Abstract
Background:
To evaluate the efficacy of botulinum toxin A (BoNT-A) in reducing photophobia and dry eye symptoms in individuals with chronic migraine. Additionally, we aimed to evaluate tear film volume as a potential contributor to symptoms in these patients.
Methods:
Retrospective review of seventy-six patients who received BoNT-A for chronic migraine (CM) between August 23, 2017 and December 13, 2017 at the Miami Veterans Affairs Medical Center Neurotoxin Clinic. Demographic data and all co-morbidities were queried via chart review. Standardized validated surveys were administered to assess symptoms prior to and after BoNT-A injection. Pre-injection tear volumes were obtained using the phenol red thread (PRT) test.
Results:
Pre-injection migraine, photophobia, and dry eye symptom scores were all significantly correlated, p<0.05; and none were associated with pre-injection PRT results. After BoNT-A, improvements in migraine, photophobia, and dry eye symptoms were also significantly correlated, p<0.05; and similarly did not associate with pre-injection PRT results. Photophobia scores significantly improved following BoNT-A, while dry eye symptoms significantly improved in those with severe symptoms at baseline (DEQ-5 score ≥12), p=0.027. In logistic regression analysis of all individuals with dry eye symptoms (DEQ-5≥6), individuals with more severe dry eye symptoms were more likely improve, OR 1.27, 95% confidence interval [1.06, 1.51], p<0.01.
Conclusions:
BoNT-A significantly improved photophobia in patients being treated for migraine and also improved dry eye symptoms in patients with severe symptoms at baseline, independent of baseline tear film volume. These improvements may be due to modulation of shared trigeminal neural pathways.
Precise:
We found that in migraine patients, photophobia and dry eye symptoms are not a direct consequence of reduced tear volume, and both symptoms improve following botulinum toxin A injections.
INTRODUCTION:
Photophobia is one of the most distressing ancillary symptoms of migraine resulting in high morbidity without effective treatment. Even in the least severe cases, individuals may be forced to wear sunglasses indoors. Interestingly, migraine patients are significantly more photophobic than controls between attacks, termed interictal photophobia. Given that photophobia in migraineurs is potentiated by application of heat to V1 nerve distribution,1 sensitization of the trigeminal system is thought to underlie this photophobia.2 A similar pathophysiology is believed to underlie the photophobia that can accompany dry eye symptoms in some individuals.3 Similar to other conditions that involve nerve sensitization, we found that patients with photophobia had more severe and persistent dry eye symptoms4 and were less responsive to topical therapy than their counterparts with dry eye symptoms but no photophobia.5
Despite its negative impact on quality of life, few studies have evaluated therapies for photophobia. One retrospective study found that autologous serum tears improved photophobia in 16 individuals with debilitating symptoms due to a variety of ocular co-morbidities, including dry eye syndrome.6 Another study found that tinted lenses, such as FL-41, improved photophobia in a variety of clinical situations, including migraine.7 Recently, our group demonstrated that botulinum toxin A (BoNT-A) injections significantly reduced photophobia and dry eye symptoms in chronic migraine patients.8 However, this study was limited by its cross-sectional design, making pre- and post-injection measurements difficult to interpret in the setting of possible recall bias, and lacked data regarding tear parameters. Therefore, this study evaluated pre- and post-injection data with a temporal component thereby eliminating recall bias and evaluated low tear volume as a potential contributor to photophobia and dry eye symptoms in this patient population.
METHODS:
Study methodology:
Retrospective review of patients who received BoNT-A for chronic migraine (CM) between August 23, 2017 and December 13, 2017 at the Miami Veterans Affairs Medical Center. Inclusion criteria for BoNT-A treatment included: a diagnosis of CM (defined as 15 or more headache days per month for at least 3 months with at least 8 days per month having migrainous features) and a least 2 failed trials of prophylactic medications (i.e. triptans) or contraindications to prophylactic medications. Patients generally receive BoNT-A injections every 3 months regardless of migraine activity at the time of the clinic visit. Our routine algorithm is to administer 100U of BoNT-A with escalation to 150U if individuals report minimal migraine relief after 3 injections of 100U. Injection sites include: the procerus, corrugators, frontalis, temporalis, occipitalis, cervical, paraspinals, and trapezius muscles. (Supplementary File 1)
In total, 76 individuals were evaluated during the study window. Of those, 2 patients did not receive BoNT-A injections and 2 patients did not complete the pre-injection survey, leaving 72 individuals for analysis. Patients were contacted 2 to 8 weeks post-injection to re-assess migraine, photophobia, and dry eye symptoms. In total, this information was obtained in 62 (86.1%) of individuals. One individual did not complete every survey in its entirety; though partial survey data was still analyzed where available.
The project was started as a quality improvement study. Miami VA Institutional Review Board approval was later obtained to allow the evaluation of questionnaires and charts. The study was conducted in accordance to the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act.
Data collected:
Demographic data collected included: age, gender, race (white, black, or other), and ethnicity (Hispanic or non-Hispanic). Systemic co-morbid conditions were determined by examining the ‘active’ diagnosis list within the patient’s chart. Medications listed as ‘active’ at the time of chart review were grouped by marketed indication including anxiolytics, anti-depressants, anti-histamines, anti-inflammatory, and dry eye medications. Date of botulinum treatment initiation, total number of treatments, total days elapsed since 1st injection, and total days elapsed since last injection were also identified.
Pre-injection surveys were administered in the neurotoxin clinic during a regularly scheduled clinic visit for BoNT-A. Migraine characteristics and severity were queried using a simplified version of the Migraine Symptom Severity Score (MSSS) (scale 0–21).9 A numerical rating scale (NRS) anchored at ‘0’ indicating “no pain” and ‘10’ indicating “very severe pain” was also administered. Photophobia severity was measured using the validated Visual Light Sensitivity Questionnaire-8 (VLSQ-8) (scale 8–40).10 Additionally, intensity of interictal photophobia was measured using a NRS (range 0–10) using the question “In the past month, how severe has your sensitivity to light been when you DO NOT have a migraine?” Dry eye symptoms were quantified using the Dry Eye Questionnaire-5 (DEQ-5).11 Additional information obtained included: current contact lens wear, a self-reported history of dry eye, history of refractive surgery, history of cataract surgery, artificial tear usage, and smoking history. Finally, pre-injection tear volumes were obtained using the phenol red thread (PRT) test. The PRT test is performed by inserting a 75mm long phenol red coated thread into the lower fornix of each eye for 15 seconds. After 15 seconds the thread is removed, and color indicator change from white to red along the thread is measured. Normal tear volume is defined as >20mm, borderline volume as between 10 and 19mm, and severe reduction as <10mm.12 Two to 8 weeks after injection, the same surveys were administered via phone survey.
Statistical analysis:
Data were entered into a standardized database. Statistical analyses were performed using SPSS 25.0 (SPSS Inc. Chicago, IL) statistical package. Descriptive statistics were used to describe the study population and response to BoNT-A with respect to migraine, photophobia, and dry eye symptoms. Pearson and Spearman coefficients were calculated to quantify correlations between pre-injection MSSS, VLSQ-8, DEQ-5 scores, and PRT (eye with lower tear volume) and to quantify correlations between improvement in scores. Independent t-tests and chi-square methodologies were used to assess which factors associated with baseline dry eye symptoms and signs (VSLQ-8, DEQ-5, PRT). Paired t-tests were used to compare change in symptom scores before and after treatment. Finally, univariable and multivariable forward step wise logistic regression analyses were used to determine which factors (listed in Supplementary File 2) predicted a positive response in symptoms. Our outcome variables were: (1) ≥2-categorical improvement in any one question of the VLSQ-8 post- vs pre-injection, and (2) ≥3-point improvement in DEQ-5 scores post- vs pre-injection. We performed this analysis twice, once including all individuals and then including only those with dry eye symptoms (DEQ ≥6) at baseline.
RESULTS:
Study population:
Of the 72 patients reviewed during this study, 43 (59.7%) were male with a mean age of 48.0 years, standard deviation (SD) 10.1. The majority of patients identified as white and non-Hispanic, and the most common systemic co-morbidities were depression (56.9%), post-traumatic stress disorder (PTSD) (45.8%), and sleep apnea (38.9%). The average time of having migraine pain was 16.8 years SD 10.8, and the average total number of BoNT-A treatments was 10.15 (SD 7.6). Using the date of visit as an anchor, mean time elapsed since initiating BoNT-A treatment was 2.7 years (SD 2.3). The mean time since the last BoNT-A injection was 3.9 months (SD 4.4). Anti-depressants, triptans, and non-steroidal anti-inflammatory agents were the most commonly reported ancillary migraine medication therapy. (Supplementary File 2)
Pre-treatment symptoms:
The majority of patients rated their pre-treatment photophobia as severe (80.6%, n=58 with a score ≥7; mean 8.01 (SD 2.2). These findings were supported by the VLSQ-8 survey data, which revealed a mean score of 29.9 (SD 5.1), range 17–40. All but 2 patients reported dry eye symptoms (97.2%, n=70 with a DEQ-5 score ≥6; mean 12.9 (SD 4.2), and 45 (63.4%) individuals reported severe dry eye symptoms (DEQ-5 score ≥12).11 Despite the high symptom burden, only 14 (20.3%) patients demonstrated objective evidence of severe low tear volume (PRT<10mm in either eye). Mean wet PRT length of right and left eye were 16.6 millimeters (SD 7.1) and 17.0 millimeters (SD 7.7), respectively. (Supplementary File 2)
Pre-injection migraine severity, photophobia, and dry eye symptom severity scores were all significantly correlated with one another; while PRT results did not correlate with these pre-injection scores. (Table 1) As expected, PRT length of right and left eye were strongly correlated with one another, r=0.808 and rho=0.817, p<0.01.
Table 1:
Associations between pre-injection migraine severity, photophobia, dry eye symptom severity, and phenol red thread testing
| Pre-Injection MSSS | Pre-Injection VLSQ-8 | Pre-Injection DEQ-5 | |
|---|---|---|---|
| Pre-Injection MSSS | -- | 0.460**/0.434** | 0.097/0.249* |
| Pre-Injection VLSQ-8 | 0.460**/0.434** | -- | 0.346**/0.403** |
| Pre-Injection DEQ-5 | 0.097/0.249* | 0.346**/0.403** | -- |
| Baseline Phenol Red Thread of Worst Eye (mm) | 0.112/0.055 | 0.119/0.150 | -0.225/−0.179 |
All data presented as Pearson r/ Spearman rho. mm=millimeters. MSSS=Migraine symptom severity score. VLSQ-8=Visual Light Sensitivity Questionnaire-8. DEQ-5=Dry Eye Questionnaire-5
p<0.05
p<0.01
We next examined which baseline factors associated with pre-injection VLSQ-8, DEQ-5, and PRT scores. None of the factors listed in Supplementary File 2 were significantly associated with pre-injection VLSQ-8 scores. We found that patients taking prazosin, gabapentin, and topiramate reported higher baseline DEQ-5 scores, mean difference 3.39 (p=0.005), 2.20 (p=0.003) and 2.67 (p=0.032) respectively; as did patients with a history of cataract surgery and insomnia, mean difference 5.72 (p=0.020) and 3.28 (p=0.003). We found that patients with fibromyalgia and patients taking antipsychotic medications had lower pre-injection PRT results, mean differences 4.80 (p=0.007) and 3.52 (p=0.047) respectively.
Clinical course:
62 (86.1%) of individuals were included in post-injection survey analysis and all but 1 individual completed all post-surveys. Mean follow-up time was 30.5 days (SD 7.65), range 19–56 days. Photophobia scores, as measured by both the VLSQ-8 and interictal photophobia NRS, significantly improved following BoNT-A injections (p<0.05). Question by question analysis of the VLSQ-8 demonstrated significant improvement in questions 2, 3, and 4, questions that quantify the frequency and severity of photophobia symptoms. (Table 2)
Table 2:
Comparison of Visual Light Sensitivity Questionnaire-8 (VLSQ-8) Pre/Post Botulinum Toxin A Injection
| Pre-injection, mean (SD) | Post-injection, mean (SD) | Mean ∆ (SD) | 95% CI of ∆ | p-value | |
|---|---|---|---|---|---|
| Total VLSQ-8 | 29.8 (5.13) | 27.7 (6.45) | −2.08 (5.12) | [−3.39, −0.77] | 0.002 |
| Q1. How often do you have light sensitivity outdoors during daylight? | 3.82 (0.96) | 3.69 (1.10) | −0.13 (1.01) | [−0.39, 0.13] | 0.314 |
| Q2. How often did you have a sense of glare in your eyes? | 3.44 (0.86) | 2.87 (1.27) | −0.57 (1.29) | [−0.89, −0.24] | 0.001 |
| Q3. How often did you have light sensitivity from flickering lights or bright colors? | 3.71 (1.01) | 3.08 (1.30) | −0.63 (1.65) | [−1.05, −0.21] | 0.004 |
| Q4. Rate the severity of worst light sensitivity in the past month? | 4.19 (0.88) | 3.73 (1.00) | −0.47 (1.04) | [−0.73, −0.21] | 0.001 |
| Q5. When sensitive to light, do you also experience headache? | 3.97 (0.86) | 3.75 (1.25) | −0.21 (1.20) | [−0.52, 0.09] | 0.170 |
| Q6. When you have sensitivity to light, how often is your vision blurry? | 3.39 (0.95) | 3.28 (1.29) | −0.12 (1.20) | [−0.42, 0.19] | 0.452 |
| Q7. How often does sensitivity limit ability to read, watch TV, or use a computer? | 3.64 (0.91) | 3.72 (1.19) | 0.08 (1.23) | [−0.23, 0.40] | 0.604 |
| Q8. How often did you need to wear dark glasses on cloudy days or indoors? | 3.75 (1.16) | 3.59 (1.42) | −0.16 (0.95) | [−0.41, 0.08] | 0.184 |
| Interictal Photophobia | 4.89 (2.97) | 3.37 (2.54) | −1.52 (2.95) | [−2.26, −0.77] | <0.001 |
The above questions are abbreviated for display purposes. See reference 10 for full survey. VLSQ-8 scale 8 to 40. Interictal Photophobia Scale 0 to 10. SD=standard deviation.
Additionally, individuals with more severe dry eye symptoms (DEQ-5 score ≥12) reported statistically significant improvements in their dry eye symptoms at follow up. (Table 3)
Table 3:
Dry Eye Questionnaire-5 (DEQ-5) Pre/Post Botulinum Toxin A Injection
| Before injection, mean (SD) | After injection, mean (SD) | Mean ∆ (SD) | 95% CI of ∆ | p-value | |
|---|---|---|---|---|---|
| Any Dry Eye Symptoms† n=60 | |||||
| DEQ-5 | 13.0 (3.84) | 12.3 (4.39) | −0.68 (4.09) | [−1.74, 0.37] | 0.200 |
| Mild-Moderate Dry Eye Symptoms† n=22 | |||||
| DEQ-5 | 8.86 (1.64) | 9.77 (3.90) | 0.91 (3.19) | [−0.51, 2.32] | 0.196 |
| Severe Dry Eye Symptoms† n=38 | |||||
| DEQ-5 | 15.4 (2.47) | 13.8 (4.02) | −1.61 (4.30) | [−3.02, −0.19] | 0.027 |
Dry eye symptoms defined as DEQ-5 score ≥6. Mild-moderate dry eye symptoms defined as DEQ-5 score 6 to 11. Severe dry eye symptoms defined as DEQ-5 score≥12. SD=standard deviation. CI=Confidence interval. ∆=change in.
Lastly, improvements in MSSS, VLSQ-8, and DEQ-5 were significantly associated; meaning, individuals demonstrating improvements in photophobia were also more likely to demonstrate improvements in dry eye symptoms. Improvements in MSSS, VLSQ-8, and DEQ-5 were not significantly associated with pre-injection PRT testing. (Table 4)
Table 4:
Correlations in symptom improvement with BoNT-A injections
| Difference in MSSS r/rho | Difference in VLSQ-8 r/rho | Difference in DEQ-5 r/rho | |
|---|---|---|---|
| Difference in MSSS | -- | 0.256*/0.236 | 0.262*/0.280* |
| Difference in VLSQ-8 | 0.256*/0.236 | -- | 0.302*/0.341** |
| Difference in DEQ-5 | 0.262*/0.280* | 0.302*/0.341** | -- |
| Baseline Phenol Red Thread of Worst Eye (mm) | 0.209/0.192 | 0.201/0.219 | 0.083/0.086 |
All data presented as Pearson r/ Spearman rho. mm=millimeters; MSSS=Migraine Symptom Severity Score. VLSQ-8=Visual Light Sensitivity Questionnaire-8. DEQ-5=Dry Eye Questionnaire-5
p<0.05
p<0.01
Predictors of Response:
All factors listed in Supplementary File 2 were examined by logistic regression analysis to evaluate which predicted a positive response to BoNT-A with respect photophobia, as defined by at least a 2-categorical improvement in any one question of the VLSQ-8, and with respect to dry eye symptoms, defined by at least a 3-point decrease in DEQ-5 scores. Concomitant use of artificial tears decreased the likelihood of improvement in photophobia, OR 0.18, 95% CI [0.05, 0.69], p=0.012. In those with mild or greater dry eye symptoms (DEQ-5 ≥6), more severe dry eye symptoms predicted improved dry eye symptoms, OR 1.27, 95% CI [1.06, 1.51], p<0.01. (Supplementary File 3) No other factors listed in Supplementary File 2 (including PRT, history of cataract or refractive surgery, contact lens use, depression, anxiety, sleep apnea, insomnia, diabetes, or migraine medication) were found to significantly predict improvement of photophobia or dry eye symptoms. To evaluate the effect of time from injection on treatment response, we binned individuals into two groups by follow up time (14–28 days versus 29–56 days) and found no difference in response frequency by these time points.
DISCUSSION:
Migraine and dry eye symptoms adversely affect millions of Americans and place substantial pressure on healthcare professionals to improve disease co-morbidities, particularly photophobia. Several studies have found that patients with migraine are more likely to report dry eye symptoms than controls13–15 and that severity of dry eye symptoms correlates with migraine severity.8 Interestingly, some studies have reported that dry eye symptoms correlate with dry eye signs, such as decreased tear break-up times and reduced Schirmer’s testing,14,15 while others have not found such significant differences.13 Despite mixed evidence, these two disease entities are clearly associated with one another. In this study, we found that BoNT-A significantly improved photophobia in patients treated for migraine pain. We also found that dry eye symptoms improved in those with severe symptoms at baseline. Both improvements occurred independent of baseline tear volume. Taken together, these findings support our hypothesis that photophobia and dry eye symptom improvement following BoNT-A injections occurs due to a modulation of trigeminal neural pathways.
Migraine is hypothesized to originate from activation of the trigeminal-vascular system via nociceptive C- and Aδ-fibers innervating the meninges and dural blood vessels that, when triggered during migraine, release neuroinflammatory substances, including calcitonin gene-related peptide (CGRP).2 Activation of first-order afferent neurons transmit nociceptive signals through the ophthalmic division of the trigeminal nerve (V1) before synapsing in the trigeminal nucleus caudalis (TNC) and its cervical extension, collectively known as the trigeminal cervical complex (TCC).2 Second-order neurons originating in the TCC synapse in the ventro-posteromedial, posterior and lateral posterior thalamic nuclei. Third order neurons then terminate in the somatosensory cortex producing conscious sensations of pain and a variety of nociceptive responses.2,16 (Figure 1)
Figure 1: Hypothesized pathway for the association of migraine, photophobia, dry eye and the effects of botulinum toxin A (BoNT-A).
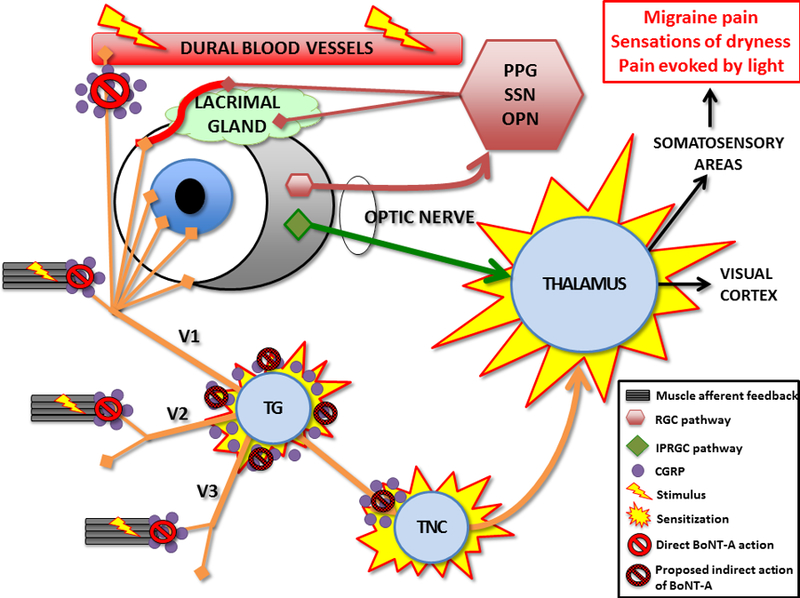
Sensations of migraine, photophobia, and dryness are all related to abnormalities in trigeminal nerve pathways. Various stimuli (inflammation, light, ocular surface disruption) can stimulate primary afferents within the ophthalmic division of the trigeminal nerve (V1). Primary afferents then synapse at the trigeminal nucleus caudalis (TNC) and later posterior thalamus before giving off somatosensory feelings of pain. Migraine originates from activation of nociceptive C- and Aδ-fibers innervating the meninges and dural blood vessels that, when activated, release neuroinflammatory substances, including calcitonin gene-related peptide (CGRP). Photophobia originates from two main circuits (1) retinal ganglion cells (RGCs) that act via parasympathetic outflow to ocular blood vessels and the lacrimal gland (pink lines), and (2) intrinsically photosensitive retinal ganglion cells (ipRGCs) that project directly to the posterior thalamus (green line). Sensations of dryness originate from corneal peripheral afferents that also synapse in the TNC. Chronic peripheral stimulation of these neural networks may lead to central sensitization. BoNT-A inhibits muscle contraction, unmyelinated C-fibers activation, and CGRP release peripheral and centrally at the TNC; leading to reduced peripheral stimulation and, overtime, potentially improved nerve function. Figure design adapted from references: 7, 17, 20, 23, and 25.
CGRP=Calcitonin gene-related peptide; OPN=olivary pretectal nucleus; SSN=superior salivatory nucleus; PPG=pterygopalatine ganglion; TG=trigeminal ganglion; TNC=trigeminal nucleus caudalis
The above-described pathway is also critical in photophobia17 and dry eye.3 Three pathways of photophobia have been documented; two of which are well described.7 The first pathway involves retinal ganglion cells (RGCs). RGC impulses travel to the olivary pretectal nucleus (OPN), superior salivatory nucleus (SuS), and pterygopalatine (PPG) before exerting a parasympathetic vasodilatory effect on ocular blood vessels (Figure 1). This nociceptive input is transmitted by V1 to the TNC, posterior thalamus, and to higher centers, including the cortex.7 A reflex circuit between the SuS and TNC has also been identified further strengthening this relationship.18 The second pathway is mediated by melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs).19 These signals are processed directly in the posterior thalamus, an important site of convergence of multimodal nociceptive signaling, sensitization, and signal amplication.20 Regarding dry eye, ipRGCs have been shown to play a role in corneal mechanosensitivity, particularly when there is ocular surface disruption.21 These findings suggest a complex interplay between the neural circuits of migraine pain, photophobia, and corneal nociception at the TNC and posterior thalamus that helps explain the associations between migraine pain, photophobia and dry eye symptoms seen in ours and other studies.8,13
CGRP may be the unifying mediator that underlies these diverse sensations. CGRP is a well-studied contributor to migraine pain2 and photophbia,22 and there is biologic plausibility that CGRP is involved in propagating chronic dry eye symptoms.3 Within the trigeminal neuronal network, CGRP is released from the afferent nerve terminals (e.g. corneal, meninges), the efferent nerve terminals, and cell bodies located within the trigeminal ganglion.23 Hyperstimulation of peripheral nociceptors, from a multitude of insults, including ocular surface disruption, leads to the release of CGRP and other inflammatory mediators that sensitizes peripheral and central neurons. Sensitized neurons can cause spontaneous unpleasant sensations from normally innocuous inputs (i.e. allodynia, which includes photophobia), hyperalgesia, and receptive field expansion.2,24 These features are hallmarks of central sensitization. With central sensitization, migraine pain, and dry eye symptoms can occur independent of peripheral pathology, as seen in our study.2,4,5
A major novel finding in our study is that BoNT-A significantly improves photophobia in patients being treated for migraine pain and also improves dry eye symptoms in patients with severe symptoms at baseline, independent of tear film volume at baseline. There are two potential explanations for these effects, neither mutually exclusive. First, BoNT-A reduces facial muscle contractions through its actions at the neuromuscular junction thereby decreasing signaling through the primary trigeminal afferents. In support of this notion, persistent muscle fiber activation leads to mechanical hyperalgesia and eventual central sensitization via CGRP,23 an effect that is quickly alleviated by BoNT-A injections.25 Second, BoNT-A may decrease the release of inflammatory mediators, such as CGRP, by interfering with calcium/synaptosomal-associated protein 25 (SNAP-25) mediated vesicle release,25,26 and inhibiting unmyelinated C-fiber nociceptors in the meninges.27 Given associations of CGRP with light-aversion22 and ocular surface inflammation,3 we believe that modulation of CGRP is of greatest significance. In both scenarios, decreased peripheral nerve signaling can stabilize the afferent processing system, and with time can indirectly reverse changes associated with both peripheral and central sensitization thereby reducing photophobia and symptoms of dry eye.26 The above mechanisms likely explain the positive effects of BoNT-A in other chronic neuropathic pain syndromes.25
This pathophysiology may explain why individuals with more severe dry eye symptoms were more likely to report an improvement in dry eye symptoms after BoNT-A injections, even after adjusting for artificial tear use, PRT, and all other risk factors for dry eye. We hypothesize that in patients with chronic migraine, severe dry eye symptoms may be a clinical marker of secondary hyperalgesia and a surrogate for the presence of central sensitization. Patients with dry eye symptoms secondary to suspected central sensitization may be more responsive to the neuromodulatory effects of BoNT-A.
Potential limitations to this study include: a unique patient population, reliance on chart review for co-morbid conditions, a variable length of time between last injection and post-injection survey administration, and possibility of active migraine at the time of injection. Furthermore, we did not perform a full ocular surface work-up as we did not have access to a slit-lamp in the neurotoxin clinic; however, other studies have demonstrated a disconnect between the signs and symptoms of dry eye in patients with chronic migraine.13 Additionally, the VLSQ-8 has not been specifically evaluated for use in chronic migraine patients. Finally, improvements in dry eye symptoms were marginal, although these differences were statistically significant. These limitations inhibit our ability to generalize our findings to patients without CM, and without a control group we cannot prove that our findings do not represent a regression to the mean.
Despite these limitations, we found that photophobia and dry eye symptoms, in this patient population, are not a direct consequence of reduced tear volume. This may be explained by convergence of several neural pathways within the central nervous system. The finding that BoNT-A improved both photophobia and severe dry eye symptoms suggests that other therapies that modulate somatosensory function and neuropathic pain could be considered in patients whose dry eye symptoms are recalcitrant to conventional treatment modalities or whose symptoms cannot be explained by ophthalmic exam findings.
Supplementary Material
Acknowledgments
Financial Support: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006–15S (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant; NIH NIDCR RO1 DE022903 and R21 NS105880 (Dr. Levitt), and the Department of Anesthesiology, Perioperative Medicine, and Pain Management, University of Miami Miller School of Medicine, Miami, FL.
Footnotes
Meeting Presentation: Accepted as an oral presentation at the American Academy of Ophthalmology Annual Meeting, 2018.
Competing interests: No conflicting relationship exists for any author.
Ethics approval: Miami VAMC IRB.
References
- 1.Boulloche N, Denuelle M, Payoux P, et al. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. Journal of neurology, neurosurgery, and psychiatry 2010;81(9):978–84. doi: 10.1136/jnnp.2009.190223 [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiological reviews 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmonte C, Acosta MC, Merayo-Lloves J, et al. What Causes Eye Pain? Current ophthalmology reports 2015;3(2):111–21. doi: 10.1007/s40135-015-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. The British journal of ophthalmology 2015;99(5):665–8. doi: 10.1136/bjophthalmol-2014-306057 [DOI] [PubMed] [Google Scholar]
- 5.Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. The British journal of ophthalmology 2016;100(6):745–9. doi: 10.1136/bjophthalmol-2015-307094 [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. The ocular surface 2015;13(3):250–62. doi: 10.1016/j.jtos.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Survey of ophthalmology 2016;61(4):466–77. doi: 10.1016/j.survophthal.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Diel RJ, Kroeger ZA, Levitt RC, et al. Botulinum Toxin A for the Treatment of Photophobia and Dry Eye. Ophthalmology 2018;125(1):139–40. doi: 10.1016/j.ophtha.2017.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buse D, Manack A, Serrano D, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012;52(1):3–17. doi: 10.1111/j.1526-4610.2011.02046.x [DOI] [PubMed] [Google Scholar]
- 10.Verriotto JD, Gonzalez A, Aguilar MC, et al. New Methods for Quantification of Visual Photosensitivity Threshold and Symptoms. Transl Vis Sci Technol 2017;6(4):18. doi: 10.1167/tvst.6.4.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact lens & anterior eye : the journal of the British Contact Lens Association 2010;33(2):55–60. doi: 10.1016/j.clae.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 12.Vashisht S, Singh S. Evaluation of Phenol Red Thread test versus Schirmer test in dry eyes: A comparative study. International journal of applied & basic medical research 2011;1(1):40–2. doi: 10.4103/2229-516x.81979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinard KI, Smith AG, Singleton JR, et al. Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache 2015;55(4):543–9. doi: 10.1111/head.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarac O, Kosekahya P, Yildiz Tasci Y, et al. The Prevalence of Dry Eye and Sjogren Syndrome in Patients with Migraine. Ocular immunology and inflammation 2016:1–6. doi: 10.3109/09273948.2015.1132739 [DOI] [PubMed] [Google Scholar]
- 15.Koktekir BE, Celik G, Karalezli A, et al. Dry eyes and migraines: is there really a correlation? Cornea 2012;31(12):1414–6. doi: 10.1097/ICO.0b013e318247ec2a [DOI] [PubMed] [Google Scholar]
- 16.Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. Journal of clinical neurology (Seoul, Korea) 2012;8(2):89–99. doi: 10.3988/jcn.2012.8.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Digre KB, Brennan KC. Shedding light on photophobia. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society 2012;32(1):68–81. doi: 10.1097/WNO.0b013e3182474548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto K, Tashiro A, Chang Z, et al. Bright light activates a trigeminal nociceptive pathway. Pain 2010;149(2):235–42. doi: 10.1016/j.pain.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science (New York, NY) 2002;295(5557):1065–70. doi: 10.1126/science.1069609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Current opinion in neurology 2011;24(3):197–202. doi: 10.1097/WCO.0b013e3283466c8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matynia A, Parikh S, Deot N, et al. Light aversion and corneal mechanical sensitivity are altered by intrinscally photosensitive retinal ganglion cells in a mouse model of corneal surface damage. Experimental eye research 2015;137:57–62. doi: 10.1016/j.exer.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 22.Kaiser EA, Kuburas A, Recober A, et al. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012;32(44):15439–49. doi: 10.1523/jneurosci.3265-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durham PL. Diverse Physiological Roles of Calcitonin Gene-Related Peptide in Migraine Pathology: Modulation of Neuronal-Glial-Immune Cells to Promote Peripheral and Central Sensitization. Current pain and headache reports 2016;20(8):48. doi: 10.1007/s11916-016-0578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Annals of neurology 2010;68(1):81–91. doi: 10.1002/ana.21994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler A, Smith HS. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology 2013;306:124–46. doi: 10.1016/j.tox.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 26.Aoki KR, Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism & related disorders 2011;17 Suppl 1:S28–33. doi: 10.1016/j.parkreldis.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 27.Burstein R, Zhang X, Levy D, et al. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia : an international journal of headache 2014;34(11):853–69. doi: 10.1177/0333102414527648 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


