Abstract
INTRODUCTION
Angiogenesis and EGFR signaling are potential therapeutic targets in triple negative breast cancer (TNBC). We hypothesized that targeting these critical pathways may prolong progression-free survival in first-line therapy for metastatic TNBC.
PATIENTS AND METHODS
We conducted a phase II trial of nab-paclitaxel and bevacizumab followed by maintenance therapy with bevacizumab and erlotinib in patients with metastatic TNBC. During induction patients received nab-paclitaxel 100 mg/m2 IV (days 1,8,15) and bevacizumab 10 mg/kg IV (days 1,15) every 28 days for 6 cycles. Patients free of progression at 24 weeks received maintenance therapy with bevacizumab 10 mg/kg IV q2weeks and erlotinib 150 mg PO daily until disease progression. The primary end point was progression free survival (PFS). Secondary endpoints included best overall response, overall survival (OS) and adverse events. Circulating tumor cells (CTCs) were exploratory.
RESULTS
There were 55 evaluable patients enrolled. The median PFS and OS for the cohort was 9.1 months (95% CI=7.2, 11.1) and 18.1 months (95% CI =15.6, 21.7) respectively. Of 53 patients with measurable disease, 39 (74%) patients had a partial response and 10 (19%) had stable disease by RECIST criteria. The majority of toxicities were uncomplicated neutropenia, fatigue, and neuropathy. Decreased CTCs from baseline to first assessment correlated with longer PFS and OS.
CONCLUSION
Nab-paclitaxel and bevacizumab followed by maintenance targeted therapy with bevacizumab and erlotinib showed a PFS similar to other trials. The majority of patients had PR by RECIST (74%). Most patients received maintenance therapy (55%) providing a break from cytotoxic chemotherapy.
Keywords: Triple Negative Breast Cancer, Metastatic, Nab-Paclitaxel Bevacizumab, Erlotinib, Circulating Endothelial Cells, Circulating Tumor Cells
MICROABSTRACT
Chemotherapy remains the mainstay of metastatic triple negative breast cancer treatment, but angiogenesis and EGFR are potential targets. This phase II clinical trial of nab-paclitaxel and bevacizumab followed by maintenance therapy with bevacizumab and erlotinib demonstrated a PFS similar to other regimens. Most patients had a partial response and received maintenance therapy resulting in a significant break from cytotoxic chemotherapy.
INTRODUCTION
Breast cancer is the second leading cause of cancer-related death in women with an estimated 40,920 deaths in 2018 alone.[1] It is widely recognized that patients with breast cancer which expresses the basaloid or “triple negative” phenotype (ER negative, PR negative, HER2 non-overexpressing) have a particularly aggressive form of the disease with hallmarks including younger age of onset, advanced stage at diagnosis, and high rates of metastasis.[1–6] Over the past decade, better molecular characterization of triple negative breast cancer (TNBC) has led to the development of new treatment strategies including DNA damaging agents, PARP inhibitors, androgen receptor blockade, and most recently immunotherapy.[7,8] Despite these advances there are no approved targeted therapies for sporadic TNBC and the mainstay of treatment remains cytotoxic chemotherapy, highlighting the need for more effective treatment options.
Angiogenesis appears to have a central role in the progression of breast cancer and therefore has been studied as a potential targeted therapy for the treatment of metastatic TNBC. [3, 9] The ECOG 2100 trial showed the combination of paclitaxel and bevacizumab was associated with a significant improvement in PFS for patients with HER2 negative disease (11.4 months for paclitaxel and bevacizumab versus 6.11 months for paclitaxel alone HR 0.51, p<0.0001) leading[10] to FDA approval in 2008. During subsequent years numerous trials were done of bevacizumab in combination with other agents which showed only marginal improvements on PFS.[11] The use of bevacizumab has in part been hampered by lack of validated prognostic or predictive biomarkers of response. Variations in Circulating Tumor Cells (CTCs) and Circulating Endothelial Cells (CECs) are promising surrogate markers, but this has yet to be implemented as a clinical tool for predicting response to targeted therapies.[12, 13]
Another common alteration in TNBC is the overexpression of EGFR.[14–16] Overexpression of EGFR is associated in chemo-resistance, large tumor size, and poor prognosis.[17, 18] Many anti-EGFR therapies are already used for cancer treatment including non-small cell lung cancer (NSCLC) and colorectal cancer (CRC); EGFR is another attractive target for TNBC treatment.[19–21] We hypothesized that leveraging these pathways in combination (bevacizumab targeting angiogenesis and erlotinib directed against EGFR) may offer a novel treatment strategy for patients with metastatic TNBC and importantly, provide a treatment option that would spare patients from the toxicity of uninterrupted cytotoxic chemotherapy. We also hypothesized that decreases in level of CTCs may predict longer time to disease progression and increases in apoptotic CECs may be predictive of response to chemotherapy and bevacizumab.
The most commonly studied agents in combination with bevacizumab have been taxanes. Nab-pclitaxel has demonstrated activity in metastatic breast cancer similar to other taxanes (ref 22). Additionally, when administered weekly, nab-paclitaxel itself appears to be anti-angiogenic.[23, 24] In contrast, erlotinib combined with chemotherapy appeared to be deleterious for patients with non–small-cell lung cancer. A sequential approach has, therefore, been preferred, with chemotherapy, followed by the small molecule inhibitor.[25] In the present study, we report the safety and efficacy for an “induction phase” of nab-paclitaxel and bevacizumab, followed by a “maintenance phase” with targeted therapies alone of bevacizumab and erlotinib for patients with advanced TNBC and the effect of this treatment on the CTC and CEC levels.
PATIENTS AND METHODS
Patient eligibility
Eligible patients had histologically-confirmed invasive breast cancer that was triple negative (ER negative (≤10%), PR negative (≤10%), HER2 negative by IHC or FISH), were receiving first line therapy for metastatic disease, had either measurable disease by RECIST criteria OR non-measurable disease with rising serum CA15-3 or CA 27.29 or CEA documented by two consecutive measurements taken at least 14 days apart, adequate organ function, were not pregnant, and had no contraindications to bevacizumab. Patients who had recurrent disease within 12 months after completion of adjuvant chemotherapy containing a weekly taxane were excluded or if they had active, untreated CNS metastases. Additional exclusion criteria are included in the supplementary material. All patients signed informed consent prior to any study-related procedures. This trial is registered with ClinicalTrials.gov, NCT0073340.
Drug administration
For the induction phase patients were treated with Nab-paclitaxel 100 mg/m2 on days 1,8,15,21 and bevacizumab 10 mg/kg intravenously on days 1 and 15 for 24 weeks (28 day cycles × 6). In January 2014, Nab-paclitaxel schedule was modified to 100 mg/m2 IV on days 1, 8, and 15 every 28 days to improve tolerance. Eighteen patients were enrolled after this modification. Patients who were free of progression at 24 weeks began maintenance therapy with bevacizumab 10 mg/kg IV Q2 weeks and erlotinib 150 mg PO daily until progression with radiographic assessment every 8 weeks (Figure 1).
Figure 1: Treatment Schema.
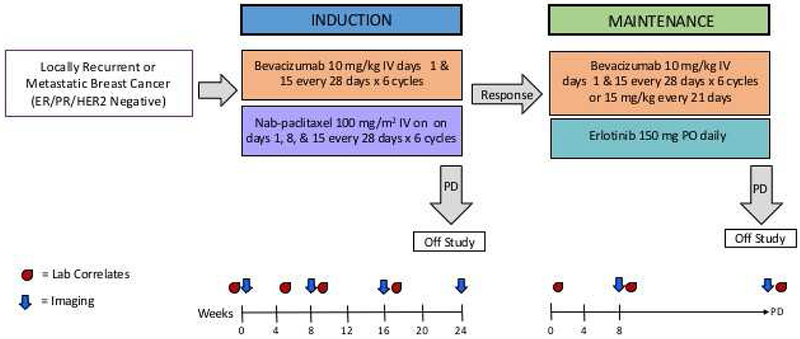
Schema for a Phase II trial of induction therapy with Nab-Paclitaxel and Bevacizumab followed by maintenance targeted therapy with Bevacizumab and Erlotinib, NCT0073340.
Toxicity
Patients were evaluated for adverse events at each study visit for the duration of their participation in the trial and for 30 days after the discontinuation. Vital signs including blood pressure were checked prestudy, every two weeks during induction and maintenance, and in follow-up. Toxicity notation and lab studies (CBC with ANC, platelets, metabolic panel, SGOT/SGPT, Alkaline Phosphatase, and bilirubin) were checked pre-study, monthly during induction and maintenance, and during follow-up. Urinalysis with protein and creatinine was pre-study, every 8 weeks, and in follow-up. For treatment related toxicity and adverse event reporting, the study used the NCI CTC (Common Terminology Criteria) Version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Doses were adjusted or delayed per protocol according to the system showing the greatest degree of treatment related toxicity. Adverse events that meet severity grade 2 or greater were collected and reported.
Clinical Response:
The primary endpoint was PFS defined as the time from date of registration to date of first documentation of progression or symptomatic deterioration or death due to any cause. Secondary endpoints included best overall response in patients with measurable disease, overall survival (OS), and safety and toxicity. Changes in levels of circulating tumor cells (CTCs) and circulating endothelial cells (CECs) as potential predictors of treatment response were exploratory endpoints. Patients were evaluated with baseline staging scans performed pre-study, every 8 weeks during induction and maintenance therapy, and in follow-up. The response was evaluated among patients with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 with central review. Patients provided consent and review of medical records was used to confirm date of death and last contact.
CTC and CEC Counts
Two whole blood samples (one for CTC and one for CEC analysis) were collected using CellSave tubes at each specified time point. Samples were collected prior to the initiation of study treatment (baseline), on weeks 5, 9, and 17 of induction with nab-paclitaxel and bevacizumab treatment, and on weeks 1 and 9 of maintenance treatment with bevacizumab and erlotinib. All samples were obtained prior to infusion of chemotherapy or before taking the first of oral therapy for the interval. The protocol was later modified to include a collection at the time of progression. For patients who did not have a sample collected at the time of progression, the value of the sample collected closest to the time of progression was assigned for the purpose of data analysis. After collection, the tubes were inverted a minimum of 8 times to ensure proper mixing of additives and were stored at ambient temperature (between 10-30° C). The standardized CellSearch technique was used which has been well reports in the literature: CTC’s expressing the CTCs expressing the epithelial cell adhesion molecule were stained with 4,2-diamidino-2-phenylindole dihydrochloride (DAPI) (+), cytokeratin 8,18,19 (+) and CD45 (−). CECs expressing CD146 were stained with DAPI (+), CD105 (+) and CD45 (−). CTC and CEC morphology was confirmed in all cases. [12, 26, 27]
Statistical Analysis
The study was planned for a sample size of n=59, with 80% power to detect a median PFS of 13 months as superior to a historical control of 8 months with combination chemotherapy and bevacizumab.[10] Descriptive statistics, such as frequency and percentage for categorical variables, mean, median, range and standard deviation (SD) for continuous variables, were calculated for patient and disease characteristics, treatment completion, reason for stopping, and CTC/CEC measurements. The Kaplan-Meier (KM) method was used to estimate PFS and OS, with censoring at date of last tumor assessment (PFS) or date of last follow up (OS). Median (95% and 90% CI) times of PFS and OS were estimated from KM curves. The log-rank test was used to assess survival differences among subgroups (e.g. time from diagnosis to metastasis > 3 years vs <= 3 years). Grade 3/4 toxicities were summarized by induction and maintenance phase with both number of subjects and number of incidences.
RESULTS
Patient and disease characteristics
From April 2009 through January 2016, a total of 59 patients (safety population) were enrolled from academic center and community network oncology practices and initiated study therapy. Four of these patients failed to complete a single cycle of induction treatment and are not included in efficacy analysis as their progression thought to be related to a failure in initial disease assessment rather than treatment.
Of the 55 in the efficacy population, 53 (96%) had measurable disease and were included in analysis of best overall response. Patient characteristics are summarized in Table 1. All patients enrolled were female and the average age at registration was 54.9 years old (ranging from 33 to 83). Most patients had a high functional status at registration with an ECOG of 0 or 1 (51 patients, 92.8%). Tumor characteristics were taken from metastatic biopsy (if available) or from breast primary tumor. Forty-one (75.5%) patients had received previous (neoadjuvant or adjuvant) chemotherapy whereas 14 (24.5%) where chemotherapy naïve.
Table 1. Patient Characteristics.
Selected patient and tumor characteristics from 55 patients. Treatment sites listed as other included Bend Memorial Clinic (2), Bozeman Deaconess Cancer Center (2), Columbia Basin Hematology Oncology (2), Group Health Cooperative (1), Kadlec Clinic Hematology & Oncology (1), Katmai Oncology Group (1), Skagit Valley Hospital (1), and Spokane Valley Cancer Center (1).
| Overall (N=55) |
|
|---|---|
| Race | |
| American Indian or Alaska Native | 2 (3.64%) |
| Asian | 1 (1.82%) |
| Black or African American | 2 (3.64%) |
| Caucasian or White | 47 (85.5%) |
| Other or Multiple | 1 (1.82%) |
| Unknown | 2 (3.64%) |
| Ethnicity | |
| Hispanic or Latino | 6 (10.9%) |
| Not Hispanic | 47 (85.5%) |
| Unknown | 2 (3.64%) |
| Stage at Diagnosis | |
| I | 6 (10.9%) |
| II | 22 (40%) |
| III | 12 (21.8%) |
| IV | 9 (16.4%) |
| Unknown, < IV | 6 (10.9%) |
| Histology at Diagnosis | |
| Ductal | 47 (85.5%) |
| Lobular | 1 (1.82%) |
| Unknown | 7 (12.7%) |
| ECOG at registration | |
| 0 | 37 (67.3%) |
| 1 | 14 (25.5%) |
| 2 | 1 (1.82%) |
| Unknown | 3 (5.45%) |
| Conversion to TNBC from other histology | |
| Yes | 11 (20.0%) |
| No | 42 (76.4%) |
| Unknown | 2 (3.6%) |
| Prior Chemotherapy | |
| Yes | 41 (74.5%) |
| No | 14 (25.5%) |
| Treatment Site | |
| UWMC/SCCA | 26 (47.2%) |
| Other | 11 (20.0%) |
| Wenatchee Valley Clinic (WVC) | 8 (14.5%) |
| Cascade Cancer Center (CCC) | 4 (7.27%) |
| Providence Alaska Medical Center (PAMC) | 3 (5.45%) |
| SCCA at EvergreenHealth (SCCA-EH) | 3 (5.45%) |
Toxicity profile
During induction there were 58 incidences of grade 3 or higher events that were definitely or probably related to treatment as reported using CTCAE 3.0. There were 9 grade 3 or higher events during maintenance that were definitely or probably related to treatment. Grade 3 or 4 toxicities with 2 or more events are reported in Table 2. During induction these events occurred in 27/59 patients and during maintenance these events occurred in 7/30 patients. Toxicities that were considered possibly related to treatment are reported in Supplemental Table 2. The most common toxicities during the induction phase were neutropenia (15), fatigue (11), neuropathy (7), and leukopenia (5). The most common toxicity during the maintenance phase was rash (3). No grade 5 toxicities were reported. Grade 3 and 4 toxicities were similar to other reports evaluating these agents and we did not observe any new or unexpected toxicities.
Table 2. Toxicity.
Summary of grade 3-4 toxicities during induction (N=59, safety cohort) and maintenance phase (N=30) reported using NCI CTC (Common Terminology Criteria) Version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Toxicities that were either definitely or probably related to study drugs with ≥2 incidences are shown above. The values in the Induction/Maintenance columns indicate the number and the percent of patients that experienced a given toxicity. There were no grade 5 toxicities.
| Grade 3,4 Toxicities | Induction (N=59) #, (% of patients) | Maintenance (N=30) #, (% of patients) |
|---|---|---|
| Anemia | 2 (3.4%) | |
| Dehydration | 2 (3.4%) | 1 (3.3%) |
| Fatigue | 11 (18.6%) | 1 (3.3%) |
| Lymphopenia | 5 (8.5%) | 1 (3.3%) |
| Nail Changes | 2 (3.4%) | |
| Nausea/Vomiting | 2 (3.4%) | |
| Neuropathy | 7 (11.9%) | |
| Neutropenia | 15 (25.4 %) | |
| Pain | 2 (3.4%) | 1 (3.3%) |
| Rash | 3 (10.0%) |
Of the 55 patients, 34 (62%) completed induction therapy, some with dose modifications or holds (Table 3). Thirty of these patients received maintenance therapy for 29-564 days (mean 198 days). Four patients completed induction but did not subsequently receive maintenance therapy (3 beforebecause of progression and 1 by patient choice owing to toxicity). The most common reasons for dose modifications/holds related to toxicity during induction included neutropenia (9), neuropathy (7), fatigue (3) and leukopenia (3). The most common reasons for dose modifications/holds during maintenance related to toxicity included neuropathy (6), fatigue (5), neutropenia (4), and diarrhea (4). Other reasons for dose modifications/holds included anorexia, LFT abnormalities, constipation, rash, anemia, hypertension, hemorrhage, hypotension, infection, muscle weakness, syncope, muscle weakness, nausea/vomiting, dehydration, and weight loss.
Table 3. Treatment Completion.
Summary of Patient Disposition and Reasons for Study Discontinuation in efficacy cohort
| Treatment Summary | N (%) |
|---|---|
| Completed induction without dose modification | 11 (20%) |
| Completed induction with dose modifications or holds | 23 (42%) |
| Off study during induction phase | 21 (38%) |
| Reason for Discontinuation | |
| Progressive Disease (PD) | 40 (73%) |
| Physician Recommendation | 1 (2%) |
| Toxicity | 4 (7%) |
| Patient Choice | 6 (11%) |
| Other | 4 (7%) |
Of the 55 patients, 4 (7%) discontinued treatment because of toxicity in accordance with the protocol guidelines, 6 patients (11%) discontinued study treatment by choice, 1 patient (2%) discontinued due to physician recommendation, and 4 patients (7%) discontinued study treatment for other reasons. The remaining 40 patients were removed from the study because of progressive disease (13 during the induction phase and 27 during the maintenance phase).. Most patients who removed themselves from the study by choice cited quality of life concerns and toxicity as the primary reasons. The adverse effects resulting in termination of study treatment included anorexia, dehydration, fatigue, infection, leukocytosis, nausea/vomiting, neuropathy, neutropenia, constipation, and diarrhea. One patient experience grade 3 congestive heart failure during the maintenance phase (month 16). No serious adverse events related to study therapy were observed.
Survival data
The median PFS for the efficacy cohort was 9.1 months (95%CI=7.2, 11.1, 90% CI=7.3, 9.6) and the median OS was 18.1 months (95%CI =15.6, 21.7, 90% CI=16.3, 21.3) (Figures 2 and 3). No significant difference was seen in patients who had prolonged time from initial diagnosis to metastasis (defined as greater than 3 years) for either PFS or OS (p=0.3 and p=0.47 respectively) (Supplemental Figure 1,2). Additionally, no significant difference was seen in patients who converted to triple negative status from another histopathology during the course of their disease for both PFS and OS (p=0.28 and p=0.55 respectively) (Supplemental Figure 3,4).
Figure 2: Progression Free Survival.
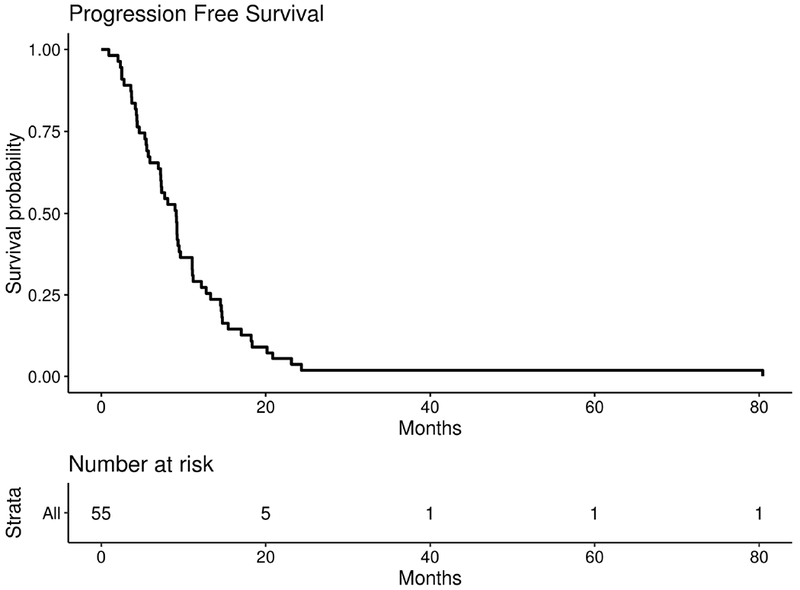
PFS for efficacy cohort (n=55). Median PFS = 9.1 months, 95%CI = (7.2, 11.1), 90%CI = (7.3, 9.6)
Figure 3: Overall Survival.
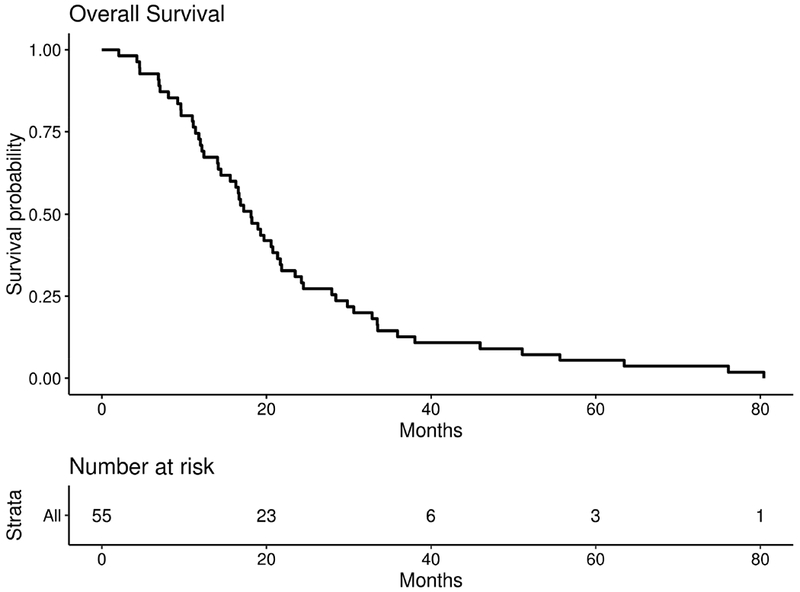
Overall survival for 55 patients with measurable and evaluable disease. Median OS = 18.1 months, 95%CI = (15.6, 21.7), 90%CI = (16.3, 21.3)
Of the 53 patients with measurable disease, 39 (74%) patients had a partial response and 10 (19%) had stable disease by RECIST criteria (Figure 4). Two patients had no response (0%) and were designated baseline (BL). Response was unknown for the remaining 4 patients. No patients had a complete response. The greatest decrease (in the sum of longest diameter of target lesion) was 87%. The PFS for this patient was 469 days and the OS was 907 days. This patient had had stage IIA disease at diagnosis but had not received previous systemic chemotherapy.
Figure 4: Best Response.
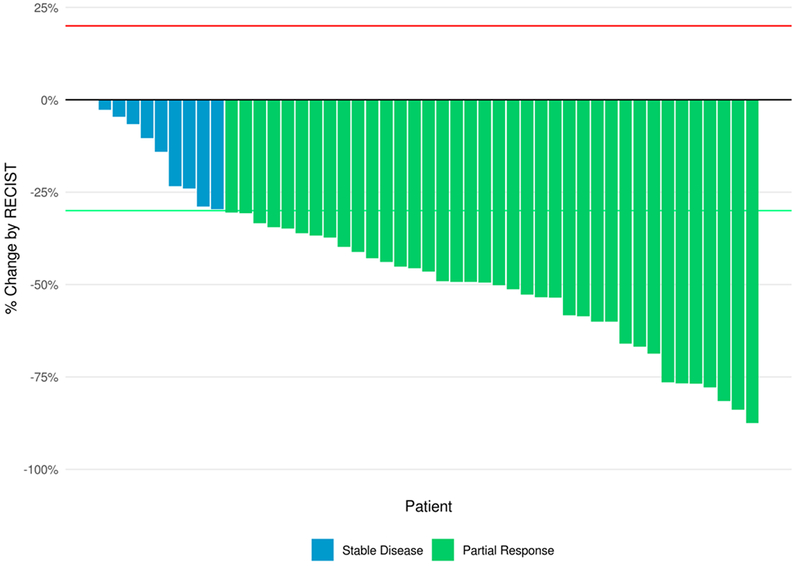
Best Response by RECIST 1.1 (% change in the sum of longest diameters of all target lesions compared to baseline) for patients with measurable disease and follow up assessment, N=47.
There were 3 patients with long term disease control who were on treatment for a long duration with 613, 660, and 740 days between enrollment and progression. The time on maintenance for these patients was 441, 476, and 564 days respectively. The best response by RECIST criteria for these patients was −77%, −60%, and −66% and the OS was 1022 (patient still living), 1156, and 1692 days. One patient had metastatic disease at diagnosis and was chemotherapy naïve. The other two patients had stage 1 and stage IIa disease respectively and had previously received chemotherapy.
Circulating Tumor Cells/Circulating Endothelial Cells
Over the course of the trial CTC and CEC samples were collected from patients at pre-specified time points as an exploratory endpoint as potential predictors of response to treatment. The number of CTCs when averaged at each point showed a general decreasing trend during the course of treatment though the sample size was not consistent (Figure 5). The number of CECs did not substantially change during the course of treatment though similarly the sample size was variable (Figure 5). Based on the SWOG 0500 trial, PFS and OS were analyzed using CTCs at baseline and after 1 cycle of induction chemotherapy (week 5).[28] Three patients from the trial either did not have a baseline or first follow-up CTC level collected and were therefore excluded from analysis. Of the 51 patients with CTCs evaluated at both time points, 28 (55%) did not have elevated CTCs at baseline (with elevated CTCs defined as five or more CTCs per 7.5 mL of whole blood[28]) (Figure 6, Arm A). Of the 23 patients with elevated CTCs at baseline, 16 (70%) had CTC levels that were no longer elevated after 5 weeks of chemotherapy (Arm B) and 7 (30%) had persistent CTC elevation (Arm C). CTC status was associated with both PFS (p=0.0037) and OS (p=0.0019), with a clear disadvantage for patients in Arm C. The median PFS was 9.3, 7.9, and 2.8 months for Arms A, B, and C respectively and the median OS was 19.5, 20.7, and 9.6 months (Figure 6). All 3 of the exceptional responders described above were in Arm A as was the the patient with a best response of −87%.
Figure 5: CTC and CEC concentrations during induction and maintenance.
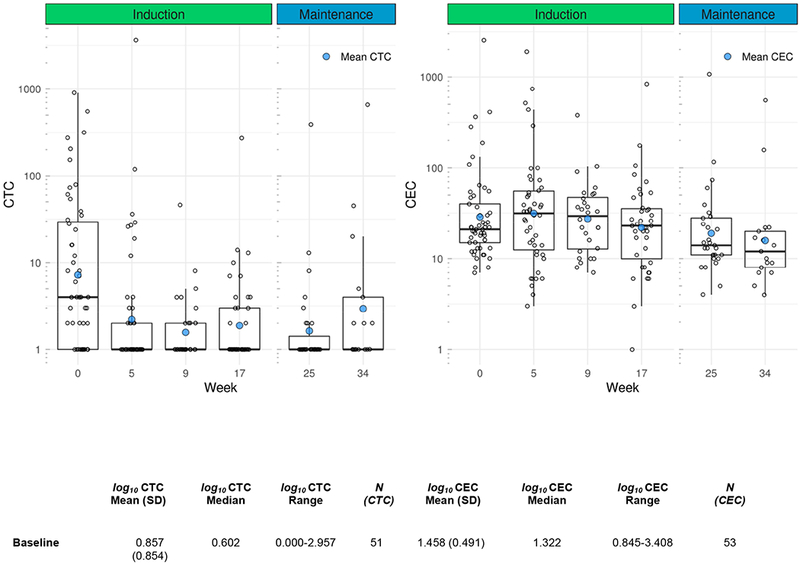
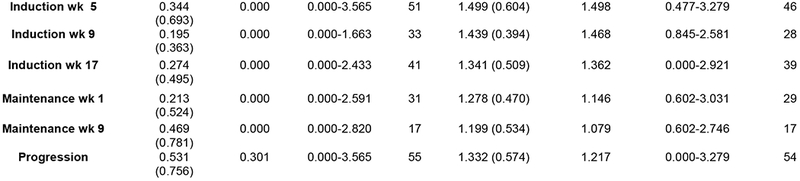
Mean, median, and range for CTC and CEC measurements during the course of treatment.
Figure 6: Progression Free Survival and Overall Survival Based on CTCs at Baseline and in Response to Treatment.
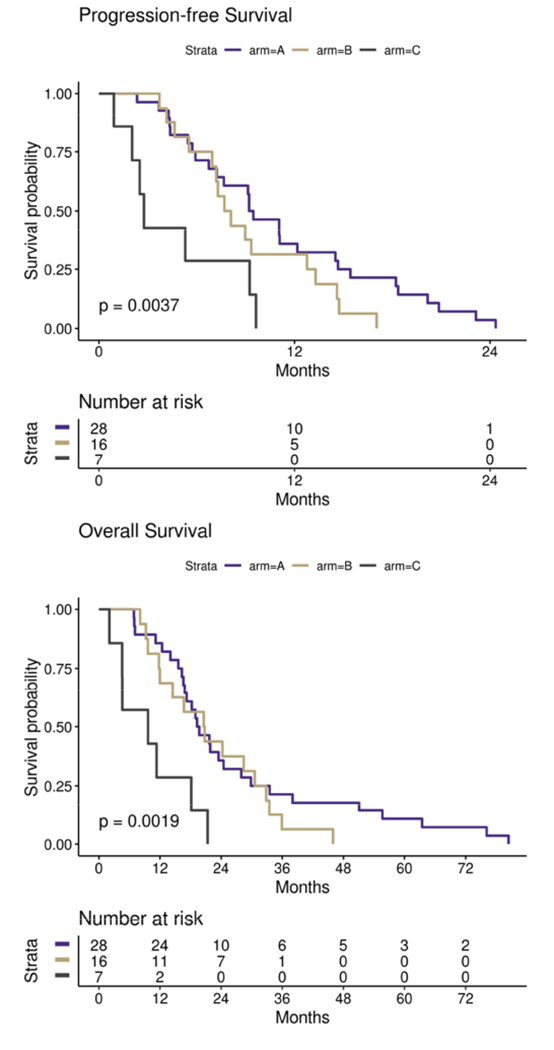
PFS and overall survival (OS) in patients with metastatic TNBC based circulating tumor cell (CTC) levels at baseline and at first follow-up after 5 weeks of treatment with induction nab-paclitaxel and bevacizumab. Arm A represents patients with no CTC elevation at baseline defined as less than five CTCs per 7.5 mL of whole blood(WB), N=28. Arm B represents patients with elevated CTC levels at baseline (defined as five or more CTCs per 7.5 mL WB) but less than five CTCs per 7.5 mL WB at first follow-up, N=16. Arm C represents patients with elevated CTCs at baseline (five or more CTCs per 7.5 mL WB) which remained elevated at first follow-up, N=7.
DISCUSSION:
Overall, we found that Nab-paclitaxel and bevacizumab followed by maintenance targeted therapy with bevacizumab and erlotinib was well tolerated as first-line therapy for metastatic TNBC. The observed median PFS (9.1 months, 95% CI=7.2, 11.1) did not meet pre-specified criteria of interest which was defined as a 60% increase in median PFS from 8 to 13 months. The PFS observed in this trial is similar to other trials which have studied the use of bevacizumab in metastatic TNBC[11]. We hypothesized that patients who took longer to progress to metastatic disease after initial diagnosis and patients who converted to TNBC, may have more favorable disease and therefore improved response. However, no difference in PFS or OS was observed among these subtypes. Even though PFS did not meet the prespecified endpoint, 30 patients were able to be treated with non-cytotoxic maintenance therapy from 29 to 564 days with an average of 198 days providing a substantial break from chemotherapy. Toxicity during this time was less with minimal grade 3 or 4 reactions. Manageable rash was the most common toxicity during maintenance therapy. There was were also 3 exceptional responders who had 613, 660, and 740 days respectively between enrollment and progression. Additionally, the majority of patients did have a partial response (PR) by RECIST criteria (39, 74%) and the best response by RECIST criteria was 87%.
The majority of toxicities were either neutropenia, leukopenia, fatigue, or neuropathy which most commonly occurred during the induction phase. During the study, 4 patients were removed due to toxicity and 5 patients went off study by choice with all but 1 citing quality of life concerns or side effects. A total of 23 (42%) of patients required dose modifications or a hold during the course of treatment. However, no grade 5 toxicities occurred and the toxicities that were observed were similar to other reports of bevacizumab, nab-paclitaxel, and erlotinib. Overall the treatment combination was found to be safe and well tolerated.
Since the promising results of the ECOG 2100 trial, numerous other studies have evaluated bevacizumab in early breast cancer in both adjuvant and neoadjuvant settings, and in advanced breast cancer. Comparable to our findings, these studies have also shown only modest improvements in PFS and no significant change in OS.[29, 30] A systematic review of bevacizumab use in breast cancer by Kumler et all in 2014 evaluated 14 phase III trials which unanimously showed increased RR and PFS, but no OS benefit in any trial.[11] Several groups of investigators have conducted studies also targeting EGFR in breast cancer, especially in TNBC where EGFR is commonly overexpressed; unfortunately, results have been similarly disappointing.[19, 20] Two trials have specifically evaluated combination therapy of bevacizumab and erlotinib. A trial reported by Dickler et al studied erlotinib in combination with bevacizumab in patients with metastatic breast cancer who had previously been treated with chemotherapy and identified no significant difference in median time to progression (11 weeks, 95% confidence interval [CI] 8-18 week).[31] Another study by Montagna et al investigated metronomic chemotherapy in combination with bevacizumab and erlotinib in patients with metastatic breast cancer and showed a response rate of 62%, a PFS of 9.6 months, and OS of 24 months’[32] However, in contrast to our study the majority of patients (55%) were chemotherapy naïve.
Several explanations have been offered for the persistent failure of these therapies despite their targets known roles in metastatic breast cancer. Lack of proper patient selection and resistance mechanisms to anti-angiogenic therapy likely contribute to bevacizumab’s inability to improve OS. For example, FGFR is mutated in up to 10% of breast cancers, but is not routinely assayed and could be driving angiogenesis independently of VEGF.[33, 34]
Additional possibilities exist for the failure of EGFR inhibition in breast cancer. For one, studies suggest that EGFR is involved in regulating epithelial-mesenchymal transition, migration, and tumor invasion and therefore may be more useful in preventing metastasis rather than producing tumor shrinkage due to cell proliferation.[19, 35] As our study focused on patients with known metastatic disease, it may have been too late in the patients’ course to capture this benefit. Additionally, at the time this study was designed erlotinib administered concurrently with chemotherapy appeared deleterious; however, a recent study by Lee at all suggests that EGFR targeted therapy may enhance the sensitivity of TNBC cells to cytotoxic therapy.[36] The chemo-sensitizing benefits of erlotinib would have also been lost during this trial because it was administered during the maintenance rather than induction phase with nab-paclitaxel. Similar to bevacizumab, resistance via alternative signaling pathways likely contributes to failure of erlotinib treatment and in fact, EGFR is known to interact with other tyrosine kinases such as c-MET and IGF-1R which could be involved in resistance [37] Finally, in contrast to malignancies such as non-small cell lung cancer (NSCLC), most TNBC exhibit overexpression of EGFR rather than activating mutations meaning they are not exclusively dependent on EGFR signaling for their survival.[20] Several studies have looked to identify activating mutations in EGFR in breast cancer as these patients might receive improved benefit from TKIs such as erlotinib. Unfortunately, the results are controversial and activating mutations in TNBC appears to be extremely rare.[20, 38]
While the clinical value of anti-VEGF and anti-EGFR targeted therapies remains in dispute for TNBC, the need for biomarkers to identify subsets of patients that might benefit from these agents is unanimous. There are currently no biomarkers to predict which patients are most likely to respond to EGFR inhibitors and unfortunately, the level of EGFR expression has not been predictive of response[19, 31]
For bevacizumab, there is some evidence that CEC and/or CTC levels may be independent predictors of responsiveness. No study has looked at the combined predictive value of CTCs/CECs. Other biomarkers such as VEGF-A level have had not been shown to be predictive of response.[39] While the available literature regarding CECs have been inconsistent regarding the utility of this measure in part due to numerous technical differences, [12, 13, 40] there are studies to support their continued evaluation. For example, using the same CellSearch platform in this trial, Bidard et al found that increased CEC counts were associated with improved time to progression (threshold of 20 CECs/4 ml, P < 0.01).[12] Calleri et al also found that higher baseline CECs were associated with an increased time to progression (P = 0.021) and in this study CECs were markedly reduced at the time of progression (P = 0.0002).[40] In our study, There was not a substantial change in the number of CECs during treatment and CECs could not be correlated with response to treatment though analysis was limited due to missing samples.
The results for CTCs have been more promising. Cristofanilli et al showed that the number of CTCs before treatment is an independent predictor of PFS and OS in patients with metastatic breast cancer.[41] In the SWOG 0500 trial Smerage et all showed that patient with metastatic breast cancer who had increased CTCs at baseline (defined as 5 or more per 7.5 mL of whole blood) that persisted after 21 days of first line chemotherapy had a significantly worse prognosis compared to those who either responded to treatment or did not have elevated CTCs at baseline.[28] Our study did show a general downward trend in the number of CTCs during treatment. Similarly, to the study by Smerage et al, our trial showed that patients with elevated CTCs at baseline that remained elevated at first follow-up after 5-weeks induction therapy (Arm C) had significantly worse PFS and OS compared to patients who either did not have elevated CTCs at baseline (Arm A) or whose CTCs were no longer elevated at first follow-up (Arm B). Interestingly, all exceptional responders to study treatment were in Arm A. While no biomarker has yet been implemented clinically, this data highlights the promise of using biomarkers to identify which patients will respond best to certain therapies including targeted agents.
CONCLUSION:
Induction therapy with Nab-Paclitaxel and bevacizumab followed by maintenance therapy with bevacizumab and erlotinib was found to be safe and well tolerated, but efficacy was limited. While this study did not show a significant increase in PFS or OS, most patients showed a partial response and received a substantial break from chemotherapy toxicity. There were several exceptional responders who remained on maintenance therapy for well over a year and therefore received a significant holiday from cytotoxic chemotherapy. This adds to the growing body of evidence that challenges the use of both bevacizumab and erlotinib in TBNC treatment. However, as studies have shown some degree of tumor response for bevacizumab, it remains a part of the discussion for TBNC treatment strategies[42] and with the advent of immunotherapy there has been renewed interest in the possibility of combination strategies. For both bevacizumab and erlotinib, failure to identify patients who are more likely to benefit from anti-VEGF and anti-EGFR therapy has hindered development of these drugs in breast cancer. To date, no biomarker has been identified to reliably follow patient response to bevacizumab or erlotinib. Survivability of advanced TNBC remains poor underscoring the need for novel therapies and the need to identify which patients would benefit from these treatments.
Supplementary Material
CLINICAL PRACTICE POINTS.
Triple negative breast cancer remains an aggressive subtype with poor prognosis and despite extensive clinical research the mainstay of treatment remains cytotoxic chemotherapy. [43]
Angiogenesis and overexpression of EGFR have both been identified as potential targets in TNBC.[3, 14, 16]
This phase II clinical trial showed that induction therapy with nab-paclitaxel and bevacizumab followed by maintenance targeted therapy with bevacizumab and erlotinib was safe and well tolerated in patients with metastatic TNBC. While PFS did not meet the pre-specified criteria of interest, it was similar to comparable trials. Additionally, the majority of patients had a partial response by RECIST and most received maintenance therapy providing a break from cytotoxic chemotherapy.
There were several exceptional responders who were on maintenance therapy well over a year highlighting the need for biomarkers to better identify which patients will benefit from these targeted therapies.
Circulating tumor cells (CTCs) were found to be prognostic; patients with persistently elevated CTCs following 1 cycle of induction chemotherapy had a significantly worse prognosis compared to those who either had a decrease in CTCs following cycle 1 of induction or did not have elevated CTCs at baseline.
ACKNOWLEDGEMENTS
Funding: This project was supported by Genentech (OSI4266s), Celegene (AX-CL-BRST-PI-003828) and Janssen. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA015704 and supported by P30CA047904 (Biostatistics).
We thank the patients who participated in this trial and their families, the Seattle Cancer Care Alliance Network Physicians and Research Staff, Tove Thompson for support of UW Breast Oncology Research Program, Barbara Buening, Arianne Cundy, and the late Dr. Robert B. Livingston for mentorship in trial design, clinical research and patient care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors of have no conflicts of interest to disclose.
REFERENCES:
- 1.SR L, MK D, Ahmedin J: Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018, 68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al. : Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences 2001, 98(19):10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribatti D, Nico B, Ruggieri S, Tamma R, Simone G, Mangia A: Angiogenesis and Antiangiogenesis in Triple-Negative Breast cancer. Translational Oncology 2016, 9(5):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irvin WJ, Carey LA: What is triple-negative breast cancer? European Journal of Cancer 2008, 44(18):2799–2805. [DOI] [PubMed] [Google Scholar]
- 5.Foulkes WD, Smith IE, Reis-Filho JS: Triple-Negative Breast Cancer. New England Journal of Medicine 2010, 363(20):1938–1948. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani SR, Vijver MJvd, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF: The Pathology of Familial Breast Cancer: Predictive Value of Immunohistochemical Markers Estrogen Receptor, Progesterone Receptor, HER-2, and p53 in Patients With Mutations in BRCA1 and BRCA2. Journal of Clinical Oncology 2002, 20(9):2310–2318. [DOI] [PubMed] [Google Scholar]
- 7.Collignon J, Lousberg L, Schroeder H, Jerusalem G: Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer : Targets and Therapy 2016, 8:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L: Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016, 13(11):674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkman J: What Is the Evidence That Tumors Are Angiogenesis Dependent? JNCI: Journal of the National Cancer Institute 1990, 82(1):4–7. [DOI] [PubMed] [Google Scholar]
- 10.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE: Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. New England Journal of Medicine 2007, 357(26):2666–2676. [DOI] [PubMed] [Google Scholar]
- 11.Kümler I, Christiansen OG, Nielsen DL: A systematic review of bevacizumab efficacy in breast cancer. Cancer Treatment Reviews 2014, 40(8):960–973. [DOI] [PubMed] [Google Scholar]
- 12.Bidard FC, Mathiot C, Degeorges A, Etienne-Grimaldi MC, Delva R, Pivot X, Veyret C, Bergougnoux L, de Cremoux P, Milano G et al. : Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Annals of Oncology 2010, 21(9):1765–1771. [DOI] [PubMed] [Google Scholar]
- 13.Strijbos MH, Gratama JW, Kraan J, Lamers CH, Bakker MAd, Sleijfer S: Circulating endothelial cells in oncology: pitfalls and promises. British Journal of Cancer 2008, 98(11):1731–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burness ML, Grushko TA, Olopade OI: Epidermal Growth Factor Receptor in Triple-Negative and Basal-Like Breast Cancer: Promising Clinical Target or Only a Marker? The Cancer Journal 2010, 16(1):23–32. [DOI] [PubMed] [Google Scholar]
- 15.Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO: Prognostic markers in triple-negative breast cancer. Cancer 2007, 109(1):25–32. [DOI] [PubMed] [Google Scholar]
- 16.Siziopikou KP, Ariga R, Proussaloglou KE, Gattuso P, Cobleigh M: The Challenging Estrogen Receptor-Negative/ Progesterone Receptor-Negative/HER-2-Negative Patient: A Promising Candidate for Epidermal Growth Factor Receptor-Targeted Therapy? The Breast Journal 2006, 12(4):360–362. [DOI] [PubMed] [Google Scholar]
- 17.Salomon DS, Brandt R, Ciardiello F, Normanno N: Epidermal growth factor-related peptides and their receptors in human malignancies. Critical Reviews in Oncology / Hematology, 19(3):183–232. [DOI] [PubMed] [Google Scholar]
- 18.Richard J, Sainsbury C, Needham G, Farndon J, Malcolm A, Harris A: EPIDERMALGROWTH-FACTOR RECEPTOR STATUS AS PREDICTOR OF EARLY RECURRENCE OF AND DEATH FROM BREAST CANCER. The Lancet, 329(8547):1398–1402. [DOI] [PubMed] [Google Scholar]
- 19.Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT: Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast cancer research and treatment 2012, 136(2): 10.1007/s10549-10012-12289-10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai K, Hung M-C, Yamaguchi H: A perspective on anti-EGFR therapies targeting triple-negative breast cancer. American Journal of Cancer Research 2016, 6(8):1609–1623. [PMC free article] [PubMed] [Google Scholar]
- 21.Rusch V, Mendelsohn J, Dmitrovsky E: The epidermal growth factor receptor and its ligands as therapeutic targets in human tumors. Cytokine & Growth Factor Reviews 1996, 7(2):133–141. [DOI] [PubMed] [Google Scholar]
- 22.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J: Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. Journal of Clinical Oncology 2005, 23(31):7794–7803. [DOI] [PubMed] [Google Scholar]
- 23.Ng SSW, Sparreboom A, Shaked Y, Lee C, Man S, Desai N, Soon-Shiong P, Figg WD, Kerbel RS: Influence of Formulation Vehicle on Metronomic Taxane Chemotherapy: Albumin-Bound versus Cremophor EL–Based Paclitaxel. Clinical Cancer Research 2006, 12(14):4331–4338. [DOI] [PubMed] [Google Scholar]
- 24.Robidoux A, Buzdar AU, Quinaux E, Jacobs S, Rastogi P, Fourchotte V, Younan RJ,Pajon ER, Shalaby IA, Desai AM et al. : A Phase II Neoadjuvant Trial of Sequential Nanoparticle Albumin-Bound Paclitaxel Followed by 5-Fluorouracil/Epirubicin/Cyclophosphamide in Locally Advanced Breast Cancer. Clinical Breast Cancer, 10(1):81–86. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X et al. : TRIBUTE: A Phase III Trial of Erlotinib Hydrochloride (OSI-774) Combined With Carboplatin and Paclitaxel Chemotherapy in Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 2005, 23(25):5892–5899. [DOI] [PubMed] [Google Scholar]
- 26.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW, Terstappen LWMM: Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases. Clinical Cancer Research 2004, 10(20):6897–6904. [DOI] [PubMed] [Google Scholar]
- 27.Rowand JL, Martin G, Doyle GV, Miller MC, Pierce MS, Connelly MC, Rao C, Terstappen LWMM: Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry Part A 2007, 71A(2):105–113. [DOI] [PubMed] [Google Scholar]
- 28.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O’Rourke MA, Lew DL et al. : Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. Journal of Clinical Oncology 2014, 32(31):3483–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SYT, Zhou X et al. : RIBBON-1: Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Chemotherapy With or Without Bevacizumab for First-Line Treatment of Human Epidermal Growth Factor Receptor 2–Negative, Locally Recurrent or Metastatic Breast Cancer. Journal of Clinical Oncology 2011, 29(10):1252–1260. [DOI] [PubMed] [Google Scholar]
- 30.Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS: RIBBON-2: A Randomized, Double-Blind, Placebo-Controlled, Phase III Trial Evaluating the Efficacy and Safety of Bevacizumab in Combination With Chemotherapy for Second-Line Treatment of Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer. Journal of Clinical Oncology 2011, 29(32):4286–4293. [DOI] [PubMed] [Google Scholar]
- 31.Dickler MN, Rugo HS, Eberle CA, Brogi E, Caravelli JF, Panageas KS, Boyd J, Yeh B, Lake DE, Dang CT et al. : A Phase II Trial of Erlotinib in Combination with Bevacizumab in Patients with Metastatic Breast Cancer. Clinical Cancer Research 2008, 14(23):7878–7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montagna E, Cancello G, Bagnardi V, Pastrello D, Dellapasqua S, Perri G, Viale G, Veronesi P, Luini A, Intra M et al. : Metronomic Chemotherapy Combined With Bevacizumab and Erlotinib in Patients With Metastatic HER2-Negative Breast Cancer: Clinical and Biological Activity. Clinical Breast Cancer, 12(3):207–214. [DOI] [PubMed] [Google Scholar]
- 33.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, Natrajan R, Marchio C, Iorns E, Mackay A et al. : <em>FGFR1</em> Amplification Drives Endocrine Therapy Resistance and Is a Therapeutic Target in Breast Cancer. Cancer Research 2010, 70(5):2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santa-Maria CA, Gradishar WJ: Changing treatment paradigms in metastatic breast cancer: Lessons learned. JAMA Oncology 2015, 1(4):528–534. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, Lucci A, Singh B, Hung M-C, Hortobagyi GN et al. : Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Reverses Mesenchymal to Epithelial Phenotype and Inhibits Metastasis in Inflammatory Breast Cancer. Clinical Cancer Research 2009, 15(21):6639–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Michael J, Ye Albert S, Gardino Alexandra K, Heijink Anne M, Sorger Peter K, MacBeath G, Yaffe Michael B: Sequential Application of Anticancer Drugs Enhances Cell Death by Rewiring Apoptotic Signaling Networks. Cell, 149(4):780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, Iwata KK, Gibson NW, Cagnoni P, Haley JD: Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Molecular Cancer Therapeutics 2007, 6(2):532–541. [DOI] [PubMed] [Google Scholar]
- 38.Kim A, Jang MH, Lee SJ, Bae YK: Mutations of the Epidermal Growth Factor Receptor Gene in Triple-Negative Breast Cancer. J Breast Cancer 2017, 20(2):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im S-A, Canon JL, Shparyk Y, Yardley DA et al. : Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): A double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. European Journal of Cancer, 70:146–155. [DOI] [PubMed] [Google Scholar]
- 40.Calleri A, Bono A, Bagnardi V, Quarna J, Mancuso P, Rabascio C, Dellapasqua S, Campagnoli E, Shaked Y, Goldhirsch A et al. : Predictive Potential of Angiogenic Growth Factors and Circulating Endothelial Cells in Breast Cancer Patients Receiving Metronomic Chemotherapy Plus Bevacizumab. Clinical Cancer Research 2009, 15(24):7652–7657. [DOI] [PubMed] [Google Scholar]
- 41.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM et al. : Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. New England Journal of Medicine 2004, 351(8):781–791. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Yan H, Zhao P, Yang Y, Cao B: Efficacy and Safety of Bevacizumab Combined with Chemotherapy for Managing Metastatic Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. 2015, 5:15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elias AD: Triple-Negative Breast Cancer: A Short Review. American Journal of Clinical Oncology 2010, 33(6):637–645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


