment
The research is part of an ongoing cohort study with the goal of understanding the health impacts of fluoride and metals on Ethiopian Rift Valley populations. We are immensely grateful for the funding from the NIEHS's career development grant (K99 and R00 ES023472). We thank all the children and parents who participated in this study, and the local water bureaus for their help in recruiting them and guiding us during the fieldwork. Dr. Keith Levine from the Research Triangle Institute (RTI) International (Research Triangle Park, United States) for measurement of trace elements in urine of selected samples for interlaboratory comparison. We also thank Drs. Avner Vengosh and Gary Dwyer for access to the laboratory and support of the analytical analysis. The content expressed in this paper is the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Abbreviations
- Ag
Silver
- Al
Aluminum
- As
Arsenic
- B
Boron
- Be
Beryllium
- Ca
Calcium
- Cd
Cadmium
- Co
Cobalt
- Cu
Copper
- F−
Fluoride
- Li
Lithium
- Mg
Magnesium
- Mn
Manganese
- Mo
Molybdenum
- Ni
Nickel
- Pb
Lead
- Rb
Rubidium
- Sb
Antimony
- Sr
Strontium
- Th
Thorium
- TI
Thallium
- Zn
Zinc
- U
Uranium
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- DL
Detection limit
- EU
directives: European Union Directives
- ICP-MS
Inductively Coupled Plasma Mass Spectrometry
- IRB
Institutional Review Board
- ISE
Ion Selective Electrode
- LGC
LoGiCal® reference materials
- mg/L
milligram per liter
- pg/L
microgram per liter
- TISAB
Total Ionic Strength Adjuster Buffer
- U.S. EPA
U.S. Environmental Protection Agency
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Shakra FR, Havercroft JM, Ward NI, 1989. Lithium and boron in biological tissues and fluids. Trace Elem Med 6:142. [Google Scholar]
- Angerer J, Ewers U, Wilhelm M, 2007. Human biomonitoring: state of the art. Int. J. Hyg. Environ. Health 210, 201e228. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Aposhian MM, 2006. Arsenic toxicology: five questions. Chem Res Toxicol 19:1–15. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Manganese. Atlanta (GA): US Department of Health and Human Services; 2012. [PubMed] [Google Scholar]
- Apel. P, Angerer J, Wilhelm M, Kolossa-Gehring M, 2017. New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health. 220(2): 152–156. [DOI] [PubMed] [Google Scholar]
- Aprea MC, Apostoli P, Bettinelli M, Lovreglio P, Negri S, Perbellini L, Perico A, Ricossa MC, Salamon F, Scapellato ML, lavicoli I, 2018. Urinary levels of metal elements in the non-smoking general population in Italy: SIVR study 2012–2015. Toxicol Lett. 298:177–185. [DOI] [PubMed] [Google Scholar]
- Åkesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Strömberg U, Skerfving S, 2005. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 113, 1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimonti A, Bocca B, Mannella E, Petrucci F, Zennaro F, Cotichini R, D'lppolito C, Agresti A, Caimi S, Forte G, 2005. Assessment of reference values for selected elements in a healthy urban population, Ann. Delllstituto Super. Sanità 41:181–187. [PubMed] [Google Scholar]
- Aral H, Vecchio-Sadus A, 2008. Toxicity of lithium to humans and the environment-a literature review. Ecotoxicol. Environ. Saf, 70 (3), 349–356. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), 2013. ToxFAQsTMforThallium. U.S. Department of Health and Human Services Atlanta, GA, Atlanta,GA, USA. [Google Scholar]
- Aylward LL, Hays SM, Vezina A, Deveau M, St-Amand A, Nong A, 2015. Biomonitoring Equivalents for interpretation of urinary fluoride. Regul Toxicol Pharmacol, 72(1):158–167. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Bowler R, Roels HA, 2007. Neurobehavioral functioning after cessation of manganese exposure: a follow-up after 14 years. Am J Ind Med 50(11): 831–40. [DOI] [PubMed] [Google Scholar]
- Coelho P, Costa S, Costa C, Silva S, Walter A, Ranville J, Pastorinho MR, Harrington C, Taylor A, Dall'Armi V, Zoffoli R, Candeias C, da Silva EF, Bonassi S, Laffon B, Teixeira JP, 2013. Biomonitoring of several toxic metal(loid)s indifferent biological matrices from environmentally and occupationally exposed populations from Panasqueira mine area Portugal, Environ. Geochem. Health 36: 255–269. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2018. Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables, Volume One, U.S. Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2015. Fourth National Report on Human Exposure to Environmental Chemicals. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2012. National Report on Human Exposure to Environmental Chemicals – Fourth Report, February 2012. [Google Scholar]
- Caroli S, Alimonti A, Coni E, Petrucci E, Senofonte O, Violante N, 1994. The assessment of reference values for elements in human biological tissues and fluids: a systematic review. Crit. Rev. Anal. Chem. 24, 363–398. [Google Scholar]
- Černá M, Krsková A, Čejchanová M, Spĕváčková V, 2012. Human biomonitoring in the Czech Republic: an overview. Int. J. Hyg. Environ. Health 215, 109–119. [DOI] [PubMed] [Google Scholar]
- Chorowicz J, 2005. The East African Rift System. J Afr Earth Sci. 43:379–410. [Google Scholar]
- Dlugaszek M, Kaszczuk M, Mularczyk-Oliwa M 2011. Magnesium, calcium, and trace elements excretion in 24-h urine. Biol Trace Elem Res ; 142:1–10. [DOI] [PubMed] [Google Scholar]
- Fréry N, Vandentorren S, Etchevers A, Fillol C, 2012. Highlights of recent studies and future plans for the French human biomonitoring (HBM) programme. Int. J. Hyg. Environ. Health 215, 127–132. [DOI] [PubMed] [Google Scholar]
- García-Esquinas E, Pollan M, Umans JG, Francesconi KA, Goessler W, Guallar E, et al. 2013. Arsenic exposure and cancer mortality in a US-based prospective cohort: the Strong Heart Study. Cancer Epidemiol Biomarkers Prev 22(11):1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Bouige D, Lacroix C. Metal and metalloid multi-elementary ICP-MS validation in whole blood plasma, urine and hair. Reference values, Forensic Sci. Int. 153 (2005)39–44. [DOI] [PubMed] [Google Scholar]
- Ganzleben C, Antignac J-P, Barouki R, Castano A, Fiddicke U, Klánová J, Lebret E, Olea N, et al. 2017. Human biomonitoring as a tool to support chemicals regulation in the European Union Int J Hyg Environ Health 2017; 220(2A):94–7. [DOI] [PubMed] [Google Scholar]
- Haines DA, Gurusankar S, Werry K, Cheryl K. 2017. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int. J. Hyg. Environ. Health, 220, 13–28. [DOI] [PubMed] [Google Scholar]
- Hays SM, Macey K, Poddalgoda D, Lu M, Nong A, Aylward LL, 2016. Biomonitoring Equivalents for molybdenum. Regul Toxicol Pharmacol. 77:223–9. [DOI] [PubMed] [Google Scholar]
- Heitland P, and Köster HD, 2006. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clinica Chimica Acta 365:310–318. [DOI] [PubMed] [Google Scholar]
- Hoet P, Jacquerye C, Deumer G, Lison D, Haufroid V, 2013, Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin Chem Lab Med. 51(4):839–49. [DOI] [PubMed] [Google Scholar]
- Heinrich-Ramm R, Mindt-Prüfert S, Szadkowski D, 2002. Arsenic species excretion after controlled seafood consumption. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 778, 263–273. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Makelainen I, Vaisanen SB, Penttila IM, Komulainen H, 2002. Renal effects of uranium in drinking water. Environ. Health Perspect. 110, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlifi R, Olmedo P, Gil F, Feki-Tounsi M, Hammami B, Rebai A, HamzaChaffai A, 2014. Biomonitoring of cadmium, chromium, nickel and arsenic in general population living near mining and active industrial areas in Southern Tunisia. Environ. Monit. Assess. 186, 761e779. [DOI] [PubMed] [Google Scholar]
- Khaliq H, Juming Z, Ke-Mei P, 2018. The Physiological Role of Boron on Health. Biol Trace Elem Res. March 15. doi: 10.1007/s12011-018-1284-3. [DOI] [PubMed] [Google Scholar]
- Kapusta ND, Mossaheb N, Etzersdorfer E, Hlavin G, Thau K, Willeit M, Praschak-Rieder N, Sonneck G, Leithner-Dziubas K, 2011. Lithium in drinking water and suicide mortality. Br. J. Psychiatry, 198, 346–350. [DOI] [PubMed] [Google Scholar]
- Leonardi G, Vahter M, Clemens F, Goessler W, Gurzau E, Hemminki K, Hough R, Koppova K, Kumar R, Rudnai P, Surdu S, Fletcher T, 2012. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: a case-control study. Environ. Health Perspect. 120:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy LS, Jones K, Cocker J, Assem FL, Capleton AC, 2007. Background levels of key biomarkers of chemical exposure within the UK general population – pilot study. Int. J. Hyg. Environ. Health 210, 387–391. [DOI] [PubMed] [Google Scholar]
- Laamech J, Bernard A, Dumont X, Benazzouz B, Lyoussi B, 2014. Blood lead, cadmium and mercury among children from urban, industrial and rural areas of Fez Boulemane Region (Morocco): relevant factors and early renal effects. Int. J. Occup. Med. Environ. Health 27, 641e659. [DOI] [PubMed] [Google Scholar]
- Meacham SL, Taper LJ, Volpe SL, 1994. Effects of boron supplementation on bone mineral density and dietary, blood, and urinary calcium, phosphorus, magnesium, and boron in female athletes. Environ Health Perspect.; 102 Suppl 7:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoia C, Sabbioni E, Apostoli P, Pietra R, Pozzoli L, Gallorini M, Nicolaou G, Alessio L, Capodaglio E, 1990. Trace element reference values in tissues from inhabitants of the European community. I. A study of 46 elements in urine, blood, and serum of Italian subjects. Sci. Total Environ. 95, 89–105. [DOI] [PubMed] [Google Scholar]
- Moawad EM, Badawy NM, Manawill M, 2016. Environmental and occupational lead exposure among children in cairo, Egypt: a community-based crosssectional study. Med. Baltim. 95, e2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med 159(10):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E, 2008. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300:814–822. [DOI] [PubMed] [Google Scholar]
- Newnham RE, 1994. Essentiality of boron for healthy bones and joints. Environ Health Perspect. 102 Suppl 7:83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FH, Stoecker B, 2009. Boron and fish oil have different beneficial effects on strength and trabecular microarchitecture of bone. J Trace Elem Med Biol 23: 195–203. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Fowler BA, Nordberg M, 2007. Handbook on the Toxicology of Metals. Third Edition. Burlington: Academic Press. [Google Scholar]
- Ohgami H, Terao T, Shiotsuki I, Ishii N, Iwata N, 2009. Lithium levels in drinking water and risk of suicide. Br. J. Psychiatry, 194, 464–465. [DOI] [PubMed] [Google Scholar]
- Paschal DC, Ting BG, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ, Miller DT, Caldwell KL, 1998. Trace metals in urine of United States residents: reference range concentrations. Environ Res. 76(1):53–9. [DOI] [PubMed] [Google Scholar]
- Paul CJ, Jeuland MA, Godebo TR, Weinthal E, 2018. Communities Coping with Risks: Household Water Choice and Environmental Health in the Ethiopian Rift Valley. Environmental Science and Policy. 86: 85–94. [Google Scholar]
- Pérez-Gómez B, Pastor-Barriuso R, Cervantes-Amat M, Esteban M, Ruiz-Moraga M, Aragonés N, Pollán M, Navarro C, Calvo E, Román J, López-Abente G, Castaño A, 2013. BIOAMBIENT.ES study protocol: rationale and design of a crosssectional human biomonitoring survey in Spain. Environ. Sci. Pollut. Res. Int. 20, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Peter AJ, Viraraghavan T, 2005. Thallium: a review of public health and environmental concerns. Environ. Int. 31, 493–501. [DOI] [PubMed] [Google Scholar]
- Rango T, Bianchini G, Beccaluva L, Ayenew T, Colombani N, 2009. Hydrogeochemical study in the main Ethiopian rift: new insights to source and enrichment mechanism of fluoride. Environ. Geol. 58, 109–118. [Google Scholar]
- Rango T, Kravchenko J, Atlaw B, McCornick PG, Jeuland MA, Merola B, Vengosh A, 2012. Groundwater quality and its health impact: an assessment of dental fluorosis in rural inhabitants of the main Ethiopian Rift. Environ. Int. 43, 37–47. [DOI] [PubMed] [Google Scholar]
- Rango T, Vengosh A, Dwyer G, Bianchini G, 2014. Mobilization of arsenic and other naturally occurring contaminants in groundwater of the main Ethiopian Rift aquifers. Water Res. 47, 5801–5818. [DOI] [PubMed] [Google Scholar]
- Rango T, Vengosh A, Jeuland M, Whitford GM, Tekle-Haimanot R, 2017. Biomarkers of chronic fluoride exposure in groundwater in a highly exposed population. Sci. Total Environ. 596, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann C, de Caritat P, 1998. Chemical Elements in the Environment. Springer-Verlag, Berlin. [Google Scholar]
- Reimann C, Bjorvatn K, Frengstad B, Melaku Z, Tekle-Haimanot R, Siewers U, 2003. Drinking water quality in the Ethiopian section of the East African Rift Valley I—data and health aspects. Sci Total Environ; 311:65–80. [DOI] [PubMed] [Google Scholar]
- Reis MF, Sampaio C, Melim M, Miguel JP, 2004. Environmental Health Survey Programs (ProVEpAs) in Portugal The European Environment and Health Action Plan 2004-2010. The Netherlands, Egmond aan Zee. [Google Scholar]
- Rodushkin I, Ödman F, Olofsson R, Burman E, Axelsson MD, 2001. Multi-element analysis of body fluids by double-focusing ICP-MS. Transworld Res. Network Recent Res. Dev. Pure Appl. Chem. 5, 51–66. [Google Scholar]
- Schmidt CW, 2014. Low-dose arsenic: in search of a risk threshold. Environ Health Perspect 122:A130 A134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauzer GN Shrestha KP. 1990. Lithium in drinking water and the incidences of crimes, suicides, and arrests related to drug addictions. Biol. Trace Elem. Res. 25, 105–113. [DOI] [PubMed] [Google Scholar]
- Schulz C, Wilhelm M, Heudorf U, Kolossa-Gehring M, 2012. Reprint of “Update of the reference and HBM values derived by the German Human Biomonitoring Commission”. Int. J. Hyg. Environ. Health 215, 150–158. [DOI] [PubMed] [Google Scholar]
- Saravanabhavan G Weey K, Walker M, Haines D, Malowany M, Khoury C, 2017. Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013. Int. J. Hyg. Environ. Health, 220, 189–200. [DOI] [PubMed] [Google Scholar]
- Susheela AK, Bhatnagar M, 2002. Reversal of fluoride induced cell injury through elimination of fluoride and consumption of diet rich in essential nutrients and antioxidants. Molec. Cell Biochem. 234/235: 335–340. [PubMed] [Google Scholar]
- Skerfving S, Gerhardsson L, Schütz A, 1998. Lead-Biological monitoring of exposure and effects. J. Trace Elem. Exp. Med. 11,289–301. [Google Scholar]
- Sexton K, Needham LL, Pirkle JL, 2004. Human biomonitoring of environmental chemicals. Am Sci 92:38–45. [Google Scholar]
- Smolders R, Koch HM, Moos RK, Cocker J, Jones K, Warren N, et al. 2014. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 1: metals. Toxicol Lett 231:249–260. [DOI] [PubMed] [Google Scholar]
- Ting BG, Paschal DC, Jarrett JM, Pirkle JL, Jackson R,J, Sampson EJ, Miller DT, Caudill SP, 1999. Uranium and thorium in urine of United States residents: reference range concentrations. Environ Res. 81:45–51. [DOI] [PubMed] [Google Scholar]
- Tuakuila J, Kabamba M, Mata H, Mbuyi F, 2015. Tentative reference values for environmental pollutants in blood or urine from the children of Kinshasa. Chemosphere 139, 326e333. [DOI] [PubMed] [Google Scholar]
- White MA, Sabbionia E, 1998. Trace element reference values in tissues from inhabitants of the European Union. X. A study of 13 elements in blood and urine of a United Kingdom population. Sci Total Environ. 216:253–70. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ewers U, Schulz C, 2004. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine, Int. J. Hyg. Environ. Health. 207, 69–73. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Pesch B, Wittsiepe J, Jakubis P, Miskovic P, Keegan T, Nieuwenhuijsen MJ, Ranft U, 2005. Comparison of arsenic levels in fingernails with urinary arsenic species as biomarkers of arsenic exposure in residents living close to a coal-burning power plant in Prievidza District, Slovakia. J. Expo. Anal. Environ. Epidemiol. 15, 89–98. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119(6):878–885; doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedomon. B, Menudier A, Etangs FLD, Anani L, Fayomi B, Druet-Cabanac M, Moesch C, 2017. Biomonitoring of 29 trace elements in whole blood from inhabitants of Cotonou (Benin) by ICP-MS. J Trace Elem Med Biol. 43:38–45. [DOI] [PubMed] [Google Scholar]