Abstract
Rationale:
“Bath salts” preparations often contain combinations of synthetic cathinones (e.g., 3,4methylenedioxymethcathinone [methylone], 3,4-methylenedioxypyrovalerone [MDPV]) and caffeine, and evidence suggests that mixtures of synthetic cathinones and caffeine (e.g., MDPV+caffeine or methylone+caffeine) can be more potent and/or effective reinforcers than predicted for an additive interaction.
Objective:
To use demand curve analyses to compare the reinforcing effectiveness of MDPV and methylone to mixtures of MDPV+caffeine and methylone+caffeine.
Methods:
Male Sprague-Dawley rats acquired methylone self-administration (0.32 mg/kg/inf) under a fixed ratio (FR) 1 schedule of reinforcement, and generated full dose-response curves for methylone (0.01–1 mg/kg/inf) under an FR5 schedule of reinforcement. Demand curves were then obtained for methylone, MDPV, caffeine, and methylone+caffeine and MDPV+caffeine mixtures by increasing the FR across sessions according to the following series: 3, 10, 18, 32, 56, 100, 178, etc.
Results:
Self-administration of methylone was rapidly acquired by 87.5% of rats and was maintained across a range of doses, producing an inverted U-shaped dose-response curve. Rank order demand for the individual constituents was MDPV>methylone>caffeine. Demand for the 3:1 (but not 10:1) methylone+caffeine mixture was greater than that for methylone alone, and demand for MDPV alone was similar to both MDPV+caffeine mixtures evaluated.
Conclusions:
These studies provide additional evidence that although methylone is an effective reinforcer, combining methylone with caffeine results in an enhanced reinforcing effectiveness compared to methylone alone. Thus, abused “bath salts” preparations containing synthetic cathinones and caffeine may have higher abuse liability than preparations containing only synthetic cathinones.
Keywords: methylone, MDPV, caffeine, bath salts, drug mixtures, demand curves, self-administration
INTRODUCTION:
Use and abuse of new psychoactive substances (NPSs) has become a global concern over the past decade, and designer stimulants, including synthetic cathinones, are the largest subgroup of NPSs (UNODC 2017). Synthetic cathinones are commonly detected in “bath salts” preparations that are often marketed as safe and legal alternatives to illicit stimulants, such as cocaine, methamphetamine, or 3,4-methylenedioxymethamphetamine. Similar to other stimulants, synthetic cathinones interact with dopamine, norepinephrine, and serotonin transporters (DAT, NET, and SERT; respectively) where they function as either amphetamine-like transporter substrates (e.g., 3,4methylendedioxymethcathinone [methylone]) or cocaine-like transporter inhibitors (e.g., 3,4methylenedioxypyrovalerone [MDPV]) (Baumann et al. 2013; Eshleman et al. 2013; Simmler et al. 2013). Consistent with these mechanisms of action, methylone and MDPV have also been shown to increase locomotor activity, produce cocaine-, methamphetamine-, or 3,4-methylenedioxymethamphetamine-like discriminative stimulus effects, and maintain intravenous self-administration (e.g., Collins et al. 2016; Dolan et al. 2018; Fantegrossi et al. 2013; Gannon et al. 2016, 2017a, b, 2018b; Gatch et al. 2013).
The exact composition of “bath salts” preparations is quite variable; however, analyses of seized and purchased “bath salts” suggest that they often contain more than one psychoactive constituent, including multiple synthetic cathinones and/or combinations of synthetic cathinones and caffeine (Caudevilla-Galligo et al. 2013; Davies et al. 2010; Schneir et al. 2014; Seely et al. 2013; Shanks et al. 2012; Spiller et al. 2011; Zuba and Byrska 2013). Given this variability, it is perhaps not surprising that “bath salts” users report their effects to be variable, ranging from quite favorable (e.g., alertness and intense euphoria) to extremely undesirable (e.g., agitation, paranoia, and death) (Forrester et al. 2012; Johnson and Johnson 2014; Ross et al. 2011; Spiller et al. 2011). Although “bath salts” preparations typically contain more than one psychoactive constituent, the majority of “bath salts” research has focused on characterizing the effects of individual cathinones.
Caffeine, an adenosine A1/A2 receptor antagonist, is often mixed with stimulants (e.g., cocaine, methamphetamine, 3,4-methylenedioxymethamphetamine) on the illicit drug market (e.g., Lapachinske et al. 2015; Vidal Gine et al. 2016), in part because it is cheap and legal, but also because its mild stimulant properties are thought to mimic those of illicit stimulants. Indeed, humans report the subjective effects of intravenous caffeine to be similar to amphetamine (Garrett and Griffiths 2001), and studies in rats suggest that the discriminative stimulus effects of caffeine are not only similar to those of cocaine, but when administered as a drug mixture, caffeine can also enhance the discriminative stimulus effects of cocaine (Collins et al. 2016; Harland et al. 1989). Moreover, we have recently shown that mixtures of synthetic cathinones and caffeine can exhibit supra-additive interactions with regard to their reinforcing effects. For instance, when evaluated under a progressive ratio (PR) schedule of reinforcement, mixtures of MDPV and caffeine were found to be more potent than MDPV alone, and mixtures of methylone and caffeine were found to be more effective than methylone alone. Although these findings suggest that caffeine can enhance the abuse-related effects of synthetic cathinones, it is important to note that additive interactions were observed for the majority of the “bath salts” mixtures, with supra-additive interactions observed only at 1:1 and 3:1 mixtures of methylone+caffeine, and at a 3:1 mixture of MDPV+caffeine (Gannon et al. 2018b). Although these findings suggest mixtures of synthetic cathinones and caffeine can function as more effective reinforcers than the synthetic cathinone alone, because these studies used a PR schedule of reinforcement to characterize these interactions, it is possible that the conclusions were influenced by factors such as drug accumulation and potential pharmacokinetic differences amongst the drug(s) (Griffiths et al, 1979; Lile et al, 2003; Panlilio and Schindler, 2000; Wee et al, 2006).
Thus, the present study employed a complementary, behavioral economic method for determining reinforcing effectiveness (i.e., demand curve analyses) that avoids these confounds to compare the reinforcing effects of MDPV and methylone alone to those of mixtures of MDPV+caffeine and methylone+caffeine. Because demand curve analyses can provide a single estimate of value (i.e., α) for data generated with multiple doses of a given commodity (e.g., drug or food), they provide a largely dose-independent method for assessing reinforcing effectiveness (e.g., Hursh and Silberberg 2008; Hursh and Winger 1995; Winger et al. 2006), but are less useful for comparing differences in potency amongst drug reinforcers (e.g., Koffarnus et al. 2012). Accordingly, based on the results of our previous study that evaluated the relative reinforcing effects of MDPV, methylone, caffeine, and their binary mixtures under the PR schedule of reinforcement (Gannon et al. 2018b), we hypothesized that 1) the rank order demand for the “bath salts” constituents would be MDPV > methylone > caffeine; and 2) “bath salts” mixtures that exhibited supra-additive increases in reinforcing effectiveness (methylone+caffeine) would be greater than the cathinone alone, whereas demand for “bath salts” mixtures that exhibited supra-additive increases in reinforcing potency (MDPV+caffeine) would not differ from demand for the cathinone alone.
METHODS:
Subjects
Male Sprague-Dawley rats (275–300g upon arrival) purchased from Envigo (Livemore, CA, USA) were singly housed, maintained in a temperature- and humidity-controlled room on a 10/14-h dark/light cycle, and provided with ad libitum access to water and Purina rat chow. All experiments were conducted in accordance with the Institutional Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio and the Eighth Edition of the Guide for Care and Use of Laboratory Animals (National Research Council 2011).
Surgical Procedures
Rats were implanted with catheters in the left femoral vein while anesthetized with 2% isoflurane as previously described (Gannon et al. 2017a, b, 2018a, b, c, d). Immediately after the surgery, catheters were flushed with 0.5 ml heparinized saline (100 U/ml) via a vascular access port located between the scapulae, and penicillin G (60,000 U/rat) was administered subcutaneously. Rats were provided at least 5 days to recover from the surgery, during which catheters were flushed daily with 0.5 ml heparinized saline.
Self-Administration
Self-administration sessions were conducted 7 days/week in operant conditioning chambers situated within noise-reduction containers (Med Associates, St Albans, VT, USA). Each chamber was equipped with two sets of red, yellow, and green light emitting diodes that were located directly above each of two response levers, and a house light was located at the top center of the opposite panel. Drug infusions were delivered by a variable speed syringe pump that was attached to a plastic fluid swivel connected to the rat via a spring tether that was held in place by a counterbalance arm.
A catheter-loading infusion was delivered 30-s before the start of each session, and illumination of the yellow light above the active lever (left or right; counterbalanced) signaled the start of the session and drug availability. Completion of the fixed ratio (FR) resulted in a drug infusion (0.1 ml/kg over ~1 s) that was paired with the illumination of the houselight and all three lights above the active lever for the duration of a 5-s timeout (TO). Responses made during the TO or on the inactive lever were recorded but had no scheduled consequence. Catheters were flushed before (0.2 ml saline) and after (0.5 ml heparinized saline) daily operant sessions to verify and maintain catheter patency. If pressure was noted when catheters were flushed, an infusion of methohexital (3 mg/kg) was administered to further test whether a catheter was still functional. If methohexital failed to produce a rapid loss of righting reflex, a second surgery was performed to place a catheter in the right femoral vein.
Acquisition
Rats (n=42) were initially allowed to respond for methylone (0.32 mg/kg/inf) under an FR1:TO 5-s schedule of reinforcement during 10 daily 90-min sessions. After 10 days, the response requirement was increased to an FR5 schedule for all rats that met our acquisition criteria (>20 infusions earned for 2 consecutive days with ≥80% of the responses made on the active lever). Rats that failed to acquire (n=6) were provided additional sessions at FR1 until criteria were met before increasing the response requirement. All rats responded on the FR5 schedule of reinforcement for at least 10 days and until stability criteria (±20% of the mean of three consecutive days with no increasing or decreasing trend) were met before moving on to other experiments. For all experimental procedures, if lethality was observed in (A) three out of the first four rats, or (B) >25% of the total number of rats assigned to a particular dose/dose pair, testing of that particular dose/dose pair was terminated.
FR5 Dose-Response Curve
Previous behavioral economic studies of drug self-administration suggest that a single function (i.e., a demand curve) can be used to describe responding maintained by a range of doses for a given drug (e.g., Hursh and Winger, 1995, Winger et al, 2006). While this provides a largely dose-independent approach to quantifying demand for a drug, it is important to note that doses that fall on the ascending limb, or peak of a FR dose-response curve are sometimes unable to be fit to the same function as larger doses, resulting in these “threshold” doses having a larger α (i.e., lower value) (e.g., Hursh and Silberberg, 2008). Thus, in order to ensure that sufficiently large doses of methylone and MDPV were used, and because there is some discrepancy in the literature regarding the doseresponse curve for methylone self-administration in rats (Creehan et al, 2015; Javadi-Paydar et al, 2018; Schindler et al, 2016; Vandewater et al, 2015; Watterson et al., 2012), a full dose-response curve was generated for methylone under a FR5:TO 5-s schedule of reinforcement in approximately half of the rats (n=23) in order to ensure that the appropriate doses of methylone were studied. For the dose-response study, the first dose tested was always 0.32 mg/kg/inf, with the remaining doses of methylone (0.01 – 1 mg/kg/inf) evaluated by substitution in a random order. All doses were evaluated until stability criteria were met (± 20% of the mean number of infusions for 3 consecutive days, with no increasing or decreasing trend). Doses for MDPV used in the demand experiments were selected based on historical data from our laboratory (Gannon et al., 2017).
Demand Curves
The remaining rats (n=19) were used to generate demand curves for methylone (0.32 and 1 mg/kg/inf), MDPV (0.032 mg/kg/inf), caffeine (0.32 mg/kg/inf), mixtures of methylone+caffeine at ratios of 1:1 (0.3 mg/kg/inf + 0.89 mg/kg/inf), 3:1 (0.45 mg/kg/inf + 0.44 mg/kg/inf), and 10:1 (0.54 mg/kg/inf + 0.16 mg/kg/inf) methylone+caffeine, mixtures of MDPV+caffeine at ratios of 3:1 (0.038 mg/kg/inf + 0.44 mg/kg/inf), and 10:1 (0.046 mg/kg/inf + 0.16 mg/kg/inf) MDPV+caffeine, and saline during daily sessions that lasted 120 min. As previously described (Gannon et al. 2018a), demand curves were generated under a FRx:TO 5-s schedule of reinforcement where the FR increased across sessions according to the following series: 3, 10, 18, 32, 56, 100, 178, 320, 560, 1000, etc. Each ratio was in place until the stability criterion was met (± 15% of the mean number of infusions for 2 consecutive days), and ratios were increased until a rat failed to earn a reinforcer for two consecutive sessions. With the exception that methylone (0.32 mg/kg/inf) was assessed first in all rats, the order of testing was randomized across rats. The doses of the individual constituents were selected based on their relative position on the descending limb of a FR dose response curve (i.e., ½ log larger than the peak). The specific fixed dose ratios for the “bath salts” mixtures were selected based on a previous study in which mixtures of MDPV+caffeine yielded smaller ED50 values than predicted for an additive interaction (i.e., supra-additive increase in potency), and mixtures of methylone+caffeine yielded larger Emax values than predicted for an additive interaction (i.e., supra-additive increases in effectiveness) under a PR schedule of reinforcement (Gannon et al. 2018b). In order to determine whether a history of responding for more effective drug reinforcers affected demand for methylone, demand for methylone (0.32 mg/kg/inf) was reassessed after demand for MDPV and the binary “bath salts” mixtures were evaluated.
Drugs
Methylone and MDPV were synthesized as HCl salts in the laboratory of Kenner Rice (Bethesda, MD). Caffeine was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in sterile saline.
Data Analysis
Acquisition data are presented as the mean ± SEM of the number of responses emitted on the active and inactive levers, as well as the percentage of rats that met acquisition across the 10-day acquisition period. Doseresponse data obtained under the FR5 schedule of reinforcement are presented as the mean ± SEM of the number of infusions earned at each unit dose of methylone. The “peak dose” of methylone (i.e., the unit dose that maintained the most responding) was determined on an individual subject basis and is presented as the group mean ± the 95% confidence intervals (CIs).
Data from the demand curve analyses are presented as the proportional consumption (Q/Q0) across the standardized price (the FR value X Q0), where Q is the mean number of infusions earned for a given drug/drug mixture at any FR and Q0 is the mean number of infusions earned for a given drug/drug mixture when the FR was set to 3. The data were then fit according to the following equation from Hursh and Silberberg (2008):
where C is the FR and α estimates the rate of decline in consumption as the price increases (i.e., elasticity coefficient). As such, α can serve as a quantitative measure of the value (or reinforcing effectiveness) of each drug/drug mixture. Any two drugs/drug mixtures were considered to differ significantly in their reinforcing effectiveness if the 95% CIs surrounding the α value of each drug were non-overlapping. A one-way ANOVA with repeated measures followed by a Holm-Sidak’s test for multiple comparisons was used to detect differences in Q0 among the drugs/drug mixtures. All statistical analyses were performed on individual subject data; however, group means were used for graphical representations. Prism 7 software (GraphPad Software Inc., La Jolla, California, USA) was used to conduct statistical analyses and plot all figures.
RESULTS:
Acquisition
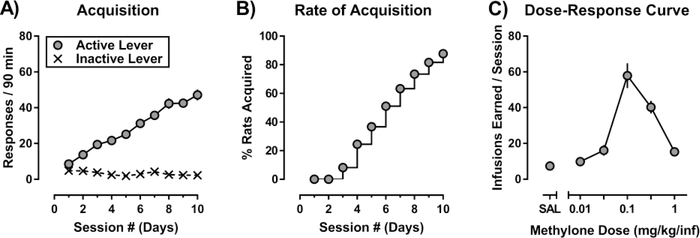
The majority of rats (85.7% [36/42]) acquired responding for methylone (0.32 mg/kg/inf) by the end of the 10-day acquisition period (Figure 1). Acquisition criteria for methylone self-administration were met in 6.3 ± 0.3 days, with rats earning an average of 48.5 ± 3.4 infusions per session by the end of the 10-day acquisition period.
Figure 1:
Methylone self-administration in male Sprague-Dawley rats. (A) Mean±SEM of the total responses emitted on the active (filled symbols) or inactive (Xs) lever during the active portion of the 90-minute session (n=42 rats). (B) Cumulative percent of rats to acquire self-administration of 0.32 mg/kg/infusion methylone across the 10-day acquisition period. Abscissa: numbers refer to consecutive days during the acquisition period. (C) Dose-response curve for methylone self-administration obtained in rats (n=21) under the FR5 schedule of reinforcement. Abscissa: SAL represents infusions of saline, while the numbers refer to dose of methylone available during each session, expressed as mg/kg/inf on a log scale. Ordinate: Mean±SEM of the total infusions earned during the 90-minute session.
Dose-Response Curve
Figure 1C shows the number of infusions earned for each unit dose of methylone under an FR5 schedule of reinforcement (n=21). The mean dose (95%CI) that maintained the maximal number of infusions (i.e., the peak dose) was 0.12 (0.1, 0.15) mg/kg/inf methylone, with rats earning on average 61.0 ± 6.4 infusions. Selfadministration of the largest unit dose of methylone tested (1 mg/kg/inf) was lethal in 2 out of 23 rats (Table 1).
Table 1:
Intake and lethality data for all of the drugs/drug mixtures evaluated in the present study.
| Constituent dose(s) (mg/kg/inf) | Lethality | Mean non-lethal intake (mg/kg ± SEM) | Mean lethal intake (mg/kg ± SEM) | |||||
|---|---|---|---|---|---|---|---|---|
| Drug 1 | Drug 2 | n / total | % lethality | Drug 1 | Drug 2 | Drug 1 | Drug 2 | |
| Dose-Response Curve | ||||||||
| FR5:TO 5-sec, 90 min session | ||||||||
| methylone | ||||||||
| 0.01 | 0/22 | 0 | 0.1 ± 0.0 | |||||
| 0.032 | --- | 0/23 | 0 | 0.5 ± 0.1 | --- | --- | --- | |
| 0.1 | --- | 0/23 | 0 | 6.2 ± 0.8 | --- | --- | --- | |
| 0.32 | --- | 0/23 | 0 | 13.0 ± 1.1 | --- | --- | --- | |
| 1 | --- | 2/23 | 9 | 15.3 ± 0.8 | --- | 65 ± 6.0 | --- | |
| Demand Curves | ||||||||
| FR3:TO 5-sec, 120 min session | ||||||||
| methylone | ||||||||
| 0.32 | 0/19 | 0 | 16.9 ± 1.5 | |||||
| 1 | --- | 4/15 | 26.7 | 23.2 ± 1.5 | --- | 47.8 ± 8.4 | --- | |
| MDPV | ||||||||
| 0.032 | --- | 0/9 | 0 | 2.3 ± 0.8 | --- | --- | --- | |
| caffeine | ||||||||
| 0.32 | --- | 0/9 | 0 | 7.6 ± 1.1 | --- | --- | --- | |
| methylone | caffeine | |||||||
| 10:1 methylone:caffeine | 0.54 | 0.16 | 0/12 | 0 | 17.1 ± 1.2 | 5.0 ± 0.4 | ||
| 3:1 methylone:caffeine | 0.45 | 0.44 | 3/12 | 25 | 17.5 ± 1.5 | 17.2 ± 1.4 | 48.6 ± 4.5 | 48.0 ± 4.5 |
| 1:1 methylone:caffeine | 0.30 | 0.89 | 3/5 | 60 | 15.6 ± 1.1 | 46.3 ± 3.3 | 24.8 ± 3.1 | 73.5 ± 9.2 |
| MDPV | caffeine | |||||||
| 10:1 MDPV:caffeine | 0.046 | 0.16 | 0/8 | 0 | 2.3 ± 0.5 | 8.1 ± 1.7 | ||
| 3:1 MDPV:caffeine | 0.038 | 0.44 | 1/9 | 11.1 | 1.8 ± 0.3 | 21.0 ± 3.1 | 11.6 | 135.5 |
Demand Curves
Individual Constituents
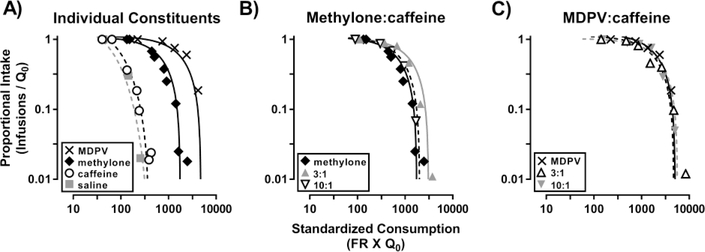
Results from the demand curve analyses of FR responding for 0.32 mg/kg/inf methylone (diamonds), 0.032 mg/kg/inf MDPV (Xs), 0.32 mg/kg/inf caffeine (circles), and saline (squares) are shown in Figure 2A (n=8). Data obtained from the demand curves (i.e., the elasticity coefficients [α], initial consumption [Q0], and fit of the curve [R2]) are shown in Table 2. Demand data for 0.32 mg/kg/inf methylone before and after responding for the mixtures were evaluated separately and when combined; however, because demand for the two determinations were not significantly different from one another and because a single curve could be fit to the data when both determinations were combined, only the combined values are reported. Comparisons of the elasticity coefficients (α) from the bestfit curves for these data indicate all three of the constituents functioned as reinforcers (i.e., demand for each was greater than that for saline) with MDPV being the most effective reinforcer (α=1.2×10−5 [0.9, 1.4]), followed by methylone (α=4.2×10−5 [3.8, 4.7]), and caffeine (α= 19.1×10−5 [17.8, 20.5]). A one-way ANOVA with repeated measures did not detect a main effect of self-administered drug on Q0 (F[1.06, 7.42)=3.19; p>0.05); however, a posthoc Holm-Sidak test revealed Q0 for methylone (47.6 ± 3.4 infusions) was significantly different from that of saline (14.3 ± 2.3 infusions) (t=7.11; p<0.001).
Figure 2:
Demand curve analyses of self-administration data obtained during 2-hr sessions in which the FR response requirement was increased across sessions. Abscissa: standardized price of each drug presented on a log scale. Left ordinate: proportional consumption expressed on a log scale. Note: the data presented are from only the rats (n=8) that responded for all of the drugs/drug mixtures shown.
Table 2:
Data obtained from the demand curve analyses (n=8).
| Drug/Drug Mixture | α (95% CIs) | Q0 (±SEM) | R2 |
|---|---|---|---|
| saline | 22.6 × 10−5 (20.8, 24.4) | 14.3 (2.3) | 0.96 |
| caffeine | 19.1 × 10−5 (17.8, 20.5)* | 21.6 (3.3) | 0.93 |
| methylone | 4.2 × 10−5 (3.8, 4.7)* | 47.6 (3.4)* | 0.75 |
| MDPV | 1.2 × 10−5 (0.9, 1.4)* | 74.7 (29.3) | 0.59 |
| 10:1 methylone:caffeine | 3.5 × 10−5 (3.1, 4.0)* | 30.1 (3.0)# | 0.79 |
| 3:1 methylone:caffeine | 2.6 × 10−5 (2.3, 2.8)*,# | 37.5 (3.3) | 0.86 |
| 10:1 MDPV:caffeine | 1.5 × 10−5 (1.3, 1.8)* | 50.3 (10.7) | 0.70 |
| 3:1 MDPV:caffeine | 1.3 × 10−5 (1.1, 1.6)* | 47.4 (7.0) | 0.59 |
indicates that value for the drug/drug mixture is significantly different from saline.
indicates that value for the mixture is significantly different from the cathinone alone.
Because self-administration of a unit dose of 1 mg/kg/inf methylone was lethal in 4 out of 15 rats (Table 1), demand for this dose of methylone was not determined for all subjects and is therefore not included in Figure 2 or Table 2.
Methylone+caffeine mixtures
Demand curves for the self-administration of methylone alone (diamonds) as well as the 3:1 (upward triangles) and 10:1 (downward triangles) mixtures of methylone+caffeine are shown in Figure 2B. The rank order of reinforcing effectiveness for these drugs/drug mixtures was 3:1 methylone:caffeine (α=2.6×10−5 [2.3, 2.8]) > methylone (α=4.2×10−5 [3.8, 4.7]) ≈ 10:1 methylone:caffeine (α=3.5×10−5 [3.1, 4.0]).
Self-administration of the methylone+caffeine mixtures was associated with lethality (Table 1). For the 1:1 mixture of methylone+caffeine (0.3 mg/kg/inf methylone + 0.89 mg/kg/inf caffeine), 3 out of 5 rats died. Thus, evaluations of demand for the 1:1 mixture were terminated and therefore are not included in Figure 2B. For the 3:1 mixture of methylone+caffeine (0.45 mg/kg/inf methylone + 0.44 mg/kg/inf caffeine), 3 out of 12 rats died. Because demand for the 3:1 mixture of methylone+caffeine had already been assessed in all other rats before the third rat died, these data were included in Figure 2B.
MDPV+caffeine mixtures
Demand curves for the self-administration of MDPV alone (Xs) as well as mixtures of MDPV+ caffeine at ratios of 3:1 (upward triangles), and 10:1 (downward triangles) are shown in Figure 2C. The 95% CIs surrounding the α values for all three of the drugs/drug mixtures shown in Figure 2C overlapped (Table 2), so all three were considered to be equally effective reinforcers.
Although self-administration of MDPV alone (or a 10:1 mixture of MDPV+caffeine) was not lethal in any subjects, self-administration of the 3:1 mixture of MDPV+caffeine (0.038 mg/kg/inf MDPV + 0.44 mg/kg/inf caffeine) was lethal in 1 out of 9 rats (Table 1).
DISCUSSION:
“Bath salts” preparations typically contain multiple psychoactive drugs including more than one synthetic cathinone or synthetic cathinones and caffeine. However, because most preclinical research on “bath salts” has focused on the effects of individual cathinone constituents relatively little is known about how the composition of these “bath salts” preparations impacts their abuse-related and toxic effects. The current study evaluated the reinforcing effects of three common “bath salts” constituents (i.e., MDPV, methylone, and caffeine) and used demand curve analyses to determine whether “bath salts” mixtures containing either methylone+caffeine, or MDPV+caffeine were more effective reinforcers than the synthetic cathinone alone (methylone and MDPV, respectively). The present study provides evidence that the composition of “bath salts” can significantly impact their reinforcing (and toxic) effects, with mixtures of methylone+caffeine functioning as more effective reinforcers, and mixtures of either methylone+caffeine or MDPV+caffeine resulting in more lethality than comparable unit doses of the synthetic cathinone alone.
Consistent with what has previously been reported (Creehan et al. 2015; Nguyen et al. 2017; Schindler et al. 2016; Vandewater et al. 2015; Watterson et al. 2012), acquisition of responding for methylone was gradual, with rats earning on average ~50 infusions of 0.32 mg/kg methylone by the end of the 10-day acquisition period. This level of intake (~15 mg/kg/session) is comparable to that reported by others (Watterson et al.2012; Creehan et al. 2015; Nguyen et al. 2017; Vandewater et al. 2015), but greater than that reported by Schindler and colleagues (2016) for rats responding for a similar dose (0.3 mg/kg/inf) of methylone. Upon substitution of other doses of methylone, an inverted U-shaped dose-response curve emerged for methylone self-administration, with a peak at 0.1 mg/kg/inf, and doses smaller than this maintaining responding at levels no different than saline. Although dose-response curves for methylone self-administration have been reported by others, these have tended to be either flat (Schindler et al. 2016), or only encompassed the ascending- (Watterson et al. 2012) or descending-limb; (Creehan et al. 2015; Vandewater et al. 2015).
Consistent with reports that have used PR schedules of reinforcement to quantify the relative reinforcing effectiveness of MDPV and methylone (e.g., Dolan et al. 2018; Gannon et al. 2017a, b, 2018b; Watterson et al. 2012, 2014), demand analyses indicated that MDPV is a more effective reinforcer than methylone, which is more effective than caffeine. Moreover, when taken together with α values reported for other synthetic cathinones (e.g., alpha-pyrrolidinopentiophenone [α-PVP], α-pyrrolidinopropiophenone [α-PPP], 3,4-methylenedioxy-αpyrrolidinobutiophenone [MDPBP], 3,4-methylenedioxy-α-pyrrolidinopropiophenone [MDPPP], 4-methyl-alphapyrrolidinopropiophenone [4-MePPP], and 4-methyl-N-ethylcathinone [4-MEC]; Huskinson et al. 2017; Gannon et al. 2018a), the α values reported in the present study for MDPV (α=1.2×10–5) and methylone (α=4.2×10–5) are consistent with the notion that the reinforcing effects of synthetic cathinones exist on a continuum, with MDPV functioning as a highly effective reinforcer and methylone functioning as a moderately effective reinforcer. Although caffeine was the least effective of the three “bath salts” constituents, the confidence intervals surrounding its α value (α = 19.1 × 10–5 [17.8, 20.5]) do not overlap with those for saline (α = 22.6 × 10–5 [20.8, 24.4]) suggesting that caffeine functioned as a reinforcer in rats with a history of responding for synthetic cathinones, an effect that is consistent with our previous study in which caffeine maintained more responding than saline in cathinone-experienced rats responding under a PR schedule of reinforcement (Gannon et al. 2018b). Thus, albeit with very different levels of effectiveness, each of the “bath salts” constituents functioned as a reinforcer when administered alone.
We have previously reported supra-additive interactions between MDPV and caffeine with respect to the reinforcing potency of 3:1 mixtures of MDPV+caffeine in rats responding under a PR schedule of reinforcement (Gannon et al. 2018b). Because demand analyses provide a largely dose-independent measure for assessing the reinforcing effectiveness (e.g., demand) for drug reinforcers (Hursh and Silberberg 2008; Hursh and Winger 1995), these types of interactions (i.e., changes in potency) would not be expected to result in differences in demand for drug mixtures relative to the drug alone. Indeed, demand for both the 10:1 and 3:1 mixtures of MDPV+caffeine were comparable to that of MDPV alone, consistent with a strictly additive interaction with respect to reinforcing effectiveness as reported previously (Gannon et al. 2018b). In addition, even though demand for MDPV was found to be significantly greater than demand for methylone (or caffeine), we have evidence from studies with other synthetic cathinones (e.g., α-PVP) that the current procedures are capable of detecting even greater levels of demand (i.e., smaller α value) (Gannon et al. 2018a). Thus, when taken together with our previous findings (Gannon et al. 2018b), the present data provide strong evidence that interactions between the reinforcing effects of MDPV and caffeine can result in “bath salts” mixtures that are more potent (ED50 values from PR dose-response curves are smaller than predicted for an additive interaction; Gannon et al. 2018b), but not more effective, reinforcers than preparations containing MDPV alone.
Conversely, we have previously shown that mixtures of methylone and caffeine could function as more effective, but not more potent, reinforcers when evaluated under a PR schedule of reinforcement (Gannon et al. 2018b). In the current studies, demand for the 10:1 methylone+caffeine mixture was not different than that of methylone alone, while demand for the 3:1 methylone+caffeine mixture was found to be greater than that for methylone alone. Not only are these findings consistent with the supra-additive interactions reported for rats responding for mixtures of methylone+caffeine under a PR schedule of reinforcement (Gannon et al. 2018b), but when considered together they provide strong and convergent evidence that “bath salts” mixtures containing methylone+caffeine can function as more effective reinforcers than preparations containing methylone alone.
Although the current studies were not designed to determine the mechanism(s) that contribute to the supraaddictive increases in reinforcing potency (MDPV+caffeine) or effectiveness (methylone+caffeine), these interactions are likely occurring at the postsynaptic receptor level. Indeed, adenosine receptors are thought to form heteromeric complexes with dopamine receptors where they serve to reduce binding affinities of dopamine receptor agonists and dampen dopamine signaling (e.g., Ferre 2016; Ferre et al. 2016). Thus, by antagonizing adenosine receptors, caffeine can disinhibit dopamine receptors, effectively increasing the potency of dopamine or dopamine receptor agonists to produce their behavioral effects. A caffeine-induced enhancement in the potency of dopamine provides a straightforward explanation for the supra-additive increases in reinforcing potency observed with mixtures of MDPV+caffeine, because the relative ratio of activity at DAT and SERT (i.e., DAT/SERT selectivity) is thought to be a primary determinant of the relative reinforcing effectiveness of stimulants (e.g., Lile et al. 2003; Roberts et al. 1999; Wee et al. 2005; Gannon et al. 2018a), and such an effect is also consistent with the supraadditive increases in reinforcing effectiveness, at least for drugs that have significant serotonergic activity, such as methylone (Baumann et al. 2013; Eshleman et al. 2013; Simmler et al. 2013). While the capacity of caffeine to selectively enhance dopaminergic (relative to serotoninergic) signaling would effectively increase the DAT/SERT selectivity of methylone, and thereby its reinforcing effectiveness, it is also possible that the differential effects of caffeine (i.e., enhanced potency when mixed with MDPV, and enhanced effectiveness when mixed with methylone) are related to the fact that MDPV functions as a cocaine-like inhibitor, whereas methylone functions as an amphetamine-like substrate at monoamine transporters. Indeed, caffeine has been shown to potentiate the release of dopamine by drugs such as 3,4-methylenedioxymethamphetamine (Gorska and Golembiowska 2015), an effect that is thought to be mediated by antagonism of presynaptic adenosine A1 receptors (e.g., Cauli and Morelli, 2005). Although the functional consequences of such an interaction would be similar to that described above (i.e., a selective increase in dopaminergic relative to serotonergic signaling), it is possible that the combined effect is necessary to observe caffeine-induced increases in reinforcing effectiveness. Such conclusions are speculative, and further studies aimed at determining the precise mechanism(s) that underlie the interactions between synthetic cathinones and caffeine are clearly needed.
In addition to evidence suggesting that the reinforcing effects of “bath salts” mixtures can differ significantly from the reinforcing effects of the individual constituents, increased incidence of toxicity (e.g., lethality) have also been observed in rats that are allowed to self-administer mixtures of multiple cathinones (MDPV+methylone) or cathinones and caffeine (MDPV+caffeine, or methylone+caffeine) under either FR (present study) or PR schedules of reinforcement (Gannon et al. 2018b). Importantly, even though this study was not designed to investigate the toxic/lethal effects of synthetic cathinones, or “bath salts” mixtures, post-hoc analyses of drug intake were used to provide insight into the factors that may have contributed to the observed lethality. For instance, although lethality was not associated with the self-administration of either MDPV, or caffeine alone, lethality was observed in 6 of the 23 rats that self-administered the 1 mg/kg/inf dose of methylone (2 under FR5; 90 min sessions, and 4 under FR3; 120 min session), and these rats exhibited unusually high levels of intake (~2–4 fold) relative to rats in which this unit dose was self-administered safely (~10 mg/kg/hr), suggesting that lethality resulted from an overdose of methylone rather than a differential sensitivity to the toxic effects of methylone. A similar trend was also observed in the 3 rats that died after self-administering a mixture of 3:1 methylone+caffeine. Although it is impossible to rule out a contribution of relatively high levels of caffeine intake (~50 mg/kg/session), because the level of methylone intake (~50 mg/kg/session), and incidence of lethality (~25%) was comparable to that which was associated with lethality in the 1 mg/kg/inf methylone alone condition, it is likely that these instances of lethality were attributed to the effects of methylone alone. Conversely, although only a subset of rats (n=5) responded for the 1:1 mixture of methylone+caffeine, the incidence of lethality was disproportionately high (60%), despite the fact that the level of methylone intake (~25 mg/kg/session) was comparable to those observed in rats that safely selfadministered methylone, either alone, or in combination with caffeine. Moreover, because the average level of caffeine intake (~75 mg/kg/session) was smaller than the LD50 for intravenous caffeine (105 mg/kg; Scott and Chen 1944), it seems likely that the lethality resulted from an interaction between the toxic effects of methylone and caffeine.
Interestingly, although the single death associated with the self-administration of the 3:1 mixture of MDPV+caffeine was associated with extremely high levels of caffeine intake (135 mg/kg/session), this rat also earned ~5-fold more MDPV than all other rats, regardless of whether MDPV was administered alone, or in combination with caffeine. While this complicates interpretations, it should be noted that we have not previously observed lethality with MDPV self-administration (Gannon et al. 2017a, b, 2018b, d), despite some rats selfadministering ~3-fold more MDPV than was associated with lethality (or ~15-fold more than the non-lethal intakes) in the current study. Taken together with a previous report that experimenter-administered caffeine enhances the toxic effects of amphetamine (a methylone-like substrate) to a greater degree than those of cocaine (an MDPV-like inhibitor) (Derlet et al. 1992), it seems likely that this single incident resulted from the self-administration of a lethal dose of caffeine, rather than the effects of MDPV, or an interaction between the toxic effects of MDPV and caffeine. Nevertheless, these high rates of lethality associated with the self-administration of “bath salts” mixtures containing caffeine suggest that the interactions between cathinones and caffeine are not limited to their abuse-related effects, and clearly highlight the need for future research on the toxic effects of synthetic cathinones, and how these effects are modified when they are administered with common “bath salts” constituents, such as caffeine.
The popularity of synthetic cathinones has become a significant problem worldwide due to their use often being linked with high levels of abuse and toxicity. Although it has been established that some synthetic cathinones commonly detected in these preparations, such as MDPV, are more effective than the illicit stimulant drugs of abuse cocaine and methamphetamine when self-administered alone (Gannon et al. 2017a), “bath salts” preparations frequently contain more than one psychoactive constituent. The present study confirms and extends our previous work by using behavioral economic procedures to directly compare the reinforcing effects of “bath salts” mixtures to individual constituents. There were 4 main findings: (1) responding for methylone is readily acquired in most rats and maintains behavior across a range of doses, which results in an inverted U-shaped dose-response curve, (2) MDPV is a more effective reinforcer than methylone which is more effective than caffeine; (3) “bath salts” mixtures containing methylone and caffeine can function as more effective reinforcers than methylone alone; and (4) selfadministering “bath salts” mixtures containing either MDPV+caffeine or methylone+caffeine appeared to be more toxic (lethal) than self-administering either MDPV, or methylone alone. Although demand for most of the mixtures assessed in the present study did not differ from the synthetic cathinone alone, these findings indicate that behavioral economic procedures can be used to detect supra-additive interactions between the reinforcing effects of two drugs that function as reinforcers in their own right (i.e., methylone and caffeine). Together with our previous work, these studies provide strong evidence that the composition of “bath salts” preparations can significantly impact their abuse-related and toxic effects, and that these interactions between “bath salts” constituents likely contributes to the high levels of abuse and toxicity often reported for “bath salts” users. Given that “bath salts” preparations are often mixtures of cathinone and non-cathinone constituents, it will be important for future studies to continue to investigate interactions among binary and higher order drug “bath salts” mixtures.
Acknowledgement of financial support:
This study was supported by National Institutes of Health grants R01DA039146 (GTC) and T32DA031115 (BMG) and by the Intramural Research Programs of the National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism (KCR).
Abbreviations:
- NPSs
new psychoactive substances
- α-PVP
alpha-pyrrolidinopentiophenone
- α-PPP
α-pyrrolidinopropiophenone
- MDPV
3,4-methylenedioxypyrovalerone
- MDPBP
3,4-methylenedioxy-αpyrrolidinobutiophenone
- MDPPP
3,4-methylenedioxy-α-pyrrolidinopropiophenone
- 4-MePPP
4-methyl-alphapyrrolidinopropiophenone
- 4-MEC
4-methyl-N-ethylcathinone
- DAT
dopamine transporter
- NET
norepinephrine transporter
- SERT
serotonin transporter
- PR
progressive ratio
- FR
fixed ratio
- TO
timeout
- SEM
standard error of the mean
- CIs
confidence intervals
Footnotes
Conflict of Interest:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES:
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive bath salts products. Neuropsychopharmacology 38:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudevilla-Gálligo F, Ventura M, Iciar BI, Ruiz I, Fornís I (2013) Presence and composition of cathinone derivatives in drug samples taken from a Drug Test Service in Spain (2010–2012). Human Psychopharmacol. 28:341–344. [DOI] [PubMed] [Google Scholar]
- Cauli O, Morelli M (2005) Caffeine and the dopaminergic system. Behav Pharmacol. 16(2):63–77. [DOI] [PubMed] [Google Scholar]
- Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. (2011) Adulterants in illicit drugs: a review of empirical evidence. Drug Testing and Analysis. 3:89–96. [DOI] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP (2016) Discriminative Stimulus Effects of Binary Drug Mixtures: Studies with Cocaine, MDPV, and Caffeine. J Pharmacol Exp Ther 359(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA (2015) Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 92:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Wood DM, Smith G, Button J, Ramsey J, Archer R, Holt DW, Dargan PI (2010) Purchasing “legal highs” on the Internet--is there consistency in what you get? QJM. 103:489–493. [DOI] [PubMed] [Google Scholar]
- Derlet RW, Tseng JC, Albertson TE. (1992) Potentiation of cocaine and d-amphetamine toxicity with caffeine. American Journal of Emergency Medicine 10:211–216. [DOI] [PubMed] [Google Scholar]
- Dolan SB, Chen Z, Huang R, Gatch MB (2018) “Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology. 133:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 85:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC (2013). In vivo effects of abused ‘bath salt’ constituent 3,4methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 38(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S (2016) Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl) 233(10):1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortes A, et al. (2016) Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer. Neuropharmacology. 104:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester MB (2012) Synthetic cathinone exposures reported to Texas poison centers. Am J Drug Alcohol Abuse 38(6):609–615. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A,, Rice KC, Collins GT (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT (2018b) Reinforcing effects of binary mixtures of common bath salts constituents: studies with 3,4-methylenedioxypyrovalerone (MDPV), 3,4methylenedioxymethcathinone (methylone), and caffeine in rats. Neuropsychopharmacology. 43(4):761769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT (2018c) Relative reinforcing effects of second-generation synthetic cathinones: Acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology. 134(Pt A):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017a) Individual Differences in the Relative Reinforcing Effects of 3,4-Methylenedioxypyrovalerone under Fixed and Progressive Ratio Schedules of Reinforcement in Rats. J Pharmacol Exp Ther. 361:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, Collins GT (2017b) Reinforcing effects of abused “bath salts” constituents 3,4methylenedioxypyrovalerone (MDPV) and α-pyrrolidinopentiophenone (α-PVP) and their enantiomers. Behav Pharmacol. 28(7):578–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Sulima A, Rice KC, Collins GT (2018d) Inhibition of Cocaine and 3,4-Methylenedioxypyrovalerone (MDPV) Self-Administration by Lorcaserin Is Mediated by 5-HT2C Receptors in Rats. J Pharmacol Exp Ther. 364(2):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016) Stereoselective Effects of Abused “Bath Salt” Constituent 3,4-Methylenedioxypyrovalerone in Mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J Pharmacol Exp Ther. 356(2):615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett BE, Griffiths RR (2001) Intravenous nicotine and caffeine: subjective and physiological effects in cocaine abusers. J Pharmacol Exp Ther 296:486–494. [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 24(5–6):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska AM, Golembiowska K (2015) The role of adenosine A1 and A2A receptors in the caffeine effect on MDMA-induced DA and 5-HT release in the mouse striatum. Neurotox Res. 27(3):229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bradford LD, Brady JV (1979) Progressive ratio and fixed ratio schedules of cocaine-maintained responding in baboons. Psychopharmacology (Berl) 65:125–136. [DOI] [PubMed] [Google Scholar]
- Harland RD, Gauvin DV, Michaelis RC, Carney JM, Seale TW, Holloway FA. (1989) Behavioral interaction between cocaine and caffeine: a drug discrimination analysis in rats. Pharmacology Biochemistry and Behavior. 32:1017–1023. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. (2008) Economic demand and essential value. Psychological Review. 115:186–198. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. (1995) Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 64(3):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology (Berl). 234(4):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS and Johnson MW (2014) Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Hall A, Winger G (2012) Individual differences in rhesus monkeys’ demand for drugs of abuse. Addict Biol. 17(5):887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapachinske SF, Okai GG, dos Santos A, de Bairros AV, Yonamine M (2015) Analysis of cocaine and its adulterants in drugs for international trafficking seized by the Brazilian Federal Police. Forensic Sci Int 247:28–53. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, Nader MA (2003) The reinforcing efficacy of psychostimulants in rhesus monkeys: The role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther 307:356–366. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed., National Academy Press, Washington, DC. [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA (2017) Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict Biol. 22(5):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW (2000) Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 150:61–66. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H (1999) Self-administration of cocaine analogs by rats. Psychopharmacology (Berl). 144(4):389–397. [DOI] [PubMed] [Google Scholar]
- Ross EA, Watson M, Goldberger B (2011) “Bath salts” intoxication. N Engl J Med. 365:967–968. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH (2016) Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 233(10):1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneir A, Ly BT, Casagrande K, Darracq M, Offerman SR, Thornton S et al. (2014) Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin Toxicol (Phila). 52(7):651–658. [DOI] [PubMed] [Google Scholar]
- Scott CC, Chen KK (1944) Comparison of the Action of l-Ethyl Theobromine and Caffeine in Animals and Man. J Pharmacol Exp Ther 82: 89–97. [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE et al. (2013) Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int 233:416–422. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A (2012) Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J Anal Toxicol 36:360–371. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu L-H, Huwyler J et al. (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J (2011) Clinical experience with and analytical confirmation of bath salts and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol 49:499–505. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2017) World Drug Report 2017. United Nations publication. Sales No. E.17.XI.10.
- Vandewater SA, Creehan KM, Taffe MA (2015) Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology. 99:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal Giné C, Ventura Vilamala M, Fornís Espinosa I, Gil Lladanosa C, Calzada Álvarez N, Fitó Fruitós A et al. (2016) Crystals and tablets in the Spanish ecstasy market 2000–2014: Are they the same or different in terms of purity and adulteration? Forensic Sci Int. 263:164–168. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT et al. (2013) The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J Addict Res Ther Suppl 9:002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF et al. (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol. 19(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL (2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854. [DOI] [PubMed] [Google Scholar]
- Wee S, Carroll FI, Woolverton WL (2006) A Reduced Rate of In Vivo Dopamine Transporter Binding is Associated with Lower Relative Reinforcing Efficacy of Stimulants. Neuropsychopharmacology 31:351–362. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH (2006) Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther. 318(1):223–229. [DOI] [PubMed] [Google Scholar]
- Zuba D, Byrska B (2013) Prevalence and co-existence of active components of “legal highs.” Drug Test Anal 5:420–429. [DOI] [PubMed] [Google Scholar]