Abstract
Self-tolerance, the state of unresponsiveness to self-tissues, is maintained through central and peripheral tolerance mechanisms, and a breach of these mechanisms leads to autoimmune diseases. Foxp3+T-regulatory cells (Tregs) play an essential role in suppressing autoimmune response directed against self-antigens and thereby regulate self-tolerance. Natural Tregs are differentiated in the thymus on the basis of their higher TCR-affinity to selfantigens and migrate to the periphery where they maintain peripheral tolerance. In addition, extra-thymic differentiation of induced Tregs can occur in the periphery which can control abrupt immune responses under inflammatory conditions. A defect in Treg cell numbers and/or function is found to be associated with the development of autoimmune disease in several experimental models and human autoimmune diseases. Moreover, augmentation of Tregs has been shown to be beneficial in treating autoimmunity in preclinical models, and Treg based cellular therapy has shown initial promise in clinical trials. However, emerging studies have identified an unstable subpopulation of Tregs which expresses pro-inflammatory cytokine under both homeostatic and autoimmune conditions, as well as in ex vivo cultures. In addition, clinical translation of Treg cellular therapy is impeded by limitations such as lack of easier methods for selective expansion of Tregs and higher cost associated with GMP-facilities required for cell sorting, ex vivo expansion and infusion of ex vivo expanded Tregs. Here, we discuss the recent advances in molecular mechanisms regulating Treg differentiation, Foxp3 expression and lineage stability, the role of Tregs in the prevention of various autoimmune diseases, and critically review their clinical utility for treating human autoimmune diseases.
Keywords: Autoimmunity, Treg differentiation, Foxp3 expression, Immune tolerance, Treg immunotherapy
1. Introduction
The ability of the immune system to distinguish between self and non-self is fundamental to maintaining self-tolerance and breakdown of self-tolerance results in the positive selection and activation of self-reactive T- and B-cells resulting in autoimmunity[1]. Autoimmune diseases include a wide spectrum of (>80) systemic and organ-specific diseases such as type-1 diabetes (T1D), thyroiditis, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS), colitis, etc., and affect 3–5% of the population[2]. Foxp3+ Tregs have been shown to control self-reactive immune response by suppressing proliferation and/or effector functions of CD4+/CD8+ T cells, B cells, NK cells, and Antigen Presenting Cells (APCs), and thereby maintain the critical balance between selftolerance and autoimmunity [3, 4]. T-cell tolerance is regulated at two different levels namely, central and peripheral tolerance. Central tolerance is maintained through a negative selection process in which self-reactive thymocytes undergo clonal deletion in the thymic medulla[5]. However, negative selection is not foolproof and a proportion of self-reactive effector T-cells (Teff) often escape thymic selection and migrate to the periphery where they can recognize and initiate autoimmunity. Alternatively, a subset of self-reactive thymocytes is deviated into Foxp3+Treg lineage and migrate to the periphery, where they suppress autoimmune response elicited by self-reactive Teff cells which have escaped negative selection in the thymus and thus help maintain peripheral tolerance[5]. Additionally, induced or adaptive Tregs can be differentiated in the periphery (piTregs) from Foxp3− conventional T-cells (Tconv) which contribute to gut and graft tolerance [6, 7]. Homozygous female and hemizygous male mice harboring x-linked mutations in Foxp3 gene develop T-cell mediated lethal autoimmunity and lymphoproliferative disorder [8, 9]. Similarly, human X-linked neonatal diabetes mellitus, enteropathy, and endocrinopathy (IPEX) syndrome is linked to mutations in the human FOXP3 gene [8, 10]. These findings revealed a possible genetic basis for autoimmune diseases and led researchers to explore molecular mechanisms regulating the development and homeostasis of Treg cells under homeostatic and autoimmune conditions. In addition, significant efforts are underway to more thoroughly understand the relevance of Tregs in various autoimmune diseases and validate their potential utility in treating autoimmune diseases. Therapeutic approaches targeting Tregs showed encouraging results in the prevention of onset and amelioration of ongoing autoimmunity in many preclinical models [11]. Followed by the success of preclinical studies, human trials conducted using adoptive Treg immunotherapy have shown initial promise against T1D, and many other clinical trials are in progress [12, 13]. In spite of considerable progress, routine clinical use of Tregs is impeded by several hurdles including lack of efficient approaches to cause selective expansion of human Tregs in vivo without also expanding Teff cells, cumbersome and costly approaches used for ex vivo expansion of autologous human Tregs their infusion back into patients and uncertain lineage stability of expanded Tregs. These problems primarily stem from insufficient knowledge on human Treg development and homeostasis. These limitations have hindered our ability to translate successful murine studies into human treatments. Here, we discuss recent advances in our understanding of the development of Tregs, transcriptional and epigenetic regulation of Foxp3 expression and Treg lineage stability, various approaches being used to augment Treg numbers/functions and critically review their clinical utility for treating human autoimmune diseases.
2. Regulatory T-cell development in the thymus and periphery
Earliest studies indicating a role of Tregs in immune tolerance was published in 1969 by Nishizuka and Sakakura in which they reported identifying T-cell mediated autoimmunity in 3-day-old neonatal thymectomized mice but, not in 7-day-old thymectomized mice. Based on these findings they surmised that while self-reactive Tconv cells had emigrated from the thymus by day 3 of life, suppressor T-cells, which prevented autoimmunity in 7-day-old thymectomized mice, were absent in the periphery of 3-day-old thymectomized mice[14]. Three decades later, Sakaguchi et al. characterized these suppressor cells as IL-2 receptor alpha (IL-2Rα/CD25) expressing CD4+CD25+ immunoregulatory T-cells which appear in the periphery after 3-days of life. More importantly, supplementation of CD4+CD25+ T-cells from non-thymectomized mice prevented autoimmunity in 3-day-old thymectomized mice[15]. Subsequently, the transcription factor Foxp3, which was earlier found to be associated with autoimmune abnormalities like scurfy and IPEX[10], was identified as the lineage-specific marker for Treg cells[8, 16]. Thus, it is now accepted that Foxp3+Treg cells developed in the thymus are necessary to prevent autoimmunity.
2.1. A Two-step model of thymic Treg development
There are two models of thymic Treg (tTreg) cell development proposed based on the TCR signal strength, namely TCR instructive and stochastic models. According to the TCR instructive model, thymocytes expressing intermediate affinity TCRs for self-peptides experience higher TCR signal strength and differentiate into Foxp3+ Treg cells[17]. Treg cells express higher levels of TCR activation markers such as CD25, CD69, and CTLA4 compared to Tconv cells [18, 19]. In addition, using Nur77.GFP reporter mice (Nur77 is a highly sensitive indicator of TCR-signal strength), it has been shown that tTregs express higher levels of Nur77 compared with tTconv cells [20]. Thus, it is clear that higher TCR-signal strength is a prerequisite for tTreg differentiation. On the other hand, the stochastic model suggests that Foxp3 expression might be determined independent of TCR signal strength, perhaps during the early CD4−CD8− double negative (DN) stage and Foxp3 expression might provide a survival advantage to the cells to escape negative selection[17]. However, Foxp3+ thymocytes predominantly appear during CD4+CD8- single positive (SP) stage in wild-type mice, raising questions on the stochastic model [21]. The reason for this apparent discrepancy could be due to the use of TCR transgenic mice in most of the studies leading to the stochastic model. In TCR transgenic mice, unlike wild-type mice in which TCR α/β expression appears in CD4+CD8+ DP stage, the TCR expression was induced in early CD4−CD8− DN stages [22]. Interestingly, human Foxp3+ thymocytes differentiation starts predominantly during the early CD4+CD8+ DP stage which is strikingly different from murine thymic Treg development[21]. Thus, the timing of Foxp3 expression during Treg differentiation and its role in facilitating their selection still remain unresolved.
Treg cell development in the thymus can be divided into two phases. The first phase is a TCR-dependent phase in which thymocytes experiencing higher TCR-signal strength with costimulatory signals from CD28, OX40, GITR and TNFR2 give rise to two different subsets of Treg precursors; 1) CD4+CD25+Foxp3- and CD4+CD25-Foxp3low cells[18, 19, 23]. In the second TCR-independent phase, the Treg precursors gain Foxp3 and CD25 expression through signaling from common gamma chain cytokines like IL-2, IL-7 and IL-15 to become matured CD4+CD25+Foxp3+ Tregs [19, 21, 24]. We have observed CD25+Foxp3− and CD25−Foxp3low Treg precursors in the human thymus in both CD4+CD8+ DP and CD4+SP stages as well[25]. Thus, despite striking differences in the stages of Foxp3+ thymocyte differentiation, human and murine thymuses likely share common Treg developmental pathways. However, it is not known whether matured Tregs differentiating from two different precursors have similar or dissimilar TCR repertoire and function.
2.2. Epigenetic and transcriptional regulation of Foxp3 expression and Treg differentiation.
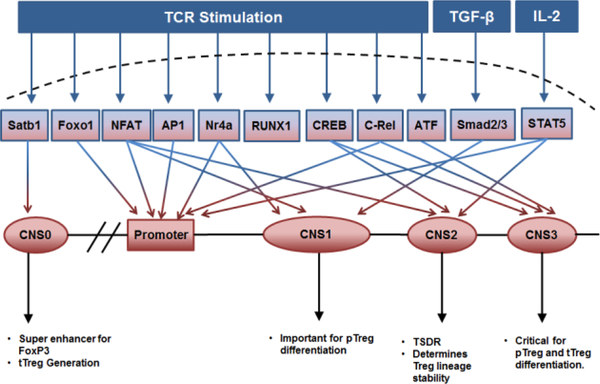
Foxp3 is the lineage-specific factor expressed exclusively on Tregs in mice. Over the years, Foxp3 gene regulation has been actively studied. Specific epigenetic events, such as nucleosome positioning, DNA methylation, and histone modification have been identified to govern Treg differentiation. The transcriptional regulation of Foxp3 expression and Treg differentiation is summarized in Fig-1. The Foxp3 gene contains a promoter region and three conserved non-coding sequences (CNS1, 2 & 3) in human, mouse, and rat [26, 27]. More recently, Kitagawa et al. reported CNS0, another conserved region bound by Satb1, which is a chromatin organizer that can activate super-enhancers even in closed chromatin where chromatin accessibility is low[28]. The human Foxp3 promoter is positioned 6.5kb upstream of exon1 and contains NFAT and AP1 binding sites. However, the Foxp3 promoter has low transcriptional activity and its expression is critically dependent on other intronic enhancer regions such as CNS regions [29]. The CNS1 (enhancer 1) contains binding sites for TGF-β and TCR driven transcription factors, such as Smad2/3 and NFAT, and has been shown to be indispensable for TGF-β-induced peripheral Treg (piTreg) differentiation but not required for thymic Treg (tTreg) differentiation[30]. Mice lacking CNS1 region were found to have normal tTreg differentiation but significantly reduced Tregs in gut-associated lymphoid tissues [31]. CNS2 (enhancer 2) contains the Treg-specific demethylated region (TSDR) harboring CpG islands, and CREB [32] and STAT5 binding sites [33]. Though CNS2 deletion did not drastically affect tTreg generation, its ablation leads to loss of lineage stability of Tregs during proliferation[34]. On the other hand, CNS3 which contains c-Rel binding sites has been shown to regulate the differentiation of both thymic and peripheral Tregs [31]. Interestingly, CNS3 deficient mice had significantly reduced tTregs indicating a relatively more critical role in tTreg differentiation [35].
Figure-1:
Schematic diagram of transcriptional and epigenetic regulation of Foxp3 expression. Inducers of various transcription factors and respective transcription factor binding sites in the Foxp3 promoter and enhancer regions like CNS1, CNS2, and CNS3 are indicated using arrowheads. Effect of corresponding regions on tTreg and pTreg differentiation and function are also indicated.
Many transcription factors have been identified to regulate Foxp3 expression, including c-Rel, Smad2/3, ATF, NFAT, AP-1, STAT5, and Nr4a. C-Rel−/− mice had 10-fold reduced Treg numbers and in addition, c-Rel−/− Tregs had impaired suppressive functions. c-Rel has been shown to form a Foxp3-specific “enhanceosome” containing c-Rel, NFAT, CREB, p65, and Smad which binds to the Foxp3 promoter to drive its transcription[36]. Moreover, c-Rel can bind to the CpG island of CNS3 enhancer region even in its methylated form whereas other transcription factors such as CREB and ATF can bind only when it is demethylated [37]. Studies using NFAT1/NFAT4 double knockout mice suggested a critical role for these two NFATs in the regulation of Foxp3 expression in nTregs [38]. NFAT binding sites were observed in CNS1, and TGF-β stimulation has been shown to recruit NFAT and Smad2/3 to the Foxp3 promoter to drive iTreg differentiation[30]. Thus, NFAT might regulate both thymic and peripheral Treg differentiation. Human Foxp3 promoter was found to contain NFAT and AP1 binding sites in close proximity and they coordinately function to drive Foxp3 transcription[26]. Another independent study has also shown a positive role for AP-1 in regulating TCR/TGF-β induced Foxp3 transcription and iTreg differentiation in co-operation with NFAT and Smad [39]. STAT5 is another unique transcription factor regulating tTreg differentiation, which is not a part of signaling downstream of TCR activation [40]. STAT5 activation is induced by common gamma chain cytokines like IL-2, IL-7 and IL-15, with IL-2 being a predominant contributor[33]. IL-2 plays a key role in Treg expansion and survival through STAT5 activation[41]. IL-2 required for Tregs survival and expansion is produced predominantly by activated Teff cells and appears to be a feedback mechanism by which Teff cell activation is controlled by Tregs [42]. Foxp3 promoter has consensus STAT5 binding sites, and a STAT5 binding site was also found in the CNS2 region of the Foxp3 gene [43]. IL-2-induced STAT5 activation plays a crucial role in the maturation of CD25+Foxp3- and CD25-Foxp3low tTreg precursors [18, 23]. IL-2, IL-2Rβ, and STAT5 knockout mice had significantly reduced Tregs and developed autoimmune symptoms [44]. Additionally, Nr4a family transcription factors were implicated in tTreg differentiation, which includes Nr4a1, Nr4a2, and Nr4a3. T-cell-specific deletion of these transcription factors resulted in abrogation of Treg differentiation and severe autoimmunity [45, 46]. Nr4a can strongly bind to the Foxp3 promoter and weakly to the CNS1 region to activate Foxp3. Additionally, Nr4a has also been reported to interact with another transcription factor called Runx1 which is necessary for Foxp3 expression and Treg function[45].
2.3. Peripheral Treg differentiation
The peripheral Treg pool consists of two different populations of Tregs; 1) Thymus-derived natural Tregs (nTregs) and peripherally induced Tregs (piTregs). However, the relative contribution of these Treg subsets to self-tolerance remains elusive. The piTregs are differentiated from CD4+CD25-Foxp3- Tconv cells in response to minute doses of antigen with suboptimal dendritic cell activation [47]. It is now widely accepted that piTregs are constituents of tissue-resident Tregs, such as gut-associated lymphoid tissue Tregs, and play an important role in gut tolerance[48]. Moreover, there are reports suggesting that piTregs might also play a role in the regulation of autoimmune responses [49, 50]. Nevertheless, it is difficult to determine the relative role of piTregs in autoimmunity because of lack of definite markers to differentiate piTregs vs tTregs. In several studies, Neuropilin−(Nrp)−1 and Helios expression were used to mark tTregs as Nrp1+Helios+Foxp3+ and Nrp1−Helios−Foxp3+ Tregs as piTregs at least in naïve mice. In line with this definition, CNS1−/− mice had a reduced frequency of Nrp1−Foxp3+ piTregs in the periphery [51]. However, this definition is true only in circulating Tregs as piTregs in the inflamed tissue might express Nrp1 upon activation[6]. Moreover, use of Nrp1 expression to mark tTregs may not be as valid for human tTregs, as the Nrp1 expression was not detected in circulating human Tregs although human splenic Tregs were positive for Nrp1[52]. Although Helios expression was observed in more than 70% of circulating Tregs, it is difficult to discriminate the site of differentiation of Helios+ vs Helios− Tregs in human[6]. Therefore, further studies on human Tregs are needed to understand the mechanism of human piTreg differentiation and homeostasis.
One of the salient features of tTregs is the demethylated TSDR in CNS2 region of Foxp3 and hypomethylation of other Treg signature genes, such as Ctla4, Il2ra, Ikzf2, and Tnfrsf18, allowing for stable expression of lineage markers [53]. However, the epigenetic regulation of the lineage stability of piTregs remains controversial. Initial studies performed using TGF-β-induced in vitro generated iTregs showed lack of TSDR demethylation [54]. Soon, it became evident that in vitro generated iTregs do not demonstrate the essential characteristics of in vivo generated piTregs [55]. Epigenetic studies using in vivo generated piTregs yielded contradicting results with some studies showing demethylated TSDR [54] and others showing methylated CpG islands in TSDR [50]. The reason for the discrepancy could be either due to differences in the animal models used or the markers used for sorting piTregs. However, Ohkura et al. showed demethylation of TSDR and Treg lineage-specific signature genes such as Ctla4, Il2ra, Ikzf2 and Tnfrsf18 in vivo generated piTregs [53], but not in in vitro generated Tregs [53]. However, it remains unclear whether piTregs represent a homogenous population or the heterogeneity of the piTreg population was the underlying reason for the observed differences in epigenetic stability in aforementioned studies. Further studies are required to demonstrate the relative contribution of piTregs in the prevention of autoimmune diseases.
3. Treg lineage stability and plasticity
One of the salient features of many immune cell types, if not all, is their ability to modulate phenotypic characteristics as an adaptive response to changing micro-environment under inflammatory conditions. There is a growing body of evidence suggesting that a subset of Tregs do not belong to a terminally differentiated cell type and tend to lose their lineage stability and trans-differentiate into pathogenic Teff cells (“ex-Foxp3” cells) [56]. As discussed in earlier sections, Treg-specific DNA demethylation at Foxp3 gene locus allows for the constitutive expression of Foxp3, which is essential for the repression of TCR activation-induced expression of inflammatory genes like Ifn-g, Il-2, and Zap70 in Tregs [53]. Thus, loss/reduced expression of Foxp3 in Tregs leads to expression of these pro-inflammatory genes by Tregs. In general, iTregs tend to have transient Foxp3 expression and considered to represent an unstable phenotype [57], whereas tTregs regarded as lineage stable Tregs. However, emerging studies show the instability of tTregs under normal and autoimmune conditions as well. Fate-mapping studies marking cells expressing Foxp3 at any time of their life cycle with yellow fluorescent protein (YFP) showed the emergence of cells with transient Foxp3 expression in the thymus [58]. More importantly, an increase in ex-Foxp3 cells was identified to be associated with pathogenesis in NOD and EAE mouse models [59, 60]. Moreover, exFoxp3 cells in NOD mice contained cells with methylated TSDR, indicating the link between epigenetic regulation of Treg lineage stability and autoimmunity [59]. Recent studies argue that Foxp3 expression alone is insufficient for optimal Treg function [61, 62]. For example, CpG hypomethylation of Il2ra (Cd25), Ctla4, Tnfrsf18 (Gitr) and Ikzf4 (Eos) gene loci in nTregs represent Foxp3-independent nTreg signature and their constitutive expression along with Foxp3, determines the Treg lineage stability and function [53]. Eos is a transcription factor which acts as a functional partner for Foxp3 mediated gene repression. Interestingly, Eos-labile Foxp3+Tregs were shown to be pathogenic T cells expressing pro-inflammatory cytokines and IL-6 was found to be the crucial factor inducing these Eos-labile Tregs [63].
Unlike murine Tregs in which Foxp3 is a definite lineage-specific marker for Tregs, a subset of activated human Teff cells were shown to transiently express Foxp3, and such transient Foxp3 expression did not confer suppressive functions[64]. Clinical studies have identified increased frequencies of CD45RA+FOXP3low and CD45RA−FOXP3low cells with impaired suppressive functions in SLE patients which correlated with disease index [65]. Hoffman et al. have demonstrated that repetitive TCR stimulation of human nTregs during in vitro expansion leads to CpG methylation of the Foxp3 gene locus in CD45RA−FOXP3+ memory-type subset, giving rise to unstable Tregs expressing pro-inflammatory cytokines like IL-2 and IFN-γ [66]. In accordance with this finding, TGF-β has been shown to antagonize TCRinduced cell-cycle dependent recruitment of DNA Methyl Transferase (DNMT1)-1 to Foxp3 locus and thereby prevent CpG methylation associated with loss of Foxp3 expression [67]. Similarly, DNMT1 inhibitors can be used to increase the fidelity and functions of nTregs in vivo [68, 69] [66]. However, it remains unknown whether TCR signaling or cell cycle induced exhaustion is responsible for the epigenetic changes leading to loss of Foxp3 expression. Taken together, it appears that Tregs exhibit different levels of heterogeneity under normal vs autoimmune conditions and further studies are warranted to determine the molecular mechanism regulating the emergence of unstable Tregs under homeostatic, proliferation induced exhaustive and autoimmune conditions.
4. Tregs: mechanism of suppressive functions.
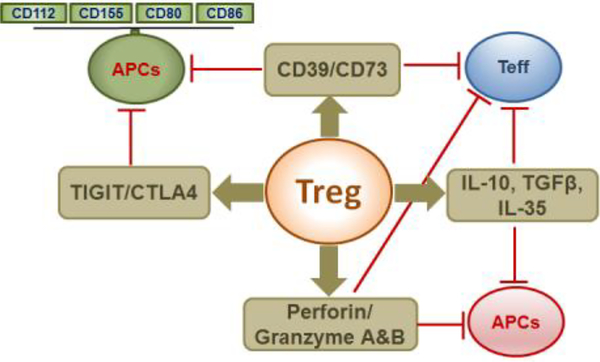
Tregs can suppress the activation, expansion, and effector functions of a wide variety of cell types including CD4+/CD8+ T-cells, B-cells, NKT cells and APCs. They exert their functions through modulation of APC functions, production of immunoregulatory cytokines such as IL-10, TGF-β, and IL-35, ATP deprivation, and cytolysis as summarized in Fig-2. Tregs express coinhibitory receptors like CTLA4 and TIGIT which can contribute to their suppressive functions. CTLA4 is constitutively expressed by Tregs and activated Teff cells, and CTLA4 deficiency or blockade has been shown to attenuate Treg functions. CTLA4 competes with co-stimulatory receptor CD28 for binding to co-stimulatory ligands CD80/CD86, which are expressed on APCs [70]. Interaction of CTLA4 with CD80/CD86 leads to trans-endocytosis of CTLA4 by APCs and down-modulation of CD80/CD86 expression [71]. Thus, CTLA4 expression by Tregs can modulate both Teff cell and APC functions. TIGIT is expressed on a subset of Tregs and contribute to their function by a mechanism similar to the CTLA4/CD28/CD80/CD86 axis. Coinhibitory receptor TIGIT competes with co-stimulatory receptor CD226 for binding to costimulation ligands CD155/CD112 expressed on APCs. Thus, binding of TIGIT with CD155 and CD112 prevents CD226 co-stimulation and inhibits Teff cell activation [72].
Figure-2:
Schematic diagram of the mechanism of Treg functions. Different arms of Treg function such as CTLA4/TIGIT co-inhibition, CD39 mediated ATP deprivation, Perforin/granzyme-A and B mediated cytolysis, and immunosuppressive cytokines IL-10, IL-35, and TGF-β, and their target cell types are shown.
Tregs produce suppressor cytokines, like TGF-β, IL-10, and IL-35, all of which can contribute to Teff cell suppression. Tregs produce TGF-β in both soluble and membrane-bound forms [73]. TGF-β can induce iTreg differentiation while suppressing Th1/Th2 differentiation[74]. However, in combination with IL-6, it can promote Th17 differentiation [75]. IL-10 is an antiinflammatory cytokine produced by Foxp3+Tregs and Foxp3−IL-10+Tr1 cells. IL-10 deficient Tregs fail to protect against T-cell transfer mediated colitis [76, 77] indicating a critical role for IL10 in the prevention of autoimmunity in a colitis model. IL-10 has also been shown to inhibit both naïve and memory T-cell responses[77] and IFN-γ production[78]. Similarly, IL-35 produced by Tregs can inhibit Teff cell proliferation and Tregs with defective IL-35 signaling had reduced suppressive functions in autoimmune IBD model as well [79].
Murine Tregs constitutively express two enzymes involved in ATP degradation: 1) CD39 (ecto-nucleoside triphosphate diphosphohydrolase-1) and 2) CD73 (Ecto-5’-nucleotidase). CD39 catalyzes the conversion of ATP to AMP, and CD73 converts AMP to adenosine[80]. Adenosine can activate signaling through A2A receptors expressed on dendritic cells (DCs) and Teff cells resulting in down-regulation of NF-kB activity and reduced production of effector cytokines and chemokines [81]. Additionally, Tregs can induce direct cytolysis of responder cells like CD4/CD8 T-cells, NK cells and APCs through granzyme-A/B and perforin-mediated mechanisms. It has been shown that granzyme-B-deficient Tregs had attenuated Treg functions and failed to induce apoptosis of Teff cells compared to granzyme-B-sufficient Tregs [82]. Similarly, Treg-mediated apoptosis of B-cells was also found to be mediated by granzyme-B and perforin-dependent mechanisms[83]. Human Tregs have been shown to induce apoptosis of Teff cells through the granzyme-B/perforin pathway. Of note, naïve Tregs do not express granzyme-B, whereas activated Tregs do express it. Moreover, activated Tregs in tumor microenvironments induce apoptosis of NK-cells and cytotoxic T-cells through the granzymeB/perforin pathway [84]. All of these suppressive mechanisms contribute to Treg mediated inhibition of function of various cell types, and loss of any one or more of these mechanisms may be compensated by the other remaining mechanisms to help sustain the competency of Tregs.
5. Approaches for targeting Tregs to treat autoimmunity
Altered Treg numbers/functions have been found to be associated with many experimental autoimmune disease models. Therefore, therapeutic approaches aimed at enhancing Tregs have been tested in experimental animal models of autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE)[85, 86], T1D[87, 88], collagen-induced arthritis (CIA)[89, 90], lupus[91], experimental autoimmune myasthenia gravis (EAMG)[92], colitis[89], etc. Phenotypic characterization of the Tregs revealed low CD25 and reduced Treg cell-specific FOXP3 demethylation as peripheral blood markers of autoimmune diseases such as SLE and T1D [93]. staggered ratio of Treg/Th17 was shown in active SLE patients, when compared to inactive Apart from FOXP3 demethylation, several other epigenetic changes such as acetylation and trimethylation of histone H3/H4 and regulatory influence of miR-155, miR-126, and miR-10a have also been noted[94]. Notably, a significantly SLE patients and normal controls [95]. Multiple preclinical studies have demonstrated that restoring Treg number/function either by using histone deacetylase inhibitor (ITF2357) [96], the active metabolite of leflunomide (A771726) [97] or inhibitor of calmodulin-dependent protein kinase IV (KN-93) [98] can rebuild immune tolerance in SLE animal models. Likewise, patients suffering from systemic sclerosis possess a lower number of CD4+CD25+Foxp3+ Tregs and reduced expression of IL-10 and TGF-β in peripheral blood as compared to healthy controls [99]. Targeting impaired suppressive function (FOXP3 hypermethylation) of Tregs by using a methylation inhibitor, 5-aza showed an increase in FOXP3 expression and in the percentages of CD4+CD25+FOXP3+ Tregs ex vivo [100]. Similarly, a lower percentage of CD4+CD25+ Tregs and reduced levels of TNF-β1 in the peripheral blood have been noted in rheumatoid arthritis patients as compared to healthy controls [101]. Patients with rheumatoid arthritis upon treatment with methotrexate showed increased FOXP3 and CTLA4 expression which corroborated with resumed Treg suppressive function [102]. Similar alterations in Treg numbers and functions were reported in Psoriasis which affects 2–3% of the world’s population. Efforts have been made to augment Treg numbers in Psoriasis by using histone deacetylase inhibitor (Trichostatin A) [103], cyclosporine [104] and TNFα antibody (Etanercept) [105] and they have yielded promising results. Reduced number of Tregs have also been reported in ulcerative colitis, T1D, and EAE which highlight the importance of Tregs in preventing chronic autoimmune diseases [94]. Apart from these indirect approaches, there are several direct approaches tested to target Treg numbers/functions in clinical trials which can be classified as follows; 1) Adoptive Treg cellular therapy, 2) Approaches to induce in vivo expansion of Tregs.
5.1. Adoptive Treg cellular therapy.
In adoptive cellular therapy, Tregs sorted from peripheral blood based on CD4+CD25hiCD127low surface phenotype (where CD25hiCD127low expression is used as a surrogate for Foxp3 expression) are expanded in vitro through repeated rounds of TCR-stimulation using anti-CD3/CD28 microbeads in the presence of IL-2, and transfused back to the patients [106]. Initially, a similar approach with adoptive transfer of Tregs in NOD mice reversed ongoing diabetes[87]. In addition, the first clinical trial using the adoptive transfer of Tregs in patients with new-onset T1D produced promising results. Eight out of twelve patients met remission criteria for reduced insulin supplementation and one patient became insulin independent [12]. More recent clinical trial (NCT01210664) in T1D patients confirmed the stability of Tregs more than a year after transfusion and improved c-peptide levels in patients [13]. Currently, another clinical trial of adoptive Treg therapy in cutaneous lupus erythematosus is underway (NCT02428309).
Adoptive Treg immunotherapy offers an advantage of expanding antigen-specific Tregs in in vitro cultures for the better suppressive functions than polyclonal Tregs. However, it would be difficult to expand antigen-specific Tregs from peripheral blood because of their minuscule numbers in PBMCs[107]. Moreover, as discussed above, the mechanism of Treg-mediated immune suppression involves different mechanisms, such as immunosuppressive cytokine production and ATP deprivation, which may not require antigen-specificity for the desired effect. Moreover, a study by Szymczak-Workman et al. Using Tregs from two different TCR-transgenic mice (i.e. AND OTII strains), which had distinct and non-cross-reactive specificities showed that that the suppressive functions of Tregs were not dependent on antigen specificity, MHC-presentation or TCR stimulation [108]. In line with this notion, the aforementioned T1D clinical trials with polyclonal Tregs achieved endpoint criteria indicating the sufficiency of polyclonal Tregs. However, further studies comparing the efficacy of polyclonal Tregs and antigen-specific Tregs are necessary to reach a firm conclusion. Another risk associated with the expansion of antigen-specific Tregs is that it might require a higher number of cycles of TCR-stimulation in ex vivo cultures to expand sufficient number of Tregs for adoptive therapy and such repetitive TCRstimulation can lead to methylation of Foxp3 TSDR and loss of lineage stability [66]. If antigenspecific Tregs contained within the expanded polyclonal population lose their lineage stability and become Teff cells, they may cause pathology and exacerbate the autoimmune disease being treated.
5.2. Approaches to augment Tregs numbers in vivo
Although adoptive Treg cellular therapy has shown promising results in clinical trials, there are several limitations which impede their routine clinical use. These limitations include 1) Need for Good Manufacturing Practice (GMP) facilities for Treg sorting, ex vivo expansion, and transfusion, and their associated cost; 2) Lack of an approach to cause selective Treg expansion as TCR-stimulation may expand contaminating Teff cell population, if any; 3) Very low frequency of Tregs in the peripheral blood; and 4) Risk of losing lineage instability of Tregs upon repeated TCR-stimulation. Therefore, an approach that can expand Tregs in vivo with sustained lineage stability would be ideal for routine clinical utility. There are several approaches tested to augment Treg numbers/functions in vivo including low dose IL-2 therapy, induction of Tregs using tolerogenic dendritic cells (DCs), anti-CD3 monoclonal antibody therapy and rapamycin, etc.
5.2.1. Low dose IL-2 therapy
IL-2 was initially discovered as a T-cell growth factor and reportedly the first cytokine to be cloned. [109]. It was identified as an indispensable effector cytokine required for Teff cell responses. Later, IL-2 was found to play a key role in Treg differentiation, survival, and proliferation as discussed in previous sections. Dysregulations in IL-2 signaling were implicated in Treg homeostasis and autoimmunity[44]. While CD25, receptor for IL-2 (IL-2RA), is expressed on Teff cells upon TCR activation, it is constitutively expressed on naïve Tregs under resting conditions. However, Tregs have a greater sensitivity (~100 fold) to IL-2 than Teff cells [110], and thus low doses of IL-2 has been proposed to selectively expand Tregs while sparing Teff cells due to their lower affinity for IL-2[110, 111]. Based on this principle, several groups tested the efficacy of low dose IL-2 in NOD mice and found it resulting in the expansion of Tregs in vivo and suppress autoimmune diabetes [110, 112, 113]. In human trials, low dose IL-2 has been shown to augment Tregs with little or no effect on other T-cell subsets. T1D clinical trials (NCT00525889 - NCT02411253) [114] are in progress to determine whether low dose IL-2 therapy can reach expected end-point criteria of reduction in insulin dependency or insulin-free survival[115].
5.2.2. Tolerogenic DCs
DCs have been found to play a crucial role in the regulation of adaptive and immune responses since their discovery as antigen presenting cells by Steinman and his colleagues[116]. Over the course of time, a subset of DCs called tolerogenic DCs (tDCs) were identified, which exhibit anti-inflammatory functions by producing peace-keeping cytokines, inducing anergy in Teff cells and promoting Treg differentiation and expansion. The tDCs are derived from immature DC precursors with appropriate stimuli, such as TSLP, retinoic acid, TGF-β, IL-10, GM-CSF, Vitamin D3, etc. The tDcs were shown to increase Tregs both in vitro and in vivo [117]. Our laboratory has shown that tolerogenic DCs derived from mouse bonemarrow DC precursors educated with GM-CSF (G-BMDCs) induced preferential proliferation of Tregs, independent of canonical antigen presentation through MHC class-II, in an IL-2 dependent manner. This TCR-independent Treg proliferation was mediated through two membrane-bound ligands, OX40L (TNFSF4 ligand) and Jagged-1 (Notch ligand), which are expressed on the surface of G-BMDCs. The cognate interactions between OX40L and Jagged-1 with their receptors OX40 and Notch3 which are preferentially expressed on Tregs over Teff cells drives the preferential proliferation of Tregs [25, 58, 118]. Moreover, these tolerogenic GBMDCs upon adoptive transfer expanded Tregs in vivo and suppressed autoimmunity in various preclinical models, including T1D [88], EAT [119, 120], and EAMG [92]. Machen et al. showed that murine BMDCs educated with anti-sense nucleotides for co-stimulatory molecules like CD80, CD86, and CD40 expanded Tregs in NOD mice and reversed ongoing diabetes [121]. They also used a similar approach to derive human monocyte-derived DCs and in a phase-I clinical trial, these tDCs were well tolerated and increased CD4+CD25hiFoxp3+ Tregs[122]. A Phase II clinical trial (NCT02354911) from the same group is underway. In addition, two more phase I clinical trials (NCT02618902) and (NCT02903537) using myelin-derived, antigenic peptide pulsed-vitamin D3-conditioned DCs are under evaluation in MS patients[123].
5.2.3. Anti-CD3 monoclonal antibodies
Anti-CD3 monoclonal antibodies were tested to expand Tregs based on the principle that α-CD3-mAb will induce fast endocytosis of TCR-CD3 complex, and later re-expression of TCR and exposure to self-antigen can produce ‘altered’ TCR signal resulting in cell death, anergy or regulatory phenotype[124]. Based on this principle, α-CD3-mAb was shown to induce TGF-β-mediated peripheral Treg generation from Tconv cells in CD28−/−mice which lacks nTregs [125]. In contrast, Fc-receptor non-binding α-CD3-mAb did not expand/induce differentiation of Tregs in NOD mice but it caused selective depletion of Teff cells while sparing Tregs. This resulted in an increase in the frequency of Tregs, with no change in absolute numbers of Tregs, but prevention of autoimmune diabetes. Moreover, Tregs from α-CD3-mAb treated NOD mice had higher Helios (Treg suppressive marker) expression indicating a functional boost [126]. In humanized NSG mice, teplizumab-Fc-receptor non-binding α-CD3-mAb induced Tregs in the gut lamina propria. In human clinical trials, teplizumab did not increase CD4+CD25+Foxp3+ Tregs in peripheral blood but increased CD8+ Tregs expressing CD25, CTLA-4, Foxp3, and TNFR2 through TNF signaling [127]. However, in another human trial involving non-alcoholic steatohepatitis, oral administration of another anti-CD3 mAb (OKT3) caused an increase in peripheral blood CD4+ Tregs and improved metabolic, hepatic and immunologic parameters and ameliorated insulin resistance [128]. Thus, it is possible that α-CD3-mAb can increase CD4+/CD8+Tregs in vivo and enhance their functions although the underlying mechanism remains unclear.
5.2.4. Rapamycin
PI3K-AKT-mTOR signaling can negatively regulate Foxp3 expression in Tregs and Foxp3 induction in Tconv cells. Tregs express higher levels of PTEN, a natural inhibitor of the PI3K-AKT-mTOR pathway, and constitutive activation of the PI3 kinase in Tregs from PTEN deficient mice can lead to negative regulation of Foxp3 expression [129, 130]. Rapamycin is an inhibitor of the mTORC1 complex and it has been shown to increase Treg differentiation/expansion and suppressive functions while inhibiting Th1/Th2/Th17 cells [131, 132]. In NOD mice, rapamycin + IL10 combination therapy increased CD4+CD25+Foxp3+ Tregs and IL-10 producing Tr1 cells and suppressed diabetes onset[133]. In a human clinical trial, rapamycin monotherapy failed to modulate CD4+CD25highFoxp3+ Treg numbers or their proliferation capacity, or effector cytokine production, but improved suppressive Treg functions [134]. In another clinical trial with rapamycin + IL-2 combination therapy, a transient impairment in pancreatic β-cell function was observed despite an increase in functional Treg numbers which was attributed to the undesired effect of rapamycin on other innate immune cell types [135].
6. Conclusion
Accumulating evidence shows that Foxp3+Treg repertoire does not constitute a homogenous population and is composed of diverse subsets of cells. However, very little is known about their unique functional attributes, if any. Further studies are warranted to understand how these different subsets of Tregs vary in their functionality and their contribution to immune homeostasis and tolerance. Moreover, Treg subsets may also include a lineage unstable population that can lose their suppressive function and contribute to autoimmunity under certain conditions. Recent studies have identified several epigenetic events regulating Foxp3 expression in Tregs. These studies have raised the possibility of altering the Treg phenotype and increasing their fidelity and thus aid in the development of protocols for the expansion of stable Tregs. Although human Treg adoptive therapy has shown promising results in early clinical trials, efforts are needed to make such an approach more feasible for routine clinical use. Moreover, the results of ongoing clinical trials will shed light on its broader applicability in different autoimmune diseases. Approaches to enhance Treg numbers and/or function in vivo are highly desirable and will be more suitable for routine clinical use. However, it has become evident from clinical trials that use of IL-2/rapamycin to selectively target Tregs under autoimmune conditions may pose problems. This could be because of the shared expression of CD25 by Tregs and activated effector-memory-T cells, which can lead to off-target effects [136] resulting in exacerbation of the disease being treated. In spite of the proven importance of Tregs in preclinical studies and their relevance in human autoimmune diseases, reports available on human Treg development and homeostasis are sparse. Most of the currently available data on human Tregs were derived using peripheral blood Tregs which are strikingly different from tissue-residentTregs. Moreover, we and others have identified several striking differences between murine and human Tregs in terms of their thymic development and heterogeneity [21, 137, 138]. Thus, relevant human studies on Treg development and homeostasis should be encouraged for the successful clinical translation of Treg based therapies.
Highlights.
Recent advances in the understanding of murine and human thymic and peripheral Treg development are discussed.
Transcriptional and epigenetic mechanisms regulating Treg differentiation and lineage stability are summarized.
Various approaches used to expand Tregs and enhance Treg functions, their advantages, and limitations, and their potential clinical utility to treat autoimmune diseases are reviewed.
Acknowledgments
This study was supported by the grant R01 AI107516–01A1 to Dr. Prabhakar from the National Institutes of Health.
Footnotes
Disclosure of conflict of interest
The authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kronenberg M, Self-tolerance and autoimmunity, Cell, 65 (1991) 537–542. [DOI] [PubMed] [Google Scholar]
- [2].Wang L, Wang FS, Gershwin ME, Human autoimmune diseases: a comprehensive update, J Intern Med, 278 (2015) 369–395. [DOI] [PubMed] [Google Scholar]
- [3].Sakaguchi S, Yamaguchi T, Nomura T, Ono M, Regulatory T cells and immune tolerance, Cell, 133 (2008) 775–787. [DOI] [PubMed] [Google Scholar]
- [4].Josefowicz SZ, Lu LF, Rudensky AY, Regulatory T cells: mechanisms of differentiation and function, Annu Rev Immunol, 30 (2012) 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xing Y, Hogquist KA, T-cell tolerance: central and peripheral, Cold Spring Harb Perspect Biol, 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yadav M, Stephan S, Bluestone JA, Peripherally induced tregs - role in immune homeostasis and autoimmunity, Front Immunol, 4 (2013) 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li L, Boussiotis VA, Physiologic regulation of central and peripheral T cell tolerance: lessons for therapeutic applications, J Mol Med (Berl), 84 (2006) 887–899. [DOI] [PubMed] [Google Scholar]
- [8].Fontenot JD, Gavin MA, Rudensky AY, Foxp3 programs the development and function of CD4(+)CD25(+) regulatory T cells, Nat Immunol, 4 (2003) 330–336. [DOI] [PubMed] [Google Scholar]
- [9].Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F, Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse, Nat Genet, 27 (2001) 68–73. [DOI] [PubMed] [Google Scholar]
- [10].Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD, The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3, Nature Genetics, 27 (2001) 20–21. [DOI] [PubMed] [Google Scholar]
- [11].Qiao YC, Pan YH, Ling W, Tian F, Chen YL, Zhang XX, Zhao HL, The Yin and Yang of regulatory T cell and therapy progress in autoimmune disease, Autoimmun Rev, 16 (2017) 1058–1070. [DOI] [PubMed] [Google Scholar]
- [12].Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, Owczuk R, Szadkowska A, Witkowski P, Mlynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P, Therapy of type 1 diabetes with CD4(+)CD25(high) CD127-regulatory T cells prolongs survival of pancreatic islets - Results of one year follow-up, Clin Immunol, 153 (2014) 23–30. [DOI] [PubMed] [Google Scholar]
- [13].Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q, Type 1 diabetes immunotherapy using polyclonal regulatory T cells, Sci Transl Med, 7 (2015) 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nishizuka Y, Sakakura T, Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice, Science, 166 (1969) 753–755. [DOI] [PubMed] [Google Scholar]
- [15].Asano M, Toda M, Sakaguchi N, Sakaguchi S, Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation, J Exp Med, 184 (1996) 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hori S, Nomura T, Sakaguchi S, Control of regulatory T cell development by the transcription factor Foxp3, Science, 299 (2003) 1057–1061. [DOI] [PubMed] [Google Scholar]
- [17].Hsieh CS, Lee HM, Lio CW, Selection of regulatory T cells in the thymus, Nat Rev Immunol, 12 (2012) 157–167. [DOI] [PubMed] [Google Scholar]
- [18].Lio CW, Hsieh CS, A two-step process for thymic regulatory T cell development, Immunity, 28 (2008) 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, Farrar MA, Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells, Nat Immunol, 15 (2014) 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA, T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse, J Exp Med, 208 (2011) 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kumar P, Marinelarena A, Raghunathan D, Ragothaman VK, Saini S, Bhattacharya P, Fan J, Epstein AL, Maker AV, Prabhakar BS, Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation, Cellular And Molecular Immunology, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baldwin TA, Sandau MM, Jameson SC, Hogquist KA, The timing of TCR alpha expression critically influences T cell development and selection, J Exp Med, 202 (2005) 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer DS, Singer A, Foxp3 Transcription Factor Is Proapoptotic and Lethal to Developing Regulatory T Cells unless Counterbalanced by Cytokine Survival Signals, Immunity, 38 (2013) 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Caramalho I, Nunes-Silva V, Pires AR, Mota C, Pinto AI, Nunes-Cabaco H, Foxall RB, Sousa AE, Human regulatory T-cell development is dictated by Interleukin-2 and −15 expressed in a nonoverlapping pattern in the thymus, J Autoimmun, 56 (2015) 98–110. [DOI] [PubMed] [Google Scholar]
- [25].Kumar P, Alharshawi K, Bhattacharya P, Marinelarena A, Haddad C, Sun Z, Chiba S, Epstein AL, Prabhakar BS, Soluble OX40L and JAG1 Induce Selective Proliferation of Functional Regulatory T-Cells Independent of canonical TCR signaling, Sci Rep, 7 (2017) 39751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB, Molecular mechanisms underlying FOXP3 induction in human T cells, J Immunol, 176 (2006) 3593–3602. [DOI] [PubMed] [Google Scholar]
- [27].Okada M, Hibino S, Someya K, Yoshmura A, Regulation of regulatory T cells: epigenetics and plasticity, Adv Immunol, 124 (2014) 249–273. [DOI] [PubMed] [Google Scholar]
- [28].Kitagawa Y, Ohkura N, Kidani Y, Vandenbon A, Hirota K, Kawakami R, Yasuda K, Motooka D, Nakamura S, Kondo M, Taniuchi I, Kohwi-Shigematsu T, Sakaguchi S, Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment, Nat Immunol, 18 (2017) 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee W, Lee GR, Transcriptional regulation and development of regulatory T cells, Exp Mol Med, 50 (2018) e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M, Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer, Nat Immunol, 9 (2008) 194–202. [DOI] [PubMed] [Google Scholar]
- [31].Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY, Role of conserved noncoding DNA elements in the Foxp3 gene in regulatory T-cell fate, Nature, 463 (2010) 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim HP, Leonard WJ, CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation, J Exp Med, 204 (2007) 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burchill MA, Yang JY, Vogtenhuber C, Blazar BR, Farrar MA, IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3(+) regulatory T cells, J Immunol, 178 (2007) 280–290. [DOI] [PubMed] [Google Scholar]
- [34].Feng YQ, Arvey A, Chinen T, Van der Veeken JD, Gasteiger G, Rudensky AY, Control of the Inheritance of Regulatory T Cell Identity by a cis Element in the Foxp3 Locus, Cell, 158 (2014) 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J, Epigenetic control of the foxp3 locus in regulatory T cells, Plos Biol, 5 (2007) 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ruan QG, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YHH, Development of Foxp3(+) Regulatory T Cells Is Driven by the c-Rel Enhanceosome, Immunity, 31 (2009) 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Long M, Park SG, Strickland I, Hayden MS, Ghosh S, Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor, Immunity, 31 (2009) 921–931. [DOI] [PubMed] [Google Scholar]
- [38].Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M, NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4(+) T lymphocytes by CD4(+) CD25(+) regulatory T cells, J Exp Med, 201 (2005) 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu LL, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W, Positive and Negative Transcriptional Regulation of the Foxp3 Gene is Mediated by Access and Binding of the Smad3 Protein to Enhancer I, Immunity, 33 (2010) 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burchill MA, Yang JY, Vang KB, Moon JJ, Chu HH, Lio CWJ, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA, Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire, Immunity, 28 (2008) 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bayer AL, Pugliese A, Malek TR, The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation, Immunol Res, 57 (2013) 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baeyens A, Saadoun D, Billiard F, Rouers A, Gregoire S, Zaragoza B, Grinberg-Bleyer Y, Marodon G, Piaggio E, Salomon BL, Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context, J Immunol, 194 (2015) 999–1010. [DOI] [PubMed] [Google Scholar]
- [43].Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu CY, O’Shea JJ, Nonredundant roles for Stat5a/b in directly regulating Foxp3, Blood, 109 (2007) 4368–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Antov A, Yang L, Vig M, Baltimore D, Van Parijs L, Essential role for STAT5 signaling in CD25(+)CD4(+) regulatory T cell homeostasis and the maintenance of self-tolerance, J Immunol, 171 (2003) 3435–3441. [DOI] [PubMed] [Google Scholar]
- [45].Sekiya T, Kashiwagi I, Inoue N, Morita R, Hori S, Waldmann H, Rudensky AY, Ichinose H, Metzger D, Chambon P, Yoshimura A, The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells, Nat Commun, 2 (2011) 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sekiya T, Kashiwagi I, Yoshida R, Fukaya T, Morita R, Kimura A, Ichinose H, Metzger D, Chambon P, Yoshimura A, Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis, Nat Immunol, 14 (2013) 230–237. [DOI] [PubMed] [Google Scholar]
- [47].Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H, Inducing and expanding regulatory T cell populations by foreign antigen, Nat Immunol, 6 (2005) 1219–1227. [DOI] [PubMed] [Google Scholar]
- [48].Bilate AM, Lafaille JJ, Induced CD4+Foxp3+ regulatory T cells in immune tolerance, Annu Rev Immunol, 30 (2012) 733–758. [DOI] [PubMed] [Google Scholar]
- [49].Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY, Extrathymically generated regulatory T cells control mucosal TH2 inflammation, Nature, 482 (2012) 395399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB, A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity, Immunity, 35 (2011) 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA, Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo, J Exp Med, 209 (2012) 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Milpied P, Renand A, Bruneau J, Mendes-Da-Cruz DA, Jacquelin S, Asnafi V, Rubio MT, MacIntyre E, Lepelletier Y, Hermine O, Neuropilin-1 is not a marker of human Foxp3(+) Treg, European Journal of Immunology, 39 (2009) 1466–1471. [DOI] [PubMed] [Google Scholar]
- [53].Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S, T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development, Immunity, 37 (2012) 785–799. [DOI] [PubMed] [Google Scholar]
- [54].Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J, DNA methylation controls Foxp3 gene expression, Eur J Immunol, 38 (2008) 16541663. [DOI] [PubMed] [Google Scholar]
- [55].Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C, Genomic definition of multiple ex vivo regulatory T cell subphenotypes, Proc Natl Acad Sci U S A, 107 (2010) 5919–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H, Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis, Nat Med, 20 (2014) 62–68. [DOI] [PubMed] [Google Scholar]
- [57].Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM, IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo, J Immunol, 186 (2011) 6329–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS, GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms, J Leukoc Biol, 89 (2011) 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang Z, Zhang W, Guo J, Gu Q, Zhu X, Zhou X, Activation and Functional Specialization of Regulatory T Cells Lead to the Generation of Foxp3 Instability, J Immunol, 198 (2017) 2612–2625. [DOI] [PubMed] [Google Scholar]
- [60].Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA, Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response, Immunity, 39 (2013) 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE, Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells, Eur J Immunol, 37 (2007) 129–138. [DOI] [PubMed] [Google Scholar]
- [62].Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S, Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor, Immunity, 30 (2009) 899–911. [DOI] [PubMed] [Google Scholar]
- [63].Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, Shi HD, Munn DH, An Inherently Bifunctional Subset of Foxp3(+) T Helper Cells Is Controlled by the Transcription Factor Eos, Immunity, 38 (2013) 998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK, Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production, International Immunology, 19 (2007) 345–354. [DOI] [PubMed] [Google Scholar]
- [65].Pan X, Yuan X, Zheng Y, Wang W, Shan J, Lin F, Jiang G, Yang YH, Wang D, Xu D, Shen L, Increased CD45RA+ FoxP3(low) regulatory T cells with impaired suppressive function in patients with systemic lupus erythematosus, Plos One, 7 (2012) e34662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M, Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation, Eur J Immunol, 39 (2009) 1088–1097. [DOI] [PubMed] [Google Scholar]
- [67].Josefowicz SZ, Wilson CB, Rudensky AY, Cutting Edge: TCR Stimulation Is Sufficient for Induction of Foxp3 Expression in the Absence of DNA Methyltransferase 1, J Immunol, 182 (2009) 6648–6652. [DOI] [PubMed] [Google Scholar]
- [68].Riley JL, June CH, Blazar BR, Human T Regulatory Cell Therapy: Take a Billion or So and Call Me in the Morning, Immunity, 30 (2009) 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hippen KL, Riley JL, June CH, Blazar BR, Clinical perspectives for regulatory T cells in transplantation tolerance, Semin Immunol, 23 (2011) 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Walunas TL, Bakker CY, Bluestone JA, CTLA-4 ligation blocks CD28-dependent T cell activation, J Exp Med, 183 (1996) 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM, Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4, Science, 332 (2011) 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Blake SJ, Dougall WC, Miles JJ, Teng MW, Smyth MJ, Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy, Clin Cancer Res, 22 (2016) 5183–5188. [DOI] [PubMed] [Google Scholar]
- [73].Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM, CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner, J Exp Med, 205 (2008) 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA, Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells, Immunology, 117 (2006) 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kumar P, Subramaniyam G, Molecular underpinnings of Th17 immune-regulation and their implications in autoimmune diabetes, Cytokine, 71 (2015) 366–376. [DOI] [PubMed] [Google Scholar]
- [76].Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F, An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation, J Exp Med, 190 (1999) 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Asseman C, Read S, Powrie F, Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: Control by CD4(+) regulatory T cells and IL-10, J Immunol, 171 (2003) 971–978. [DOI] [PubMed] [Google Scholar]
- [78].Sojka DK, Fowell DJ, Regulatory T cells inhibit acute IFN-gamma synthesis without blocking Thelper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10, P Natl Acad Sci USA, 108 (2011) 18336–18341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DAA, The inhibitory cytokine IL-35 contributes to regulatory T-cell function, Nature, 450 (2007) 566–U519. [DOI] [PubMed] [Google Scholar]
- [80].Antonioli L, Pacher P, Vizi ES, Hasko G, CD39 and CD73 in immunity and inflammation, Trends Mol Med, 19 (2013) 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Romio M, Reinbeck B, Bongardt S, Huls S, Burghoff S, Schrader J, Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells, Am J Physiol Cell Physiol, 301 (2011) C530–539. [DOI] [PubMed] [Google Scholar]
- [82].Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ, Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism, J Immunol, 174 (2005) 1783–1786. [DOI] [PubMed] [Google Scholar]
- [83].Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM, Activated CD4+CD25+ T cells selectively kill B lymphocytes, Blood, 107 (2006) 3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ, Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance, Immunity, 27 (2007) 635–646. [DOI] [PubMed] [Google Scholar]
- [85].McGeachy MJ, Stephens LA, Anderton SM, Natural recovery and protection from autoimmune encephalomyelitis: Contribution of CD4(+)CD25(+) regulatory cells within the central nervous system, J Immunol, 175 (2005) 3025–3032. [DOI] [PubMed] [Google Scholar]
- [86].Mondal S, Martinson JA, Ghosh S, Watson R, Pahan K, Protection of Tregs, Suppression of Th1 and Th17 Cells, and Amelioration of Experimental Allergic Encephalomyelitis by a Physically-Modified Saline, Plos One, 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA, In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes, J Exp Med, 199 (2004) 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cheatem D, Ganesh BB, Gangi E, Vasu C, Prabhakar BS, Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function, Clin Immunol, 131 (2009) 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY, Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40, Proc Natl Acad Sci U S A, 104 (2007) 15478–15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Morgan ME, Sutmuller RPM, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJM, Snijders A, Offringa R, de Vries RRP, Toes REM, CD25+cell depletion hastens the onset of severe disease in collagen-induced arthritis, Arthritis Rheum, 48 (2003) 1452–1460. [DOI] [PubMed] [Google Scholar]
- [91].Hayashi T, Hasegawa K, Adachi C, Elimination of CD4(+)CD25(+) T cell accelerates the development of glomerulonephritis during the preactive phase in autoimmune-prone female NZB x NZW F-1 mice, Int J Exp Pathol, 86 (2005) 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN, Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells, J Immunol, 177 (2006) 5296–5306. [DOI] [PubMed] [Google Scholar]
- [93].Ferreira RC, Simons HZ, Thompson WS, Rainbow DB, Yang X, Cutler AJ, Oliveira J, Castro Dopico X, Smyth DJ, Savinykh N, Mashar M, Vyse TJ, Dunger DB, Baxendale H, Chandra A, Wallace C, Todd JA, Wicker LS, Pekalski ML, Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity, Journal of Autoimmunity, 84 (2017) 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shu Y, Hu Q, Long H, Chang C, Lu Q, Xiao R, Epigenetic Variability of CD4+CD25+ Tregs Contributes to the Pathogenesis of Autoimmune Diseases, Clinical Reviews in Allergy & Immunology, 52 (2017) 260–272. [DOI] [PubMed] [Google Scholar]
- [95].Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG, The imbalance between regulatory and IL-17secreting CD4+ T cells in lupus patients, Clinical rheumatology, 29 (2010) 1251–1258. [DOI] [PubMed] [Google Scholar]
- [96].Regna NL, Chafin CB, Hammond SE, Puthiyaveetil AG, Caudell DL, Reilly CM, Class I and II Histone Deacetylase Inhibition by ITF2357 Reduces SLE Pathogenesis In Vivo, Clinical immunology (Orlando, Fla.), 151 (2014) 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Qiao G, Yang L, Li Z, Williams JW, Zhang J, A77 1726, the active metabolite of leflunomide, attenuates lupus nephritis by promoting the development of regulatory T cells and inhibiting IL-17producing double negative T cells, Clin Immunol, 157 (2015) 166–174. [DOI] [PubMed] [Google Scholar]
- [98].Koga T, Mizui M, Yoshida N, Otomo K, Lieberman LA, Crispin JC, Tsokos GC, KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3(+) regulatory T cells in MRL/lpr mice, Autoimmunity, 47 (2014) 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A, Osella-Abate S, De Simone C, Marzano A, Bernengo MG, Fabbri P, Caproni M, Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea, The British journal of dermatology, 162 (2010) 1056–1063. [DOI] [PubMed] [Google Scholar]
- [100].Wang YY, Wang Q, Sun XH, Liu RZ, Shu Y, Kanekura T, Huang JH, Li YP, Wang JC, Zhao M, Lu QJ, Xiao R, DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis, The British journal of dermatology, 171 (2014) 39–47. [DOI] [PubMed] [Google Scholar]
- [101].Kawashiri SY, Kawakami A, Okada A, Koga T, Tamai M, Yamasaki S, Nakamura H, Origuchi T, Ida H, Eguchi K, CD4+CD25(high)CD127(low/-) Treg cell frequency from peripheral blood correlates with disease activity in patients with rheumatoid arthritis, The Journal of rheumatology, 38 (2011) 25172521. [DOI] [PubMed] [Google Scholar]
- [102].Cribbs AP, Kennedy A, Penn H, Amjadi P, Green P, Read JE, Brennan F, Gregory B, Williams RO, Methotrexate Restores Regulatory T Cell Function Through Demethylation of the FoxP3 Upstream Enhancer in Patients With Rheumatoid Arthritis, Arthritis & rheumatology (Hoboken, N.J.), 67 (2015) 1182–1192. [DOI] [PubMed] [Google Scholar]
- [103].Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ, Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin, The Journal of investigative dermatology, 131 (2011) 1853–1860. [DOI] [PubMed] [Google Scholar]
- [104].El-Darouti MA, Hegazy RA, Abdel Hay RM, Rashed LA, Study of T helper (17) and T regulatory cells in psoriatic patients receiving live attenuated varicella vaccine therapy in a randomized controlled trial, European journal of dermatology : EJD, 24 (2014) 464–469. [DOI] [PubMed] [Google Scholar]
- [105].Quaglino P, Bergallo M, Ponti R, Barberio E, Cicchelli S, Buffa E, Comessatti A, Costa C, Terlizzi ME, Astegiano S, Novelli M, Cavallo R, Bernengo MG, Th1, Th2, Th17 and regulatory T cell pattern in psoriatic patients: modulation of cytokines and gene targets induced by etanercept treatment and correlation with clinical response, Dermatology (Basel, Switzerland), 223 (2011) 57–67. [DOI] [PubMed] [Google Scholar]
- [106].Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA, Expansion of human regulatory T-cells from patients with type 1 diabetes, Diabetes, 58 (2009) 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Perdigoto AL, Chatenoud L, Bluestone JA, Herold KC, Inducing and Administering Tregs to Treat Human Disease, Front Immunol, 6 (2015) 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Szymczak-Workman AL, Workman CJ, Vignali DA, Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress, J Immunol, 182 (2009) 5188–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Robb RJ, Interleukin 2: the molecule and its function, Immunol Today, 5 (1984) 203–209. [DOI] [PubMed] [Google Scholar]
- [110].Yu AX, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR, Selective IL-2 Responsiveness of Regulatory T Cells Through Multiple Intrinsic Mechanisms Supports the Use of Low-Dose IL-2 Therapy in Type 1 Diabetes, Diabetes, 64 (2015) 2172–2183. [DOI] [PubMed] [Google Scholar]
- [111].Bensinger SJ, Walsh PT, Zhang JD, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA, Distinct IL-2 receptor signaling pattern in CD4(+)CD25(+) regulatory T cells, J Immunol, 172 (2004) 5287–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA, Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction, Immunity, 28 (2008) 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang QZ, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E, IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells, J Exp Med, 207 (2010) 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cabrera SM, Rigby MR, Mirmira RG, Targeting Regulatory T Cells in the Treatment of Type 1 Diabetes Mellitus, Curr Mol Med, 12 (2012) 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dwyer CJ, Ward NC, Pugliese A, Malek TR, Promoting Immune Regulation in Type 1 Diabetes Using Low-Dose Interleukin-2, Curr Diabetes Rep, 16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Steinman RM, Cohn ZA, Identification of a novel cell type in peripheral lymphoid organs of mice - I. Morphology, quantitation, tissue distribution (Reprinted from J Exp Med, vol 137, pg 1142–1162, 1973), J Immunol, 178 (2007) 5–25. [PubMed] [Google Scholar]
- [117].Maldonado RA, von Andrian UH, How Tolerogenic Dendritic Cells Induce Regulatory T Cells, Advances in Immunology, Vol 108, 108 (2010) 111–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gopisetty A, Bhattacharya P, Haddad C, Bruno JC Jr., Vasu C, Miele L, Prabhakar BS, OX40L/Jagged1 cosignaling by GM-CSF-induced bone marrow-derived dendritic cells is required for the expansion of functional regulatory T cells, J Immunol, 190 (2013) 5516–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Vasu C, Dogan RN, Holterman MJ, Prabhakar BS, Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3 -ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis, J Immunol, 170 (2003) 5511–5522. [DOI] [PubMed] [Google Scholar]
- [120].Gangi E, Vasu C, Cheatem D, Prabhakar BS, IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis, J Immunol, 174 (2005) 7006–7013. [DOI] [PubMed] [Google Scholar]
- [121].Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N, Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells, J Immunol, 173 (2004) 4331–4341. [DOI] [PubMed] [Google Scholar]
- [122].Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M, Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients, Diabetes Care, 34 (2011) 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Florez-Grau G, Zubizarreta I, Cabezon R, Villoslada P, Benitez-Ribas D, Tolerogenic Dendritic Cells as a Promising Antigen-Specific Therapy in the Treatment of Multiple Sclerosis and Neuromyelitis Optica From Preclinical to Clinical Trials, Frontiers in Immunology, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chatenoud L, Bluestone JA, CD3-specific antibodies: a portal to the treatment of autoimmunity, Nature Reviews Immunology, 7 (2007) 622–632. [DOI] [PubMed] [Google Scholar]
- [125].Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L, TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes, Nature Medicine, 9 (2003) 1202–1208. [DOI] [PubMed] [Google Scholar]
- [126].Penaranda C, Tang QZ, Bluestone JA, Anti-CD3 Therapy Promotes Tolerance by Selectively Depleting Pathogenic Cells while Preserving Regulatory T Cells, J Immunol, 187 (2011) 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ablamunits V, Bisikirska B, Herold KC, Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF, Eur J Immunol, 40 (2010) 2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya’acov A, Shabat Y, Nimer A, Hemed N, Zolotarovya L, Lichtenstein Y, Lisovoder N, Samira S, Shalit I, Ellis R, Ilan Y, Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial, J Clin Immunol, 35 (2015) 399–407. [DOI] [PubMed] [Google Scholar]
- [129].Yu AX, Zhu LJ, Altman NH, Malek TR, A Low Interleukin-2 Receptor Signaling Threshold Supports the Development and Homeostasis of T Regulatory Cells, Immunity, 30 (2009) 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M, T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR, P Natl Acad Sci USA, 105 (2008) 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kopf H, de la Rosa GM, Howard OM, Chen X, Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells, Int Immunopharmacol, 7 (2007) 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yuan LF, Li GD, Ren XJ, Nian H, Li XR, Zhang XM, Rapamycin ameliorates experimental autoimmune uveoretinitis by inhibiting Th1/Th2/Th17 cells and upregulating CD4+CD25+ Foxp3 regulatory T cells, Int J Ophthalmol, 8 (2015) 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, Roncarolo MG, Induction of tolerance in type 1 diabetes via both CD4(+) CD25(+) T regulatory cells and T regulatory type 1 cells, Diabetes, 55 (2006) 1571–1580. [DOI] [PubMed] [Google Scholar]
- [134].Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo MG, Battaglia M, Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory Tcells, Diabetes, 57 (2008) 2341–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ, Tolerance DTI, Rapamycin/IL-2 Combination Therapy in Patients With Type 1 Diabetes Augments Tregs Yet Transiently Impairs beta-cell Function, Diabetes, 61 (2012) 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Sakaguchi S, Miyara M, Costantino CM, Hafler DA, FOXP3(+) regulatory T cells in the human immune system, Nature Reviews Immunology, 10 (2010) 490–500. [DOI] [PubMed] [Google Scholar]
- [137].Caramalho I, Nunes-Cabaco H, Foxall RB, Sousa AE, Regulatory T-cell development in the human thymus, Frontiers in Immunology, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, Bank I, Kloog Y, Rechavi G, Goldstein I, TNF Activates a NF-kappa B-Regulated Cellular Program in Human CD45RA(−) Regulatory T Cells that Modulates Their Suppressive Function, J Immunol, 184 (2010) 3570–3581. [DOI] [PubMed] [Google Scholar]