Abstract
Background
Surgical site infection (SSI) is one of the most common complications of abdominal surgery and is associated with substantial discomfort, morbidity and cost. The goal of this study was to describe the incidence, bacteriology and risk factors associated with SSI in patients undergoing abdominal surgery.
Methods
In this prospective cohort study, all patients aged 14 years or more undergoing abdominal surgery between Feb. 1 and July 31, 2016, at a single large academic hospital were included. Patients undergoing vascular, gynecological, urological or plastic procedures were excluded. Patients were followed prospectively for 30 days. Wound assessment was done with the Centers for Disease Control and Prevention definition of SSI. We performed multivariate analysis to identify factors associated with SSI.
Results
A total of 337 patients were included. The overall incidence of SSI was 16.3% (55/337); 5 patients (9%) had deep infections, and 25 (45%) had combined superficial and deep infections. The incidence of SSI in open versus laparoscopic operations was 35% versus 4% (p < 0.001). The bacteria most commonly isolated were extended-spectrum β-lactamase-producing Escherichia coli, followed by Enterococcus species. Only 23% of cultured bacteria were sensitive to the prophylactic antibiotic given preoperatively. The independent predictors of SSI were open surgical approach, emergency operation, longed operation duration and male sex.
Conclusion
Potentially modifiable independent risk factors for SSI after abdominal surgery including open surgical approach, contaminated wound class and emergency surgery should be addressed systematically. We recommend tailoring the antibiotic prophylactic regimen to target the commonly isolated organisms in patients at higher risk for SSI.
Abstract
Contexte
L’infection de plaie opératoire (IPO) est l’une des plus fréquentes complications de la chirurgie abdominale et elle est associée à un inconfort, une morbidité et des coûts substantiels. L’objectif de cette étude était de décrire l’incidence, les données bactériologiques et les facteurs de risque associés à l’IPO chez les patients soumis à une chirurgie abdominale.
Méthodes
Dans cette étude de cohorte, tous les patients de 14 ans ou plus soumis à une chirurgie abdominale entre le 1er février et le 31 juillet 2016 dans un seul grand établissement hospitalier universitaire ont été inclus. Les chirurgies vasculaires, gynécologiques, urologiques ou plastiques ont été exclues. Les patients ont été suivis de façon prospective pendant 30 jours. L’évaluation des plaies a été effectuée à partir de la définition de l’IPO des Centers for Disease Control and Prevention. Nous avons procédé à une analyse multivariée afin d’identifier les facteurs associés à l’IPO.
Résultats
En tout, 337 patients ont été inclus. L’incidence globale des IPO a été de 16,3 % (55/337); 5 patients (9 %) ont présenté des infections profondes, et 25 (45 %) ont présenté des infections superficielles et profondes. L’incidence des IPO lors d’interventions ouvertes c. laparoscopiques a été de 35 % c. 4 % (p < 0,001). Les bactéries les plus souvent isolées étaient Escherichia coli productrices de β-lactamases à spectre élargi, suivies du genre Enterococcus. Seulement 23 % des bactéries cultivées se sont révélées sensibles à l’antibioprophylaxie administrée avant l’intervention. Les prédicteurs indépendants d’une IPO étaient l’approche chirurgicale ouverte, le caractère urgent de l’intervention, sa durée prolongée et le fait d’être de sexe masculin.
Conclusion
Dans le contexte de la chirurgie abdominale, les facteurs de risque d’IPO indépendants potentiellement modifiables, incluant l’approche ouverte, la classification de la contamination de la plaie et le caractère urgent de la chirurgie, méritent d’être systématiquement pris en compte et corrigés. Nous recommandons une antibioprophylaxie adaptée pour cibler les agents souvent isolés chez les patients exposés à un risque plus élevé d’IPO.
Surgical site infection (SSI) is defined by the Centers for Disease Control and Prevention as a wound infection that occurs within 30 days of an operative procedure or within a year if an implant is left in place and the infection is thought to be secondary to surgery.1 It is one of the most common health-care–associated infections, occurring following 1%–3% of all surgical procedures.2 The rates of SSI are much higher with abdominal surgery than with other types of surgery, with several prospective studies indicating an incidence of 15%–25% depending on the level of contamination.2–5 Surgical site infection is preventable and is associated with high morbidity and mortality. In addition to the devastating impact on the patient’s course of treatment, it is associated with prolonged length of hospital stay and higher costs.6–8 Numerous risk factors may contribute to the development of SSI, with the most recognized factors being these incorporated in the Centers for Disease Control and Prevention and National Nosocomial Infections Surveillance System SSI risk index, including wound classification, American Society of Anesthesiologists (ASA) score and duration of the operation.9
The primary objectives of this study were to describe the incidence and risk factors associated with SSI in patients undergoing abdominal surgery. The secondary objectives were to study the microbiological pattern of SSI in our population and their antibiotic sensitivity, and to study the effect of SSI on postoperative length of stay.
Methods
Study design and participants
We conducted a prospective cohort study at King Abdulaziz University Hospital in Jeddah, Saudi Arabia. The target population consisted of all patients aged 14 years or more undergoing abdominal surgery from Feb. 1 to July 31, 2016. Patients were identified with the use of the operating room electronic registry. We excluded patients undergoing abdominal surgery for vascular, gynecological, urological or plastic indications. Patients who left the operating theatre with an open packed wound or with a vacuum-assisted dressing were also excluded. Ethics approval was obtained from the institutional review board.
Data collection
Variables included in the analysis were patient demographic characteristics, preoperative risk factors (diabetes mellitus, immunosuppression, HIV infection, use of chemotherapy and steroid use), smoking status, body mass index, ASA classification, and preoperative hemoglobin and albumin levels. Operative variables included operation performed, duration of surgery, use of prophylactic antibiotics, wound contamination class, surgical approach (open v. laparoscopic), urgency of surgery and drain use. Outcome measures studied in addition to wound infection included admission to the intensive care unit, length of stay, postoperative complications and death.
Patients were followed prospectively for 30 days in the ward, outpatient clinic or dressing clinic or through telephone interview. Wound assessment was done with the Centers for Disease Control and Prevention and National Healthcare Safety Network definition of SSI.1 Surgical site infection was classified as superficial (involving the skin and subcutaneous tissue only), deep (involving deeper soft tissues such as fascia and muscle layers) or organ space (involving any part of the anatomy that was opened or manipulated during surgery) (Table 1). The results of culture and antimicrobial sensitivity were included.
Table 1.
Surgical site infection classification according to the Centers for Disease Control and Prevention and National Healthcare Safety Network1
| Superficial incisional SSI | Deep incisional SSI |
|---|---|
| Occurs within 30 d | Occurs within 30 d |
| Only skin and subcutaneous tissue | Deep soft tissues (fascial and muscle layers) |
Patient has at least 1 of the following:
|
Patient has at least 1 of the following:
|
| And patient has at least 1 of the following: pain or tenderness, localized swelling, erythema or heat | And patient has at least 1 of the following: fever (temperature > 38°C), localized pain or tenderness |
SSI = surgical site infection.
Statistical analysis
Discrete variables were described as frequency and proportion, and continuous variables were described as mean and standard deviation or median and interquartile range. We used the Pearson and Wilcoxon univariable tests to guide the multivariable models for discrete and continuous variables, respectively. We used multivariable logistic regression models to identify preoperative and operative variables independently associated with SSI. Model fit was assured with bootstrap validation and calibration. Normality and linearity were tested, and appropriate transformation was incorporated as needed. We used the R statistical package (R Foundation for Statistical Computing) for the analyses.
Results
In total, 337 patients were enrolled in the study, 193 females (57.3%) and 144 males (42.7%) with a mean age of 43.6 years. All patients completed the 30-day follow-up apart from those who died before 30 days (n = 4). The mean body mass index was 31. Seventy patients (20.8%) were diabetic, and 41 (12.2%) were smokers. Chemotherapy, systemic steroid therapy and other immunosuppressive medications were not common in this cohort (Table 2). Most patients (279 [82.8%]) had an ASA score less than 3. The mean operative time was 145.2 minutes. A total of 199 cases (59.0%) were performed laparoscopically. Most patients (257 [76.3%]) underwent elective surgery. The most frequent type of surgery was laparoscopic cholecystectomy (111 procedures [32.9%]), followed by hernia repair (67 [19.9%]) and bariatric surgery (56 [16.6%]). Most operations (257 [76.3%]) were classified as clean-contaminated.
Table 2.
Incidence of surgical site infection by wound class following open and laparoscopic surgery
| Wound class | Surgical approach; no. of patients (% with SSI) | ||
|---|---|---|---|
| Open | Laparoscopic | All | |
| Clean | 51 (5.9) | 5 (0.0) | 56 (5.4) |
| Clean-contaminated | 69 (46.4) | 188 (3.2) | 257 (14.8) |
| Contaminated/dirty | 18 (72.0) | 6 (17.0) | 24 (58.3) |
| All | 138 (34.8) | 199 (3.5) | 337 (16.3) |
SSI = surgical site infection.
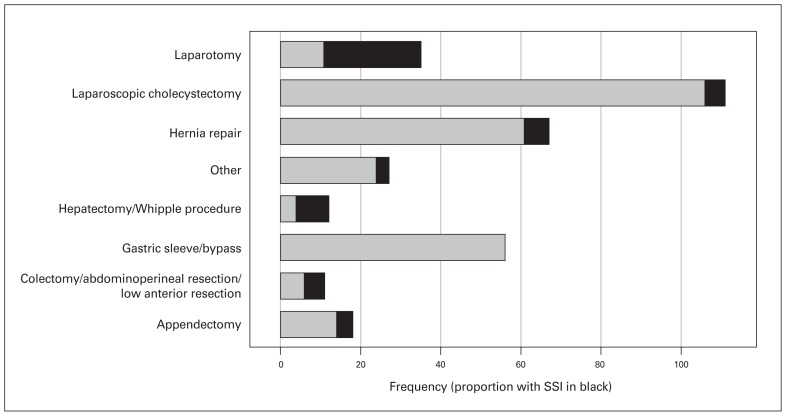
The overall rate of SSI was 16.3% (55/337). The wound classification is detailed in Table 2 for patients with and without SSI. The demographic and preoperative variables for the 2 groups are presented in Table 3. The median postoperative hospital stay was 2 days for patients without SSI, compared to 13 days for those with SSI (p < 0.001). Details of postoperative variables by SSI group are shown in Table 4. Of the 55 patients with SSI, 25 (45%) had superficial infections, 5 (9%) had deep infections, and 25 (45%) had combined superficial and deep infections. Surgical site infection rates per procedure are illustrated in Figure 1. The highest SSI rate was detected in laparotomy wounds: SSI developed in 24 (69%) of the 35 patients in this group. None of the patients who underwent bariatric surgery experienced SSI. The infection rate was 34.8% following open operations, compared to 3.5% following laparoscopic procedures (p < 0.001). Surgical site infection developed in 22 (61%) of the 36 patients with malignant disease, compared to 33 (11.0%) of the 300 patients with benign disease (p < 0.001). The 30-day mortality rate was 0.7% among non-infected patients and 3.6% among infected patients.
Table 3.
Demographic and preoperative risk factor variables of patients with and without surgical site infection
| Variable | Group; no. (%) of patients* | p value | |
|---|---|---|---|
| No SSI n = 282 |
SSI n = 55 |
||
| Age, yr, median (IQR) | 40 (31–52) | 54 (43–61) | < 0.001† |
| Sex | |||
| Male | 104 (36.9) | 40 (73) | < 0.001‡ |
| Female | 178 (63.1) | 15 (27) | |
| Body mass index, median (IQR) | 29 (25–36) | 27 (25–31) | 0.02† |
| ASA score | < 0.001‡ | ||
| 1 | 143 (50.7) | 16 (29) | |
| 2 | 107 (37.9) | 13 (24) | |
| 3 | 31 (11.0) | 18 (33) | |
| 4 | 1 (0.4) | 6 (11) | |
| 5 | 0 (0.0) | 2 (4) | |
| Diabetic | 52 (18.4) | 18 (33) | 0.02‡ |
| Smoking status | 0.004‡ | ||
| Smoker | 36 (12.8) | 5 (9) | |
| Former smoker | 8 (2.8) | 5 (9) | |
| Nonsmoker | 216 (76.6) | 34 (62) | |
| Unknown | 22 (7.8) | 11 (20) | |
| Steroid use | 11 (3.9) | 4 (7) | 0.3‡ |
| Chemotherapy | 6 (2.1) | 4 (7) | 0.04‡ |
| Preoperative hemoglobin level, g/L, median (IQR) | 13 (12–14) | 12 (11–14) | 0.1† |
| Preoperative albumin level, g/L, median (IQR) | 36 (33–38) | 31 (26–35) | < 0.001† |
| Benign disease | 267 (94.7) | 33 (60) | < 0.001‡ |
ASA = American Society of Anesthesiologists; IQR = interquartile range; SSI = surgical site infection.
Except where noted otherwise.
Wilcoxon test.
Pearson test.
Table 4.
Demographic, operative and postoperative risk factor variables of patients with and without surgical site infection
| Variable | Group; no. (%) of patients* | p value | |
|---|---|---|---|
| No SSI | SSI | ||
| Operation | < 0.001† | ||
| Laparoscopic cholecystectomy | 106 (37.6) | 5 (9) | |
| Hernia repair | 61 (21.6) | 6 (11) | |
| Gastric sleeve/bypass | 56 (19.8) | 0 (0) | |
| Appendectomy | 14 (5.0) | 4 (7) | |
| Laparotomy | 11 (3.9) | 24 (44) | |
| Colectomy/abdominoperineal resection/low anterior resection | 6 (2.1) | 5 (9) | |
| Hepatectomy/Whipple procedure | 4 (1.4) | 8 (14) | |
| Other | 24 (8.5) | 3 (5) | |
| Wound class | < 0.001† | ||
| Clean | 53 (18.8) | 3 (5) | |
| Clean-contaminated | 219 (77.6) | 38 (69) | |
| Contaminated/dirty | 10 (3.5) | 14 (25) | |
| Urgency | < 0.001† | ||
| Emergent | 50 (17.7) | 30 (54) | |
| Elective | 232 (82.3) | 25 (45) | |
| Approach | < 0.001† | ||
| Laparoscopic | 192 (68.1) | 7 (13) | |
| Laparoscopic converted to open | 3 (1.1) | 3 (5) | |
| Open midline | 35 (12.4) | 35 (64) | |
| Open nonmidline | 52 (18.4) | 10 (18) | |
| Length of operation, min, median (IQR) | 83 (110–155) | 184 (113–292) | < 0.001† |
| Drains | 78 (27.6) | 27 (49) | 0.007‡ |
| Blood transfusion | 14 (5.0) | 22 (40) | < 0.001† |
| Postoperative stay, d, median (IQR) | 2 (1–3) | 13 (6–25) | < 0.001† |
| Intensive care unit admission | 22 (7.8) | 28 (51) | < 0.001‡ |
| Death within 30 d | 2 (0.7) | 2 (4) | 0.07 |
IQR = interquartile range; SSI = surgical site infection.
Except where noted otherwise.
Pearson test.
Wilcoxon test.
Fig. 1.
Surgical site infection (SSI) rates per operation.
Generally, infected patients were older and more commonly male (40 [73%] v. 104 [36.9%], p < 0.001) than noninfected patients. Almost half (26 [47%]) of infected patients had an ASA score greater than 2. Patients with a low albumin level were more prone to SSI (p < 0.001). Notably, more than half (30 [54%]) of infected patients underwent emergency surgery, and most (48 [87%]) had an open surgical approach. Just over half (28 [51%]) were admitted to the intensive care unit. Use of steroids, preoperative hemoglobin level, prophylactic antibiotic therapy and death within 30 days were not associated with SSI on univariable analysis. Multivariable analysis revealed that patients who underwent open laparotomy were 6.5 times (95% confidence interval [CI] 2.16–19.6) more likely to experience SSI than those who had laparoscopic procedures (Table 5). Surgical site infection was 4.8 times (95% CI 1.58–14.4) more likely to develop in patients who underwent emergent operations than those who underwent elective procedures. Operation duration and male sex were also independent predictors of SSI, with odds ratios of 2.1 (95% CI 1.23–3.6) and 2.6 (95% CI 1.02–6.6), respectively.
Table 5.
Multivariate logistic regression analysis of factors associated with surgical site infection
| Risk factor | OR (95% CI) |
|---|---|
| Male sex | 2.6 (1.02–6.6) |
| Length of operation (86 min v. 181 min [25th v. 75th percentile]) | 2.1 (1.23–3.6) |
| Urgency (emergent v. elective) | 4.7 (1.58–14.4) |
| Approach (open v. laparoscopic) | 6.5 (2.16–19.6) |
| Age | 1.09 (0.57–2.1) |
| Body mass index | 1.20 (0.68–2.1) |
| Smoking | 0.55 (0.15–2.1) |
| Diabetic | 1.52 (0.52–4.5) |
| ASA score | 1.27 (0.75–2.2) |
| Blood transfusion | 0.93 (0.28–3.2 ) |
| Preoperative albumin level | 0.91 (0.63–1.3) |
| Malignant disease | 2.35 (0.65–8.5) |
| Preoperative antibiotic | 2.32 (0.71–7.6) |
| Wound type (contaminated/dirty v. clean-contaminated) | 1.59 (0.48–5.3) |
ASA = American Society of Anesthesiologists; CI = confidence interval; OR = odds ratio.
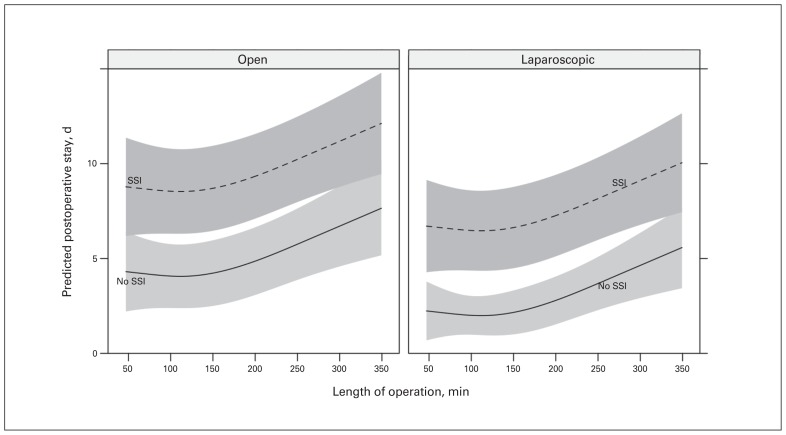
The cost of surgery is mainly related to the duration of the hospital stay. We found that length of hospital stay was predicted to increase with open versus laparoscopic procedures, long operative times (a surrogate for complexity) and the development of SSI (Fig. 2). A patient who underwent a laparoscopic operation less than 150 minutes in length and did not experience SSI was predicted to stay less than 2 days in hospital, whereas a patient who underwent an open operation that lasted 350 minutes in whom SSI developed was predicted to stay in hospital for 12 days.
Fig. 2.
Effect of surgical site infection (SSI) and open v. laparoscopic approach on length of stay.
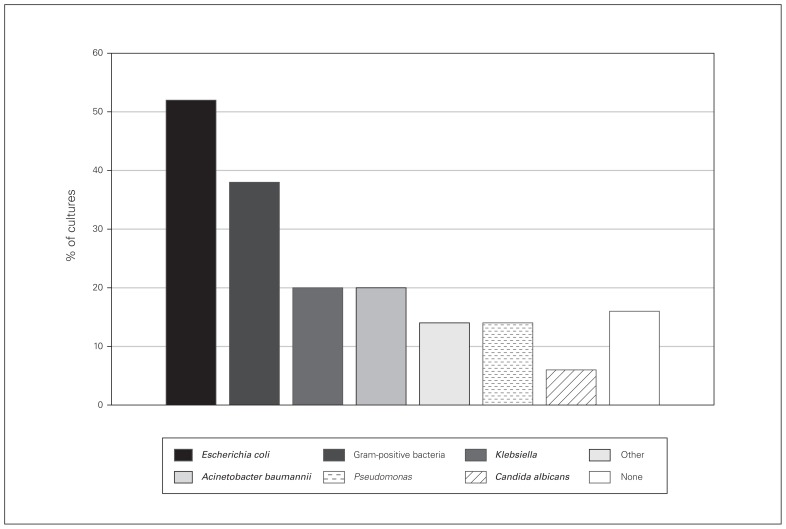
Microbiological cultures were available for 50 patients with SSI. The most commonly implicated pathogens were gram-negative bacteria, namely Escherichia coli (26 patients [52%], of whom 16 had extended-spectrum β-lactamase-producing E. coli ), followed by gram-positive bacteria (19 patients [38%]) and a considerable number of Acinetobacter baumannii and Pseudomonas (Fig. 3). Interestingly, only 23% of cultured bacteria were sensitive to the prophylactic antibiotic given preoperatively.
Fig. 3.
Culture results for 50 patients. % of cultures
All of our patients were given the antibiotic prophylaxis according to our standard hospital protocols (Table 6). All patients undergoing elective procedures take an antiseptic bath with chlorhexidine gluconate solution the night before the surgery. When hair removal is considered necessary, clipping is carried out in the operating theatre. Topically administered chlorhexidine gluconate or iodine is used for skin preparation.
Table 6.
King Abdulaziz University Hospital surgical prophylaxis guidelines
| Operation | Antibiotic prophylaxis | Antibiotic |
|---|---|---|
| Upper gastrointestinal (esophagus, stomach and small bowel) | Recommended | Cefazolin,* 1–2 g or Clindamycin,† 600 mg + gentamicin, 120 mg intravenously |
| Hepatobiliary | ||
| Bile duct, pancreatic, liver and open gallbladder | Recommended | Cefazolin,* 1–2 g or Clindamycin,† 600 mg + gentamicin, 120 mg intravenously |
| Laparoscopic cholecystectomy | Not recommended; should be considered in patients at high risk | Cefazolin,* 1–2 g or Clindamycin,† 600 mg + gentamicin, 120 mg intravenously |
| Lower gastrointestinal (appendix and colorectum) | Highly recommended | Cefoxitin, 1–2 g intravenously every 6 h preoperatively for 3 doses or Cefazolin,* 1–2 g intravenously + metronidazole, 500 mg intravenously |
| Hernia repair, groin (inguinal/femoral with or without mesh, laparoscopic or incisional | Not recommended | — |
A dose of 2 g of cefazolin is recommended for patients weighing more than 80 kg. Dosing cefazolin for renal impairment: creatine clearance 35–54 mL/min: administer full dose in intervals of ≥ 8 hours; creatine clearance 11–34 mL/min: administer half of usual dose every 12 hours; creatine clearance ≤ 10 mL/min: administer half of usual dose every 18–24 hours.
No dosage adjustment required for renal impairment.
Discussion
In this cohort, SSI developed in 55 patients (16.3%) undergoing abdominal surgery, compatible with reported rates in the literature.2,10 However, our rate is slightly higher that those reported in studies done in Saudi Arabia, 12%11 and 10.5%.12 The variation is partially attributable to the higher number of complex oncological and emergency procedures performed in our tertiary teaching hospital.
Multivariable analysis identified open surgical approach, emergency operation, length of the operation and male sex as independent predictors of SSI. Open surgical approach and emergency surgery were documented as risk factors for SSI in previous reports.13–15 We found that patients who had open surgery were 6.5 times more likely to get SSI than those who had laparoscopic surgery. Emergency surgery increased the risk of SSI fivefold compared to elective surgery. The rate of SSI was significantly higher in male patients. This finding is not novel.16 Although there is no consensus regarding why male patients are predisposed to SSI, studies have shown that, in laparoscopic cholecystectomy, male sex is a predictor of longer and more difficult operations and has a higher rate of conversion.17–19 In addition, it is known that there are sex differences in skin colonization that may be associated with differences in skin thickness, sebum production and skin pH.20,21
Among the 3 components of the National Nosocomial Infections Surveillance System risk index, only the duration of surgery was an independent predictor for SSI in our study. A patient who had an operation lasting longer than the 75th percentile (> 3 h in our cohort) had double the risk of SSI in contrast to an operation lasting less than the 25th percentile (86 min). This finding is in keeping with previous reports in the literature.5,11 Longer operative time reflects the complexity of the surgery. It would also increase the wound susceptibility to infection by increasing the exposure to potential contamination and decreasing the tissue concentration of antibiotic.22 To overcome the decreased concentration of antibiotic that occurs with prolonged operations, readministration of the antibiotics is recommended.23
The ASA score and wound class were not significant predictors of SSI in the multivariable model in our study. Certain other known risk factors for SSI such as body mass index, diabetes and smoking were also not found to be statistically significant. Obesity was not found to be a risk factor for SSI in our patients. This may be explained by the fact that obese patients had more laparoscopic bariatric operations, which have a low risk of infection.24 Patients with diabetes and smokers had higher rates of infection; however, the differences were not statistically significant. This may have been due to the sample size and the specific case-mix at our institution.
In this study, the commonest organisms isolated from patients with SSI were gram-negative bacteria, namely extended-spectrum β-lactamase-producing E. coli. This finding is contrary to those in studies that revealed more gram-positive bacteria such as Staphylococcus aureus and coagulase-negative staphylococci.2,25 However, other authors reported findings similar to ours, with more common gram-negative bacteria isolated from the infected abdominal wounds in Al-Ahsa,11 Saudi Arabia2 and Tanzania. 5 We also found that most of the pathogens were multiresistant to the commonly prescribed prophylactic antibiotics. This might explain why we found a high rate of deep SSI. Further consideration regarding the selection of appropriate prophylactic antibiotics will be needed, especially in patients at high risk.
Surgical site infection was associated with increased length of postoperative stay. Moreover, an increased operative time was associated with both higher SSI rate and prolonged postoperative stay. Although we did not evaluate the economic impact of SSI in our study, it is likely that longer postoperative stay due to SSI entails a higher cost of patient care.
Conclusion
The present study identified several independent risk factors for SSI following abdominal surgery that should be addressed systematically. We believe that these results will be helpful in updating the guidelines for preventing SSI in the region. Furthermore, we recommend tailoring the prophylactic antibiotic regimens to target the commonly isolated organisms, especially in the presence of independent risk factors.
Acknowledgements
The authors acknowledge the medical students who helped with data collection: Mohmd Alhamed, Faisal Idrees and Sundos Turostani.
Footnotes
An earlier version of this work was presented at the Canadian Surgery Forum 2017, Sept. 14–16, 2017, Victoria, BC.
Competing interests: None declared.
Contributors: A. Alkaaki, O. Al-Radi, A. Khoja, Abrar Alnawawi, A. Maghrabi, A. Altaf and M. Aljiffry designed the study. A. Alkaaki, A. Khoja, Anfal Alnawawi, Abrar Alnawawi and M. Aljiffry acquired the data, which A. Alkaaki, O. Al-Radi, Anfal Alnawawi, Abrar Alnawawi, A. Altaf and M. Aljiffry analyzed. A. Alkaaki, O. Al-Radi, Anfal Alnawawi, A. Maghrabi and M. Aljiffry wrote the article, which all authors reviewed and approved for publication.
References
- 1.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Azoury S, Farrow N, Hu Q, et al. Postoperative abdominal wound infection — epidemiology, risk factors, identification, and management. Chron Wound Care Manage Res. 2015;2:137–48. [Google Scholar]
- 3.Aga E, Keinan-Boker L, Eithan A, et al. Surgical site infections after abdominal surgery: incidence and risk factors. A prospective cohort study. Infect Dis (Lond) 2015;47:761–7. doi: 10.3109/23744235.2015.1055587. [DOI] [PubMed] [Google Scholar]
- 4.Legesse Laloto T, Hiko Gemeda D, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis. 2017;17:119. doi: 10.1186/s12879-016-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mawalla B, Mshana SE, Chalya PL, et al. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surg. 2011;11:21. doi: 10.1186/1471-2482-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–97. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–41. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 8.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–30. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 9.Gaynes RP, Culver DH, Horan TC, et al. Surgical site infection (SSI) rates in the United States, 1992–1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Nephrol Dial Transplant. 2001;33(Suppl 2):S69–77. doi: 10.1086/321860. [DOI] [PubMed] [Google Scholar]
- 10.Mihaljevic AL, Müller TC, Kehl V, et al. Wound edge protectors in open abdominal surgery to reduce surgical site infections: a systematic review and meta-analysis. PLoS One. 2015;10:e0121187. doi: 10.1371/journal.pone.0121187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawabdeh A, Rahman Saleh Al Mulhim A, Khan ZU. Surgical site infections incidence, their predictors and causative organisms in a teaching hospital. Int J Community Fam Med. 2016;1:104. [Google Scholar]
- 12.Khairy GA, Kambal AM, Al-Dohayan AA, et al. Surgical site infection in a teaching hospital: a prospective study. J Taibah Univ Med Sci. 2011;6:114–20. [Google Scholar]
- 13.de Oliveira AC, Ciosak SI, Ferraz EM, et al. Surgical site infection in patients submitted to digestive surgery: risk prediction and the NNIS risk index. Am J Infect Control. 2006;34:201–7. doi: 10.1016/j.ajic.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Segal CG, Waller DK, Tilley B, et al. An evaluation of differences in risk factors for individual types of surgical site infections after colon surgery. Surgery. 2014;156:1253–60. doi: 10.1016/j.surg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Imai E, Ueda M, Kanao K, et al. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control. 2008;36:727–31. doi: 10.1016/j.ajic.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Morikane K. Epidemiology and risk factors associated with surgical site infection after different types of hepatobiliary and pancreatic surgery. Surg Today. 2017;47:1208–14. doi: 10.1007/s00595-017-1503-0. [DOI] [PubMed] [Google Scholar]
- 17.Akcakaya A, Okan I, Bas G, et al. Does the difficulty of laparoscopic cholecystectomy differ between genders? Indian J Surg. 2015;77(Suppl 2):452–6. doi: 10.1007/s12262-013-0872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simopoulos C, Botaitis S, Polychronidis A, et al. Risk factors for conversion of laparoscopic cholecystectomy to open cholecystectomy. Surg Endosc. 2005;19:905–9. doi: 10.1007/s00464-004-2197-0. [DOI] [PubMed] [Google Scholar]
- 19.Ambe PC, Weber SA, Wassenberg D. Is gallbladder inflammation more severe in male patients presenting with acute cholecystitis? BMC Surg. 2015;15:48. doi: 10.1186/s12893-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fierer N, Hamady M, Lauber CL, et al. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A. 2008;105:17994–9. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MK, Choi SY, Byun HJ, et al. Evaluation of gender difference in skin type and pH. J Dermatol Sci. 2006;41:153–6. doi: 10.1016/j.jdermsci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Leong G, Wilson J, Charlett A. Duration of operation as a risk factor for surgical site infection: comparison of English and US data. J Hosp Infect. 2006;63:255–62. doi: 10.1016/j.jhin.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 24.Shabanzadeh DM, Sørensen LT. Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis. Ann Surg. 2012;256:934–45. doi: 10.1097/SLA.0b013e318269a46b. [DOI] [PubMed] [Google Scholar]
- 25.Sugiura T, Uesaka K, Ohmagari N, et al. Risk factor of surgical site infection after pancreaticoduodenectomy. World J Surg. 2012;36:2888–94. doi: 10.1007/s00268-012-1742-6. [DOI] [PubMed] [Google Scholar]