Abstract
Background:
Option B+ has increased the number of pregnant women initiating antiretroviral therapy for HIV, yet retention in HIV care is sub-optimal. Retention may be affected by antenatal depression. However, few data exist on antenatal depression in this population.
Aim:
Describe the prevalence and factors associated with antenatal depression among Malawian women enrolled in Option B+.
Method:
At their first antenatal visit, women with HIV provided demographic and psychosocial information, including depression as measured with the locally validated Edinburgh Postnatal Depression Scale (EPDS). Prevalence ratios (PR) for factors associated with probable depression (EPDS ≥6) were estimated with log binomial regression.
Results:
9.5% (95%CI 7.5-11.9%) of women screened positive for current depression, and 46% self-reported a history of depression or anxiety. Women were more likely to screen positive for current depression if they reported a history of depression (adjusted PR 2.42; 95%CI 1.48-3.95) or had ever experienced intimate partner violence (1.77; 1.11-2.81). Having an unintended current pregnancy (1.78; 0.99-3.21), being unmarried (1.66; 0.97-2.84), or employed (1.56; 1.00-2.44) had potential associations with probable depression.
Conclusions:
Probable antenatal depression affected a notable proportion of women living with HIV, comparable to other global regions. Screening for antenatal depression in HIV care should be considered.
Introduction:
Antenatal depression is common worldwide, affecting over one in ten pregnant women (Gaynes et al., 2005). Antenatal depression strongly predicts postpartum depression (Lancaster et al., 2010), and is associated with poor outcomes for both mothers and infants. For mothers, consequences include impaired functioning, poor quality of life, or death due to suicide (Aaron, Bonacquisti, Geller, & Polansky, 2015). Infants born to mothers with untreated antenatal depression may have increased risk of low birth weight (Evans, Heron, Patel, & Wiles, 2007), preterm birth (Field, Diego, & Hernandez-Reif, 2006; Wisner et al., 2009), and behavioral difficulties (Field et al., 2006).
Predictors of antenatal depression include history of depression or trauma, poor social support, food insecurity, recent stressful life events, intimate partner violence, and unplanned pregnancy (Adewuya, Ola, Aloba, Dada, & Fasoto, 2007; Biaggi, Conroy, Pawlby, & Pariante, 2016; Bisetegn, Mihretie, & Muche, 2016; Bonacquisti et al., 2014; Brittain, Myer, Koen, Koopowitz, & Donald, 2015; Peltzer & Shikwane, 2011). In Africa, lack of support and family/marital conflict are associated with poorer mental health, but evidence regarding sociodemographic and obstetric or medical variables is inconclusive (Sawyer, Ayers, & Smith, 2010). Notably, factors that are unique to or more prominent among HIV positive women, such as disclosure of HIV status or intimate partner violence, have not been thoroughly addressed (Msellati, 2009). Overall, depression may be more common among persons with HIV than the general population and depression negatively affects engagement in care, including retention in care, antiretroviral therapy (ART) adherence, and viral suppression (Beer & Skarbinski, 2014; Chibanda, Benjamin, Weiss, & Abas, 2014; Ciesla & Roberts, 2001; Gonzalez, Batchelder, Psaros, & Safren, 2011; Ickovics et al., 2001; Kessler et al., 2003; Leserman, 2008; Lyketsos et al., 1993; Pence, Miller, Gaynes, & Eron, 2007; Whetten et al., 2013). The risk factors for antenatal depression among women living with HIV in sub-Saharan Africa have been underexplored.
Despite the prevalence and consequences of antenatal depression, screening is not routine in many countries regardless of HIV status, including Malawi. Located in south eastern Africa, Malawi has a high prevalence of HIV: 12.9% of women are infected (NationalStatisticalOffice & ICFMacro, 2011). Malawi was the first country to implement the “Option B+” prevention of maternal to child transmission (PMTCT) of HIV program in efforts to reduce the number of perinatal HIV infections. Option B+ is novel because it provides lifelong eligibility for ART to pregnant and breastfeeding women regardless of their immune status – there are no CD4 count or AIDS-defining condition criteria for ART eligibility. While Option B+ has increased the number of women who initiated ART (Chimbwandira, Mhango, Makombe, & Midiani, 2013), retention in HIV care has been suboptimal: approximately 25–30% of women stopped returning to HIV care within 12 months of initiating ART (Haas et al., 2016; Kim et al., 2015; Tenthani et al., 2014). Antenatal depression may affect retention in HIV care, yet the prevalence of antenatal depression or its associated factors among women enrolled in Option B+ is unclear. We describe here the prevalence of and factors associated with probable antenatal depression among pregnant women enrolled in Option B+ HIV care in Malawi.
Materials and Methods:
Study setting and population:
Data are cross-sectional from baseline interviews conducted in 2015–2016 for an observational cohort study (“Safety, Suppression, Second-line, Survival - S4”, ClinicalTrials.gov identifier: NCT02249962) at the Bwaila Family Health Unit, the busiest government antenatal clinic in Lilongwe, Malawi, with over 15,000 deliveries annually. S4’s primary objectives are to evaluate the long-term safety and efficacy of Option B+. All women presenting for antenatal care were offered HIV testing with two rapid tests (Alere Determine™ and Unigold™) per the Malawi standard of care for opt-out HIV testing. Women who tested positive or were known to be HIV positive at their first antenatal care visit were approached to enroll in the S4 study. More women were available for recruitment than S4 could enroll, so nurses approached as many women with an HIV diagnosis as possible each day to describe what participation in S4 would entail, and referred interested women who could not be seen on the same day to return on a subsequent day. Women who chose not to participate or were ineligible received routine antenatal and HIV care through the government clinic.
Women considered eligible for the study were pregnant, at least age 18 years (or 16–17 years and married), planned to give birth in Lilongwe, and able to provide informed consent. S4 study nurses interviewed participants on the day of their first antenatal care visit in rooms adjacent to the antenatal clinic; all interviews were conducted in Chichewa, the predominant local language, with interview guides that had been translated and back-translated by certified professional Chichewa and English speakers, and guides were piloted with a small number of women prior to enrollment activities. Most women initiating ART (tenofovir/lamivudine/efavirenz) were newly diagnosed, but a small portion had a previous positive HIV test but had not yet started ART. Our analyses include women who at the time of study enrollment, were initiating ART through Malawi’s Option B+ program, and those who had been on tenofovir/lamivudine/efavirenz ART for at least 6 months. While an important group, women who had been on ART less than 6 months were not invited to enroll because the parent study was evaluating women for potential need to switch to second line ART, which is only done among women with at least 6 months on ART.
Measures:
Outcome:
Current antenatal depressive symptoms were evaluated with the Edinburgh Postnatal Depression Scale (EPDS). The EPDS is a 10-question instrument designed to identify symptoms of depression among women who are pregnant or postpartum (Gibson, McKenzie-Mcharg, Shakespeare, Price, & Gray, 2009). The EPDS omits physical symptom items featured on other depression screening tools because pregnant women may experience increased fatigue and appetite changes due to pregnancy and not necessarily their depression (Cox, Holden, & Sagovsky, 1987). Additionally, the EPDS features three items about anxiety symptoms, which can be particularly prominent during the perinatal period (Lee et al., 2007). Scores range from 0 to 30. The EPDS has been translated, back translated, and validated in Chichewa among antenatal women (Stewart, Umar, Tomenson, & Creed, 2013). Although typically a threshold of 13 on the EPDS is used to indicate likely major depression, the Malawian validation study recommended a threshold of 6, which had a sensitivity of 76.3% and specificity of 74.1% (Stewart et al., 2013). Thus, we considered women who scored ≥6 on the EPDS to screen positive for current probable antenatal depression.
Potential predictors:
We evaluated demographic, clinical, and psychosocial factors potentially associated with current probable antenatal depression. Demographic variables included age, education attained, and employment status; clinical variables included death of a previous child, ART history, and WHO HIV clinical stage; psychosocial variables included current relationship status, whether the current pregnancy was intended, HIV status disclosure to partner (among women who had been on ART ≥6 months), history of verbal or physical intimate partner violence (IPV; ascertained by asking if the woman’s current partner or partner from current pregnancy had ever yelled at, threatened, or physically hurt the woman), and either self-reported personal or family history of depression or anxiety. The self-reported personal history was asked as “Do you have any past history of depression or anxiety?”, and an analogous question was asked about first-degree family history. No information was available on clinical diagnoses of psychiatric conditions for participants or their family.
All potential factors were dichotomized: age above versus below the median (29 years); education as finished at least primary school (through Standard 8) versus less; unemployed versus any employment; relationship status as currently married versus not; pregnancy intendedness as wanted to become pregnant at that time versus not; HIV status disclosure as already disclosed to partner versus not; personal and family history of depression or anxiety as ever versus never; history of previous child death and of IPV as ever versus never; ART history as ART-naïve versus currently on ART; and WHO HIV clinical stage as 1 versus stages 2–4.
Statistical analysis:
We used bivariable log binomial regression models to estimate unadjusted prevalence ratios (PRs) to identify items associated with current probable antenatal depression (EPDS ≥6). All items that had a bivariable p-value <0.25 were included in a multivariable model; a higher p-value threshold was used to retain potentially important variables that may have had confounding (Sun, Shook, & Kay, 1996). We excluded variables that would have caused extremely sparse cells (n≤5) in the multivariable model regardless of p-value. We evaluated whether any pairs of variables had notable collinearity (OR ≥ 3.0) and retained the variable with a larger bivariable beta coefficient estimate in any such cases. We evaluated collinearity prior to simplifying the full model via manual backward elimination. To estimate PRs while addressing convergence issues, we used a previously described 2-step modeling process that first uses a modified Poisson model to generate starting estimates for the second-stage log binomial model (Greenland, 2004; Spiegelman & Hertzmark, 2005; Zou, 2004). For backward elimination, we omitted the variable that had the highest p-value, then re-ran the simplified model. Variables were eliminated one at a time until each remaining variable had a p-value of <0.10. As in bivariable models, a higher p-value threshold was chosen to retain variables with potentially meaningful relationships with probable antenatal depression. A variable indicating whether the woman was newly initiating ART or had been on ART for ≥6 months (referent) was retained in the multivariable model for theoretical differences between the two groups of women that may not have been captured in other variables in our dataset. We present adjusted PRs (aPR) and 95% confidence intervals (CI) for each variable retained in the final model. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethical approval:
Both the University of North Carolina at Chapel Hill institutional review board and the Malawi National Health Sciences Research Committee approved the S4 study. Any woman scoring ≥6 on the EPDS or who endorsed suicidal ideation was counseled by a study clinician and offered a referral to local mental health services.
Results:
Between May 2015 and December 2016, 22,415 women presented to the Bwaila antenatal clinic for an initial visit; most women (93%, n=20,838) received an HIV test through the standard of care opt-out testing, with 6.2% (n=1,294) testing positive, and another 6.1% (n=1,347) already known to have HIV and on ART. If approached by an S4 study nurse, 59% of women newly initiating ART and 57% of women who had been on ART for ≥6 months enrolled in the study. Overall, a total of 725 pregnant women with HIV enrolled in the S4 study during the time period; n=299 women initiated ART through Option B+ and n=426 women had been on ART for ≥6 months.
Participants’ median age was 29 years (interquartile range (IQR) 24–33), and women presented at a median 22 weeks gestation (IQR 18–26) (Table 1). Most women were married (90%), unemployed (62%), and reported that their current pregnancy was unintended (68%). Half had completed primary school or more (53%), 17% reported ever experiencing IPV, and 12% had a WHO HIV clinical stage ≥2. Many participants self-reported a history of depression or anxiety: 46% had a personal history and 20% had a family history.
Table 1:
Participant characteristics (n=725)
| Characteristic at enrollment | Median (IQR) | Total N (%) |
|---|---|---|
| Age in years | 29 (24-33) | |
| Weeks gestation | 22 (18-26) | |
| Marital status | ||
| Currently married | 654 (90) | |
| Not currently married | 71 (10) | |
| Education attained | ||
| None/some primary | 340 (47) | |
| Finished at least primary | 385 (53) | |
| Employment status | ||
| Unemployed | 452 (62) | |
| Employed | 273 (38) | |
| Current pregnancy intendedness | ||
| Intended | 230 (32) | |
| Not intended | 495 (68) | |
| Ever experienced IPV | ||
| No | 599 (83) | |
| Yes | 126 (17) | |
| Disclosed HIV status to partner* | ||
| No | 36 (8) | |
| Yes | 389 (92) | |
| Participant history of depression or anxiety | ||
| No | 394 (54) | |
| Yes | 329 (46) | |
| Family history of depression or anxiety | ||
| No | 582 (80) | |
| Yes | 141 (20) | |
| Previous child died | ||
| No | 543 (75) | |
| Yes | 179 (25) | |
| WHO HIV Clinical Stage | ||
| Stage 1 | 635 (88) | |
| Stage 2-4 | 89 (12) | |
| ART initiation status | ||
| Newly initiating ART | 299 (41) | |
| On ART for ≥6 months | 426 (59) |
Disclosure of HIV status to partner only listed among women who had been on ART for ≥6 months because some women who were newly initiating ART were diagnosed with HIV the day of the interview
Overall, 9.5% (95%CI 7.5–11.9%) (n=69) of women screened positive for depression, with EPDS scores distributed as shown in Figure 1 (mean score 1.5 (SD 2.8)). By pregnancy trimester, 12.8% (5/39), 8.7% (46/528), and 11.4% (18/158) screened positive in the first, second, and third trimester of pregnancy, respectively. The prevalence of probable antenatal depression among women newly initiating ART (10.0%) and those who had been on ART ≥6 months (9.1%) was similar in bivariable analyses (Table 2).
Figure 1.
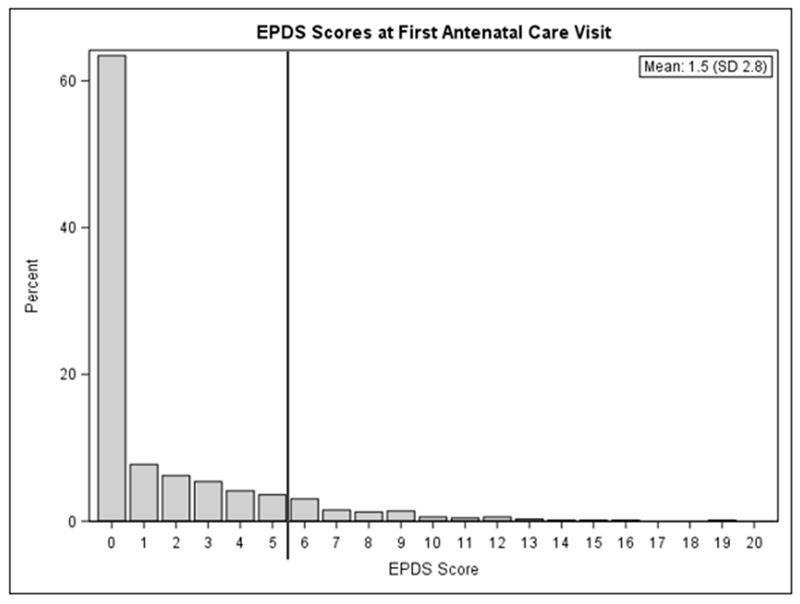
EPDS scores at first antenatal care visit.
Table 2:
Antenatal depressive symptom prevalence and prevalence ratios (unadjusted, and adjusted (aPR)) according to participant characteristics
| Variable | Prevalence (%) EPDS ≥6 | PR (95%CI) | aPR (95%CI) |
|---|---|---|---|
| Marital status | |||
| Currently married | 8.6 | 1.00 | 1.00 |
| Not currently married | 18.3 | 2.14 (1.23-3.71) | 1.66 (0.97-2.84) |
| Education | |||
| None/some primary | 9.4 | 1.00 | |
| Finished at least primary | 9.6 | 1.02 (0.65-1.60) | - |
| Employment status | |||
| Unemployed | 7.7 | 1.00 | 1.00 |
| Employed | 12.5 | 1.61 (1.03-2.52) | 1.56 (1.00-2.44) |
| Current pregnancy intendedness | |||
| Intended | 5.7 | 1.00 | 1.00 |
| Not intended | 11.3 | 2.00 (1.12-3.58) | 1.78 (0.99-3.21) |
| Ever experienced IPV | |||
| No | 8.0 | 1.00 | 1.00 |
| Yes | 16.7 | 2.08 (1.29-3.35) | 1.77 (1.11-2.81) |
| Disclosed HIV status to partner* | |||
| No | 16.7 | 1.00 | |
| Yes | 8.5 | 0.51 (0.23-1.13) | - |
| Participant history of depression or anxiety | |||
| No | 5.6 | 1.00 | 1.00 |
| Yes | 14.3 | 2.56 (1.58-4.15) | 2.42 (1.48-3.95) |
| Family history of depression or anxiety | |||
| No | 7.9 | 1.00 | |
| Yes | 16.3 | 2.06 (1.30-3.29) | - |
| Previous child died | |||
| No | 8.8 | 1.00 | |
| Yes | 10.6 | 1.20 (0.73-1.99) | - |
| WHO HIV Clinical Stage | |||
| I | 9.0 | 1.00 | |
| II-IV | 13.5 | 1.50 (0.84-2.69) | - |
| ART initiation status | |||
| On ART for ≥6 months | 9.1 | 1.00 | 1.00 |
| Newly initiating ART | 10.0 | 1.10 (0.70-1.72) | 1.39 (0.88-2.18) |
Disclosure of HIV status to partner only listed among women who had been on ART for ≥6 months because some women who were newly initiating ART were diagnosed with HIV the day of the interview
In bivariable analyses, the prevalence of probable antenatal depression was higher among women who were employed (PR 1.67; 95% CI 1.03–2.52), unmarried (2.14; CI 1.23–3.71), had a personal history of depression or anxiety (2.56; CI 1.58–4.15), had a family history of depression or anxiety (2.06; CI 1.30–3.29), had ever experienced IPV (2.08; CI 1.29–3.35), had not intended the current pregnancy (2.00; CI 1.12–3.58), and had an advanced WHO HIV clinical stage (1.50; CI 0.84–2.69) (p <0.25) (Table 2). The initial multivariable model contained a variable for ART status (newly initiating versus been on ART for ≥6 months), plus each variable from the bivariable models with p <0.25 except for family history of depression or anxiety due to collinearity with participant history of depression or anxiety. In the final multivariable model (p<0.10), women who had a personal history of depression or anxiety (adjusted PR [aPR] 2.42; CI 1.48–3.95) or had ever experienced IPV (1.77; CI 1.11–2.81) were more likely to screen positive for current depression. Being employed (1.56; CI 1.00–2.44), unmarried (1.66; CI 0.97–2.84), or having not intended their current pregnancy (1.78; CI 0.99–3.21) were associated with probable antenatal depression but had confidence intervals that included the null. While a less precise estimate, women newly initiating ART were more likely to have probable antenatal depression than women who had been on ART for ≥6 months (1.39; CI 0.88–2.18).
Discussion and Conclusions:
In this study of pregnant women living with HIV enrolled in Malawi’s Option B+ ART program, we estimated the prevalence of and identified factors associated with probable antenatal depression. About 10% of participants screened positive for current probable depression during the antenatal period. Compared to other maternal morbidity conditions during pregnancy, only anemia and HIV infection are more prevalent in Malawi (Zafar, Jean-Baptiste, Rahman, Neilson, & van den Broek, 2015).
In other sub-Saharan African countries, antenatal depression prevalence estimates range from 8.3% (Adewuya et al., 2007) to 48.7% (Peltzer, Rodriguez, & Jones, 2016), with an estimated 10.7% among antenatal Malawian women (Stewart, Umar, Tomenson, & Creed, 2014a). Among pregnant women with HIV, estimates range from 7.7% (Kaaya et al., 2002) to 55.0% (Rochat, Tomlinson, Newell, & Stein, 2013), with a weighted mean prevalence of over 20% (Sowa, Cholera, Pence, & Gaynes, 2015). The significant range of prevalence estimates could be due to heterogeneity of depression assessment methods used (screening tool versus diagnostic, self-report versus clinician interview), variability in timing of depression assessment during pregnancy, and cultural differences in depressive symptom expression. Our estimate that nearly 1 in 10 pregnant women with HIV suffer from probable antenatal depression approximates the weighted average of 11.3% (95%CI 9.5–13.1%) estimated in a systematic review of studies in Africa that did not stratify by participants’ HIV status (Sawyer et al., 2010). Some studies have found that persons living with HIV have higher prevalence of depression (Chibanda et al., 2010; Ciesla & Roberts, 2001; Kessler et al., 2003; Lyketsos et al., 1993), whereas others have shown a comparable burden of depression among those living with and without HIV (Bonacquisti et al., 2014; Rubin et al., 2011; Stewart et al., 2014a).
Our participants, pregnant women with HIV enrolled in Malawi’s Option B+ program, exhibited similar factors associated with current probable antenatal depression as those documented in previous studies: history of depression or anxiety (Biaggi et al., 2016; Biratu & Haile, 2015; Lancaster et al., 2010), history of IPV (Biaggi et al., 2016; Brittain et al., 2015; Davies, Schneider, Nyatsanza, & Lund, 2016; Dibaba, Fantahun, & Hindin, 2013; Lancaster et al., 2010; Peltzer et al., 2016; Stewart et al., 2014a), unintended pregnancy (Biratu & Haile, 2015; Brittain et al., 2015; Davies et al., 2016; Dibaba et al., 2013; Lancaster et al., 2010; Peltzer et al., 2016; Weobong et al., 2014), and being unmarried (Adewuya et al., 2007; Brittain et al., 2015; Sawyer et al., 2010; Weobong et al., 2014). About half of participants self-reported a history of depression or anxiety. While the reported depression or anxiety was not necessarily clinically diagnosed, the information serves as a marker of notable past stress, though participants with current depression may have had recall bias that led to over-reporting a history of depression or anxiety. Women in low resource settings appear to be at increased risk of depression given structural challenges such as food insecurity, unemployment, and gender-based violence, yet evidence has been inconsistent (Fisher et al., 2012). Age and having had a previous child die were not significantly associated with current probable antenatal depression among participants.
The psychosocial factors that were significantly associated with probable antenatal depression in our sample – IPV, unintended pregnancy, and being unmarried – could be indicators of a less stable social situation or having lower social support. IPV has been linked with depression in both resource-rich and resource-poor settings (Illangasekare, Burke, Chander, & Gielen, 2013; Li et al., 2014), and especially in the context of the perinatal period (Tsai, Tomlinson, Comulada, & Rotheram-Borus, 2016). Unintended pregnancy could be an acute stressor that contributes to depression (Brittain et al., 2015). Only one-third of our participants intended their current pregnancy compared to 60% of respondents in the 2015 Malawi Demographic Health Survey (NationalStatisticalOffice & ICFMacro, 2015). Becoming pregnant while unmarried is not socially desirable in Malawian culture. However, similar proportions of married and unmarried participants endorsed having an unintended pregnancy, which suggests that marital status has an association with probable depression that goes beyond pregnancy intent. Low social support has been associated with antenatal depression in Malawi; our data lacked a social support measure at baseline, which could have shed light on social support directly (Stewart, Umar, Tomenson, & Creed, 2014b). While we cannot determine the directionality between psychosocial factors and probable depression, our data highlight that some pregnant Malawian women with HIV experience probable antenatal depression in conjunction with potentially stressful social situations indicative of instability or low social support.
We considered HIV status disclosure to the participant’s sexual partner among women who had been on ART ≥6 months. Women who had not disclosed their status had a prevalence of probable antenatal depression about twice that among women who had disclosed, but did not reach statistical significance. The positive association between HIV status non-disclosure and probable antenatal depression could also be a marker of relationship instability and lack of social support from the partner (Go et al., 2015).
For theoretical reasons, we retained the variable for ART initiation status in our multivariable model to distinguish women who had been on ART from those who were initiating ART that day. Most women who were initiating ART on the day of their first antenatal visit (study enrollment) were newly diagnosed with HIV. As such, newly diagnosed women had not yet had the opportunity to fully process their HIV diagnosis or disclose their status to anyone because it is uncommon for male partners or other family members to accompany women to their antenatal visits. Newly diagnosed women may have had slightly higher EPDS scores in part due to the HIV diagnosis and ensuing acute distress. In the multivariable model, initiating ART was positively associated with probable antenatal depression, although the confidence interval for the adjusted PR includes the null. It is plausible that women who demonstrated longer-term engagement in HIV care had a lower prevalence of probable antenatal depression because women with probable antenatal depression may be less adherent to their ART (Kacanek et al., 2010). Women who had trouble adhering to ART, due to depression or otherwise, would not have met the study criterion for enrollment to the group of women who had been on ART ≥6 months.
Two indicators of socioeconomic status, education and employment, had dissimilar relationships with probable antenatal depression in bivariable models: education was not associated with depression, yet having any sort of employment was positively associated with depression. In the final multivariable model, women who were employed had an adjusted prevalence for probable antenatal depression that was 1.5 times as high as for unemployed women. It is possible that being employed was a stressor rather than representative of socioeconomic resources.
Our results have some limitations. With our cross-sectional dataset, we report the prevalence of probable antenatal depression at study enrollment, but cannot make causal inferences about how any of the considered variables affect incidence and duration of probable antenatal depression. Our data do not show whether probable antenatal depression was incident during pregnancy or preceded the pregnancy. The EPDS is a screening tool, and does not diagnose antenatal depression. While our cut-point of ≥6 based on the validation study (Stewart et al., 2013) demonstrated reasonable sensitivity and specificity, it is possible that some participants were falsely categorized as screening positive or negative for probable antenatal depression. Altering the cut-point would result in a different prevalence estimate. Applying the reported range of cut-points in the validation article (≥4 to ≥13) (Stewart et al., 2013) in our sample yields prevalence estimates of 17% and 0.8%, respectively.
All variables included in analyses were self-reported, and there could be misclassification on items such as history of depression or anxiety, history of IPV, pregnancy intendedness, or HIV status disclosure to partner. We had no information about recent stressful life events, validated social support metrics, HIV-related stigma, or food insecurity that may have contributed to probable antenatal depression (Brittain et al., 2015). The S4 study enrolled as many women as possible, but could not enroll all interested women. Some women refused participation due to lack of time, interest, or permission from their male partner. Women who suffered from severe depressive symptoms may have been less likely to participate in the S4 study.
In Malawi, women are not screened antenatally for depression, regardless of HIV status (Malawi Ministry of Health, 2015, 2016). Early recognition of antenatal depression among women living with HIV is imperative given that some women with antenatal depression may not follow through with optimal ART care (Turan et al., 2014), and could benefit from prompt depression treatment. Similarly, screening for psychosocial stressors such as IPV, social support, and HIV-related stigma could be helpful. Moreover, efavirenz, a drug commonly in first-line ART regimens, has been linked with neuropsychiatric side effects including depression (Apostolova et al., 2015). Thus, ART prescribing practices and symptom monitoring may need revision to avoid potential exacerbation of psychiatric symptoms among women with underlying depression. Good coverage for antenatal depression screening could be feasible given that >95% of Malawian women attend at least one antenatal visit (NationalStatisticalOffice & ICFMacro, 2015). Quantifying the cost effectiveness of depression screening and treatment would be useful for programmatic planning in Option B+.
In conclusion, our estimate of current antenatal depressive symptom prevalence among women enrolled in Option B+ provides evidence that Malawian women living with HIV bear a substantial burden of probable antenatal depression, comparable to the prevalence observed in other global regions. Integrating depression screening into routine HIV care such as Option B+ should be considered.
Acknowledgements:
We are appreciative of our S4 study collaborators (the Malawi Ministry of Health HIV/AIDS Unit, Baobab Health, Lighthouse Trust, Baylor College of Medicine), Bwaila Hospital Family Health Unit, and UNC Project-Malawi. Special thanks to the S4 study participants, and the S4 research team: Allan Jumbe, Trywin Phiri, Mark Maluwa, Clement Mapanje, Gift Sambiri, Linda Chikopa, Ntchindi Gondwe, Agness Gumbo, Jane Kilembe, Alvis Mvula, Juliana Ngwira, Lusubiro Paile, Chimwemwe Baluwa, Limbikani Chimndozi, Madawa Kumwenda, Chalimba Lusewa, Alice Maluwa, Kingsley Msimuko, Shaphil Wallie, Victoria Chilembwe, Tiyamike Itaye, Rob Krysiak, Gerald Tegha, Christopher Mwafulirwa, Kelvin Maziya, Madalitso Maliwichi, Frank Chimbwandira, Portia Kamthunzi, Maganizo Chagomerana, Sam Phiri, Atupele Kapito-Tembo, Nora Rosenberg, Irving Hoffman, Innocent Mofolo, Francis Martinson, Valerie Flax, Saeed Ahmed, Maria Kim, Deborah Demster Kamwendo, Michael Herce, Julie Nelson, Lameck Chinula, Robert Flick, and Austin Wesevich.
Declarations of Interest: Funding sources include: NICHD [R01HD080485], NIGMS [T32GM008719], NIMH [F30MH111370], Fulbright-Fogarty U.S. Student fellowship, NIH Fogarty International Center Grant [R25TW009340]; and the Doris Duke Clinical Research Fellowship. The authors have no other financial relationships to disclose.
Funding: This study was funded by NICHD [grant R01HD080485]; the Medical Scientist Training Program [grant T32GM008719], the NIMH individual fellowship [F30MH111370], the Fulbright-Fogarty U.S. Student fellowship, and the NIH Fogarty International Center Grant [R25TW009340]; and the Doris Duke Clinical Research Fellowship.
Footnotes
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Contributor Information
Bryna J. Harrington, University of North Carolina at Chapel Hill.
Brian W. Pence, University of North Carolina at Chapel Hill
Mathias John, UNC Project Malawi.
Caroline G. Melhado, UNC Project Malawi
Jacob Phulusa, UNC Project Malawi.
Bryan Mthiko, UNC Project Malawi.
Bradley N. Gaynes, University of North Carolina at Chapel Hill
Joanna Maselko, University of North Carolina at Chapel Hill.
William C. Miller, University of North Carolina at Chapel Hill
Mina C. Hosseinipour, UNC Project Malawi & University of North Carolina at Chapel Hill
S4 Study team, UNC Project Malawi.
References:
- Aaron E, Bonacquisti A, Geller PA, & Polansky M (2015). Perinatal Depression and Anxiety in Women with and without Human Immunodeficiency Virus Infection. Women’s Health Issues, 25(5), 579–585. 10.1016/j.whi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Adewuya AO, Ola BA, Aloba OO, Dada AO, & Fasoto OO (2007). Prevalence and correlates of depression in late pregnancy among Nigerian women. Depression and Anxiety, 24, 15–21. 10.1002/da [DOI] [PubMed] [Google Scholar]
- Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, & Esplugues JV (2015). Efavirenz and the CNS: what we already know and questions that need to be answered. Journal of Antimicrobial Chemotherapy, 70(10), 2693–2708. 10.1093/jac/dkv183 [DOI] [PubMed] [Google Scholar]
- Beer L, & Skarbinski J (2014). Adherence to Antiretroviral Therapy Among HIV-Infected Adults in the United States. AIDS Education and Prevention, 26(6), 521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggi A, Conroy S, Pawlby S, & Pariante CM (2016). Identifying the women at risk of antenatal anxiety and depression: a systematic review. Journal of Affective Disorders, 191, 62–77. 10.1016/j.jad.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biratu A, & Haile D (2015). Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: a cross-sectional study. Reproductive Health, 12(99). 10.1186/s12978-015-0092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisetegn TA, Mihretie G, & Muche T (2016). Prevalence and Predictors of Depression among Pregnant Women in Debretabor Town, Northwest Ethiopia. PLoS Medicine, 11(9), 1–10. 10.1371/journal.pone.0161108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacquisti A, Geller PA, Aaron E, Bonacquisti A, Geller PA, & Rates EA (2014). Rates and predictors of prenatal depression in women living with and without HIV. AIDS Care, 26(1), 100–106. 10.1080/09540121.2013.802277 [DOI] [PubMed] [Google Scholar]
- Brittain K, Myer L, Koen N, Koopowitz S, & Donald KA (2015). Risk Factors for Antenatal Depression and Associations with Infant Birth Outcomes: Results from a South African Birth Cohort Study. Paediatric and Perinatal Epidemiology, 29, 505–514. 10.1111/ppe.12216 [DOI] [PubMed] [Google Scholar]
- Chibanda D, Benjamin L, Weiss HA, & Abas M (2014). Mental, Neurological, and Substance Use Disorders in People Living With HIV / AIDS in Low- and Middle-Income Countries. J Acquir Immune Defic Syndr, 67(Supplement 1), 54–67. [DOI] [PubMed] [Google Scholar]
- Chibanda D, Mangezi W, Tshimanga M, Woelk G, Rusakaniko S, Stranix-Chibanda L, … Shetty AK (2010). Postnatal Depression by HIV Status Among Women in Zimbabwe. Journal of Women’s Health, 19(11), 2071–2077. 10.1089/jwh.2010.2012 [DOI] [PubMed] [Google Scholar]
- Chimbwandira F, Mhango E, Makombe S, & Midiani D (2013). Impact of an Innovative Approach to Prevent Mother-to-Child Transmission of HIV -- Malawi. Morbidity and Mortality Weekly Report. Morbidity and Mortality Weekly Report (Vol. 62). Retrieved from www.cdc.gov/mmwr/preview/mmwrhtml/mm6208a3.htm [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, & Roberts JE (2001). Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry, 158(5), 725–730. 10.1176/appi.ajp.158.5.725 [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of Postnatal Depression: Development of the 10-item Edinburgh Postnatal Depression scale. British Journal of Psychiatry, 150(JUNE), 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Davies T, Schneider M, Nyatsanza M, & Lund C (2016). “The sun has set even though it is morning”: Experiences and explanations of perinatal depression in an urban township, Cape Town. Transcultural Psychiatry, 0(0), 1–27. 10.1177/1363461516632389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibaba Y, Fantahun M, & Hindin MJ (2013). The association of unwanted pregnancy and social support with depressive symptoms in pregnancy: evidence from rural Southwestern Ethiopia. BMC Pregnancy & Childbirth, 13(135). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Heron J, Patel RR, & Wiles N (2007). Depressive symptoms during pregnancy and low birth weight at term. British Journal of Psychiatry, 191, 84–85. 10.1192/bjp.bp.105.016568 [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, & Hernandez-Reif M (2006). Prenatal depression effects on the fetus and newborn: a review. Infant Behavior and Development, 29(3), 445–455. 10.1016/j.infbeh.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, & Holmes W (2012). Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bulletin of the World Health Organization, 90(2), 139–149H. 10.2471/BLT.11.091850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, … Miller WC. (2005). Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence Report/technology Assessment (Summary), (119), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, McKenzie-Mcharg K, Shakespeare J, Price J, & Gray R (2009). A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica, 119(5), 350–364. 10.1111/j.1600-0447.2009.01363.x [DOI] [PubMed] [Google Scholar]
- Go VF, Latkin C, Le Minh N, Frangakis C, Ha TV, Sripaipan T, … Quan VM. (2015). Variations in the Role of Social Support on Disclosure Among Newly Diagnosed HIV-Infected People Who Inject Drugs in Vietnam. AIDS and Behavior. 10.1007/s10461-015-1063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Batchelder AW, Psaros C, & Safren SA (2011). Depression and HIV / AIDS Treatment Nonadherence : A Review and Meta-analysis. J Acquir Immune Defic Syndr., 58(2), 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S (2004). Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. American Journal of Epidemiology, 160(4), 301–305. [DOI] [PubMed] [Google Scholar]
- Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, … Keiser O. (2016). Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. The Lancet HIV, 3(4), e175–e182. 10.1016/S2352-3018(16)00008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, & Moore J (2001). Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA : The Journal of the American Medical Association, 285(11), 1466–1474. 10.1001/jama.285.11.1466 [DOI] [PubMed] [Google Scholar]
- Illangasekare S, Burke J, Chander G, & Gielen A (2013). The Syndemic Effects of Intimate Partner Violence, HIV/AIDS, and Substance Abuse on Depression among Low-Income Urban Women. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 90(5), 934–947. 10.1007/s11524-013-9797-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaya S, Fawzi M, Mbwambo J, Lee B, Msamanga G, & Fawzi W (2002). Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatrica Scandinavica, 106(1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, & Wilson IB (2010). Incident Depression Symptoms Are Associated With Poorer HAART Adherence: A Longitudinal Analysis From the Nutrition for Healthy Living Study. J Acquir Immune Defic Syndr., 53(2), 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas K, … Wang. (2003). The Epidemiology of Major Depressive Disorder. JAMA : The Journal of the American Medical Association, 289(23), 3095–3105. [DOI] [PubMed] [Google Scholar]
- Kim MH, Ahmed S, Hosseinipour MC, Giordano TP, Chiao EY, Yu X, … Abrams EJ. (2015). The impact of Option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. JAIDS Journal of Acquired Immune Deficiency Syndromes, 68(5), e77–e83. 10.1097/QAI.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, & Davis MM (2010). Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol, 202(1), 5–14. 10.1016/j.ajog.2009.09.007.Risk [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Lam SK, Lau SMSM, Chong CSY, Chui HW, & Fong DYT (2007). Prevalence, Course, and Risk Factors for Antenatal Anxiety and Depression. Obstetrics & Gynecology, 110(5), 8–10. [DOI] [PubMed] [Google Scholar]
- Leserman J (2008). Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine, 70(5), 539–545. 10.1097/PSY.0b013e3181777a5f [DOI] [PubMed] [Google Scholar]
- Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue EE, & Ehiri JE (2014). Intimate partner violence and HIV infection among women: a systematic review and meta-analysis. Journal of the International AIDS Society, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M, Senterfitt W, Dew MA, Wesch J, … Treisman GJ. (1993). Predictors of Medical Outcomes in HIV Infection. JAMA: The Journal of the American Medical Association, 270(21), 2563–2567. [PubMed] [Google Scholar]
- Malawi Ministry of Health. (2015). Participants Manual in Integrated Maternal and Neonatal Care. [Google Scholar]
- Malawi Ministry of Health. (2016). Malawi Guidelines for Clinical Management of HIV in Children and Adults, 3rd edition. [Google Scholar]
- Msellati P (2009). Improving mothers’ access to PMTCT programs in West Africa: A public health perspective. Social Science & Medicine, 6(1), 46–52. 10.1016/j.socscimed.2009.05.034 [DOI] [PubMed] [Google Scholar]
- NationalStatisticalOffice, & ICFMacro. (2011). Malawi Demographic and Health Survey 2010. Zomba, Malawi; Calverton, Maryland, USA. [Google Scholar]
- NationalStatisticalOffice, & ICFMacro. (2015). Malawi Demographic and Health Survey 2015. Zomba, Malawi; Calverton, Maryland, USA. [Google Scholar]
- Peltzer K, Rodriguez VJ, & Jones D (2016). Prevalence of prenatal depression and associated factors among HIV-positive women in primary care in Mpumalanga province, South Africa. Journal of Social Aspects of HIV/AIDS, 13(1), 60–67. 10.1080/17290376.2016.1189847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, & Shikwane ME (2011). Prevalence of postnatal depression and associated factors among HIV-positive women in primary care in Nkangala district, South Africa. Southern African Journal of HIV Medicine, 12(4), 24 10.4102/hivmed.v12i4.168 [DOI] [Google Scholar]
- Pence BW, Miller WC, Gaynes BN, & Eron JJ (2007). Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes, 44(2), 159–166. 10.1097/QAI.0b013e31802c2f51 [DOI] [PubMed] [Google Scholar]
- Rochat TJ, Tomlinson M, Newell M, & Stein A (2013). Detection of antenatal depression in rural HIV-affected populations with short and ultrashort versions of the Edinburgh Postnatal Depression Scale (EPDS). Archives of Women’s Mental Health, 16, 401–410. 10.1007/s00737-013-0353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Cook J. a., Grey DD, Weber K, Wells C, Golub ET, … Maki PM. (2011). Perinatal Depressive Symptoms in HIV-Infected Versus HIV-Uninfected Women: A Prospective Study from Preconception to Postpartum. Journal of Women’s Health, 20(9), 1287–1295. 10.1089/jwh.2010.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer A, Ayers S, & Smith H (2010). Pre- and postnatal psychological wellbeing in Africa: A systematic review. Journal of Affective Disorders, 123(1–3), 17–29. 10.1016/j.jad.2009.06.027 [DOI] [PubMed] [Google Scholar]
- Sowa N, Cholera R, Pence B, & Gaynes B (2015). Perinatal depression in HIV-infected African women: a systematic review. Journal of Clinical Psychiatry, 76(10), 1385–1396. 10.4088/JCP.14r09186 [DOI] [PubMed] [Google Scholar]
- Spiegelman D, & Hertzmark E (2005). Easy SAS Calculations for Risk or Prevalence Ratios and Differences. American Journal of Epidemiology, 162(3), 199–200. 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- Stewart RC, Umar E, Tomenson B, & Creed F (2013). Validation of screening tools for antenatal depression in Malawi—A comparison of the Edinburgh Postnatal Depression Scale and Self Reporting Questionnaire. Journal of Affective Disorders, 150(3), 1041–1047. 10.1016/j.jad.2013.05.036 [DOI] [PubMed] [Google Scholar]
- Stewart RC, Umar E, Tomenson B, & Creed F (2014a). A cross-sectional study of antenatal depression and associated factors in Malawi. Archives of Women’s Mental Health, 17(2), 145–154. 10.1007/s00737-013-0387-2 [DOI] [PubMed] [Google Scholar]
- Stewart RC, Umar E, Tomenson B, & Creed F (2014b). Validation of the multi-dimensional scale of perceived social support (MSPSS) and the relationship between social support, intimate partner violence and antenatal depression in Malawi. BMC Psychiatry, 14(180). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GW, Shook TL, & Kay GL (1996). Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology, 49(8), 907–916. 10.1016/0895-4356(96)00025-X [DOI] [PubMed] [Google Scholar]
- Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, … Keiser O. (2014). Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS, 28(4), 589–598. 10.1097/QAD.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Tomlinson M, Comulada WS, & Rotheram-Borus MJ (2016). Intimate Partner Violence and Depression Symptom Severity among South African Women during Pregnancy and Postpartum: Population-Based Prospective Cohort Study. PLoS Medicine, 13(1). 10.1371/journal.pmed.1001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan B, Stringer KL, Onono M, Bukusi EA, Weiser SD, Cohen CR, & Turan JM (2014). Linkage to HIV care, postpartum depression, and HIV-related stigma in newly diagnosed pregnant women living with HIV in Kenya: a longitudinal observational study. BMC Pregnancy and Childbirth, 14(1), 400 10.1186/s12884-014-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weobong B, Soremekun S, Ha A, Amenga-etego S, Danso S, Owusu-agyei S, … Kirkwood BR. (2014). Prevalence and determinants of antenatal depression among pregnant women in a predominantly rural population in Ghana: The DON population-based study. Journal of Affective Disorders, 165, 1–7. 10.1016/j.jad.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Whetten K, Shirey K, Pence BW, Yao J, Thielman N, Whetten R, … Reddy E. (2013). Trauma History and Depression Predict Incomplete Adherence to Antiretroviral Therapies in a Low Income Country. PLoS ONE, 8(10), 1–7. 10.1371/journal.pone.0074771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Sit DKY, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, … Singer LT. (2009). Major Depression and Antidepressant Treatment: Impact on Pregnancy and Neonatal Outcomes. Am J Psychiatry, 166(5), 557–566. 10.1176/appi.ajp.2008.08081170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar S, Jean-Baptiste R, Rahman A, Neilson JP, & van den Broek NR (2015). Non-Life Threatening Maternal Morbidity: Cross Sectional Surveys from Malawi and Pakistan. PLoS ONE, 10(9). 10.1371/journal.pone.0138026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G (2004). A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology, 159(7), 702–706. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]


